History of Malaria Control
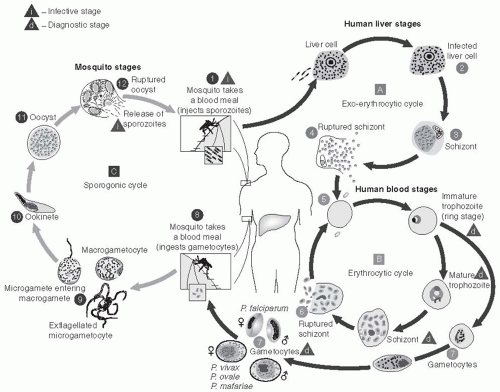
Vertebrates, mosquitoes, and plasmodia have been interacting and evolving together for tens of thousands of years, and humans have been afflicted with malaria as long as there have been humans. Documentation of what is certainly malaria dates back to 2700 bce in China, and malaria is featured in the writings of Homer, Plato, Aristotle, Chaucer, Pepys, and Shakespeare. It has been more than 100 years since the discovery that malaria is caused by a protozoan parasite that infects red blood cells and is transmitted by mosquitoes from human to human. In 1902, Ronald Ross was awarded the Nobel Prize in medicine for his work on malaria and its transmission cycle. Since then, major scientific advances have continued to understand the parasite, its life cycle within anopheline mosquitoes, and its pathogenesis in humans.
1The Greeks had known of the relationship between fever and swamps and low-lying water since the sixth century bce. Control of mosquito breeding through drainage and environmental control was a key aspect in the development of the Panama Canal and continued to be the major approach to malaria control until after World War II. During World War II, two major biochemical and pharmaceutical advances in unrelated fields revolutionized malaria control and its treatment: dichlorodiphenyltrichloroethane (DDT) as an insecticide was found to be highly effective against anopheline vectors, and chloroquine replaced quinine as the principal antimalarial drug.
With these new tools, plans for the reduction and control of malaria were envisioned, but beyond that the prospect of total eradication of this horrendous disease was proposed. Fundamentally, achieving this goal required the elimination of every parasite of all human malaria species, principally by stopping the transmission by the vector from one human to the next. Residual spraying of DDT on the walls of households was the major weapon employed and, in nearly all early trials, proved remarkably effective in killing mosquitoes that had just taken a blood meal from a sleeping household resident. The availability of chloroquine—a rapidly and uniformly curative drug with a wide margin of safety and very low cost—for treatment provided an additional tool.
Following World War II, the newly formed World Health Organization (WHO) formulated a malaria eradication plan at the 8th World Health Assembly in 1955. It was estimated that eradication using DDT residual spraying could be accomplished at a cost of less than 25 cents per person per year; the total cost for the first 5 years would be $500 million.
2Plans were put into action in many areas of the world, and truly dramatic success was achieved in some regions. By the late 1950s, the most inspirational, ambitious, complex, and costly health campaign ever undertaken was well under way.
3 Early campaign efforts in many countries in Europe, Asia, and Latin America were enormously successful. Indeed, in Malta the anopheline vector was completely eliminated. In contrast, little effect was seen in many continental tropical countries of Asia and South America. In Africa, where malaria was by far of greatest importance, virtually nothing was even attempted. Unfortunately, the emphasis on logistics and organization was accompanied by a comparable de-emphasis on scientific research.
3 Over a period extending for 20 years, virtually no innovative research on malaria was undertaken—the general opinion, frequently and loudly expressed, was that
“We know what has to be done; let us get on with it!” As a consequence, an entire generation of malaria researchers was lost.
Even by the mid-1960s, it was clear that the eradication campaign would fail. The complex logistical and operational needs were too much for the weak infrastructures in most tropical countries; moreover, basic biological developments emerged, including anopheline resistance to insecticides and parasite resistance to antimalarials.
4 Over time, the malaria eradication campaign came to be viewed as a major failure. In the wisdom of hindsight, the failure was a result of scientific arrogance and lack of foresight. Nevertheless, it should not be forgotten that large numbers of lives were saved in many countries, and major economic activities were spurred.
After the failed malaria eradication efforts, most countries greatly reduced their expenditures on malaria programs; in many areas, the result was an epidemic resurgence of malaria. However, with a shift in focus to reducing mortality and serious morbidity, rather than seeking eradication, came the gradual emergence of researchers seeking a fuller understanding of malaria parasites, the pathogenesis of malaria disease, and new approaches to control. By the end of the 1990s, major advances had been made in understanding the molecular biology of the malaria parasite, parasite and vector genomics and proteomics, vector control methods, the immunological responses to malaria infection, the rational development of novel antimalarial agents and vaccines, and a variety of innovative strategies for malaria control.
WHO, UNICEF, the United Nations Development Programme, and the World Bank came together to provide a coordinated international approach to fighting malaria when they launched the Roll Back Malaria (RBM) Partnership in 1998. The RBM Partnership is a global initiative of more than 90 partners whose activities include coordinating the many stakeholders; working toward an in-depth understanding of the ecology, biology, and epidemiology of malaria, particularly in Africa; developing comprehensive and cohesive planning; and raising funds and political support. Other important efforts to reduce malaria morbidity and mortality include the Multilateral Initiative on Malaria (MIM), the Malaria Vaccine Initiative (MVI), the President’s Malaria Initiative (PMI), and the Global Fund to Fight AIDS, Tuberculosis, and Malaria. The Bill and Melinda Gates Foundation has challenged the public health community by calling for renewed efforts to achieve malaria eradication and committed enormous resources to this goal.
The terms “control,” “elimination,” and “eradication” for malaria are now defined
5 as follows:
Malaria control: reducing the disease burden to a level at which it is no longer a public health problem.
Malaria elimination: interrupting local mosquito-borne malaria transmission in a defined geographical area—that is, zero incidence of locally contracted cases, although imported cases will continue to occur. Continued intervention measures are required.
Malaria eradication: permanent reduction to zero of the worldwide incidence of malaria infection.
Although the goal of global eradication per se remains contentious, prospects have been brightened such that organizations can now realistically work toward malaria elimination in many parts of the globe. It is vital that such malaria elimination and eradication efforts be based on detailed understanding of local malaria epidemiology and transmission dynamics, effective use of current tools and strategies, and, likely, new tools, particularly with the threat of the spread of drug and insecticide resistance.
6
Public Health Importance
The impact of malaria in human populations varies greatly in different parts of the world. Wherever there is
Plasmodium falciparum, there will be dire consequences, but the public health consequences will vary according to the intensity of transmission. Traditionally, geographical areas are classified into four levels of endemicity (intensity of transmission) as set forth in
Table 27-1.
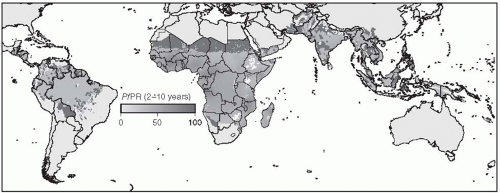
Figure 27-1 shows a map of the distribution of
Plasmodium falciparum malaria created by the Malaria Atlas Project.
7Although P. vivax malaria is a major cause of morbidity in parts of China, Southeast and Central Asia, Latin America, and the Caribbean, the overwhelming burden of disease occurs in those countries subjected to P. falciparum malaria, especially in sub-Saharan Africa. Most of the discussion of this type of malaria in this chapter will focus on issues related to tropical Africa, where malaria is of greatest importance and where approaches to control have had, until recently, the least success.
The public health significance of a disease depends on its incidence and resulting disability and mortality. In addition, this impact depends on epidemiological characteristics such as those related to person (age, sex, and other demographic variables such as occupation, education, and socioeconomic group), place (urban and rural, or particular
ecological zones), and time (including seasonal or other cyclical variation or secular trends). In infectious diseases, the incidence of a disease is commonly expressed in terms of episodes per time period or the number of persons affected per time period. In many places where malaria is hypoendemic or mesoendemic, the incidence of malaria has meaning and can be expressed as the number of episodes per thousand persons per year or as the number of persons having episodes per thousand persons per year. In holoendemic areas, however, with an entomological inoculation rate ranging from dozens to hundreds of infectious bites per person per year, everyone is infected all the time and is reinfected every few days. The very concept of incidence, or indeed prevalence, has little meaning in this context. Instead, the health status of an individual in these situations results from a balance between the parasite and host immunity. Clearance of parasites from the host occurs with reasonable certainty only when an individual is given an effective antimalarial drug. Reinfection and, therefore, “a new incident” case occurs as soon as the level
of the antimalarial drug drops below the effective therapeutic level and recently injected sporozoites develop into blood-stage parasites. Further discussion of different measures of malaria transmission is found in the “Malaria Metrics” section later in this chapter.
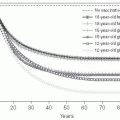
Since rates were first reported by WHO in the 1950s, childhood malaria mortality has been estimated at 1-2 million children per year. In the past decade, however, two important trends have taken place: the quality of malaria mortality data has improved and, even more notably, increased effectiveness of antimalarial efforts has become evident. In a 2004 WHO report, global deaths from malaria were estimated to have dropped to 1,272,000 (with 1,136,000 in Africa).
8 A continuing decline in mortality is supported by the estimates for 2009 of 781,000 deaths from malaria worldwide (including 709,000 in Africa).
9An indirect indicator of the importance of malaria mortality is the relative frequency of sicklecell trait (AS) in tropical Africa. This deleterious trait is maintained in the population because of the protection it provides against malaria. The high proportion of adults with the AS genotype in West Africa is a reflection of the selective mortality, 15-20%, among children with the AA genotype (see the section on “Human Genetic Factors”).
10