Introduction
Intraarterial chemotherapy has long been investigated as a potentially superior treatment modality for tumors of the cervicocephalic, thoracic and abdominopelvic regions, and the limbs. Tumors of the brain, head, and neck have attracted the most attention for several reasons. For brain tumors, particularly high-grade gliomas, the therapeutic benefits from systemic therapy are minimal, even for drugs with recognized activity and a demonstrated dose–response relationship. Theoretically, local infusion should achieve a higher drug concentration in the tumor. This would provide a potentially important palliative option for brain metastases, which are rapidly debilitating and fatal. By the time these tumors are radiologically detectable, there is impairment of the blood–brain barrier, which until then had protected the cancer cells from the action of chemotherapeutic agents administered systemically. Direct intraarterial administration should assist in overcoming drug resistance by ensuring higher concentrations of the drug at the site needed most.
For tumors of the oral cavity, hypopharynx, and larynx, other considerations arise. The early onset of local pain, interference with feeding and speech, and general deterioration call for a treatment that produces the rapid response necessary for the physical and psychological recovery of these patients, who often have advanced disease at presentation. The ability to provide a therapy that could avoid devastating operations would have a major impact on quality of life, particularly because these operations do not improve the prognosis.
Recent percutaneous catheterization techniques, deriving from neuroradiologic practice, have reopened the possibilities for intraarterial therapy. New criteria for preliminary diagnosis and response assessment, and new drug formulations, have revolutionized pharmacokinetic criteria, so higher concentrations can be achieved not only in the area of the tumor but with a significant difference between the tumor and surrounding tissue.
Background of intraarterial chemotherapy for tumors of the head, neck, and brain
Higher local concentrations
Certain characteristics must be taken into consideration when choosing drugs for locoregional treatment by direct intraarterial infusion. Drugs with a particular pharmacokinetic profile, with rapid systemic clearance, are considered to offer an advantage over intravenous administration. Single-drug or combinations of drugs such as carmustine, cisplatin, carboplatin, etoposide, methotrexate, gemcitabine, irinotecan, anthracyclines, and taxanes as macrocomplexes have long been identified as having potential for intraarterial use. Some drugs cross the blood–brain barrier intact but do not reach concentrations that can destroy micrometastases.
The potential pharmacokinetic superiority of intraarterial chemotherapy is manifested at the first pass through the tumor circulation. The positive consequences of the first pass are more evident for some drugs than others, namely, those that have a high extraction fraction by the tumor coupled with rapid systemic clearance through metabolism or excretion.
The blood–brain barrier
Primary or metastatic brain tumors merit a chapter apart because the blood–brain barrier, if intact, does not allow drugs to pass into the brain tissue at high enough concentrations to prevent the infiltration of microscopic metastases. , Intraarterial chemotherapy, when applicable, should not be considered a stand-alone option but should be combined with external radiotherapy to control microscopic disease. When the blood–brain barrier is not intact, the radiopotentiating or radiosensitizing properties of some chemotherapy drugs are potentially predictive of greater efficacy.
Chemosensitivity
Chemosensitivity must be considered, when discussing higher or lower drug concentrations and longer or shorter exposure of primary or metastatic tumors, whether in the brain or head and neck. , Chemosensitivity is generally classified as high, intermediate or low. Chemotherapy has proved effective even with systemic administration in patients with brain metastases from spindle-cell carcinomas, breast cancer, and germ cell tumors. Intraarterial infusion tends to enhance the therapeutic response even if the site—generally not just one—classifies the treatment as palliative with the aim of improving the patient’s quality of life.
New chemotherapy formulations
As for some extraencephalic tumors, a new formulation of liposome-encapsulated anthracyclines has achieved higher intratumoral drug concentrations. Anthracyclines are active in glioblastoma in vitro, with a close dose–response relationship. Recent studies with systemically administered liposomal doxorubicin for recurrent high-grade gliomas appear to provide in vivo confirmation of greater antitumoral efficacy. This takes into consideration a developing area of chemotherapy that is based on the enhanced permeability and retention effect due to the characteristics of the tumor’s capillary and lymphatic network. ,
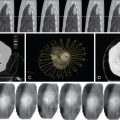
All tumors have an imperfect vascular and lymphatic architecture. Tumor neovascularity demonstrates profound alterations in the structure of the vessel walls and in the anatomic artery–capillary–vein sequence, with arteriovenous shunts and vascular lakes. These findings are clearly visible in histology slides and can be evident in vascular radiology diagnostic examinations and in high spatial and temporal resolution multislice contrast CT studies. Depending on the tumor size and histologic type, the permeability of the vascular wall allows the passage of substances in the form of macrocomplexes of considerable size, even of the order of 150–200 nm. The longer retention of the macrocomplexes in the tumor interstitium is partly due to inefficient lymphatic drainage by the tumor lymphatic network, which is also abnormal.
The majority of cytostatic drugs are phase-specific and those that have a shorter contact time do not normally have as good a chance of interfering with a significant number of tumor cells synchronized in a favorable phase for a lethal encounter. Pharmaceutical engineering has intentionally in some cases—and unintentionally in others—created a sort of camouflage for some cytostatic molecules. They can thus reach a higher intratumoral concentration, and their intracellular penetration is better too, with many more cells killed.
Liposome-encapsulated drugs provide an example of low pharmacologic signaling. An example of potentially more effective disguise is incorporation of the taxane paclitaxel into albumin microparticles. Two specific formulations have been used in intraarterial chemotherapy for tumors of the head and neck and of the brain: liposomal doxorubicin (Caelyx, Janssen-Cilag SpA, Belgium), Myocet (Chepalon, PA), and paclitaxel in albumin microparticles (Abraxane, Celegen Corporation, NJ). Anthracyclines are among the most active agents in a variety of tumors and experimentally they appear active on primary brain tumors. Liposome-encapsulated doxorubicin and conventional doxorubicin have different pharmacokinetic and therapeutic behavior. Liposomes are vesicles that closely resemble cell membranes; they can have a monolamellar or multilamellar structure, and range from 20 nm to 1 μm in diameter. Once administered into the circulation, liposomes tend to persist longer in the vessels as they travel through normal tissue. The loss of structural integrity of the liposome in the plasma is only a question of time and these corpuscles are sequestered fairly fast by the macrophage system, so when used systemically the time of drug release is not optimal; intraarterial administration helps avoid this issue.
To avoid rapid recognition by the reticuloendothelial system and subsequent destruction, monolamellar liposomes coated with chains of polyethylene glycol (peg), a hydrophilic polymer, have been designed. These “stealth” liposomes remain in the circulation longer with a greater chance of penetrating the tumor sites while respecting healthy tissue. A special formulation of nonpegylated liposomes with a size of 150 nm is available on the market. These are loaded with doxorubicin shortly before administration. In view of its size, the macrocomplex should accumulate more in the tumor interstitium because of slower drainage. These liposomes should also release doxorubicin more readily because of lower membrane resistance.
Taxanes are a class of antitumoral drugs that has had the same clinical impact as anthracyclines and cisplatin as regards its results but seemed to be completely excluded from the possibility of intraarterial administration because the original formulations contained alcohol and a surfactant agent, Cremophor, which are both irritants for the endothelium and can induce frequent, severe allergic reactions. In the late 1990s, at the Special Radiological Procedures section of the Radiology Unit of the National Cancer Institute in Milan, Damascelli and colleagues began to use the paclitaxel-albumin macrocomplex ABI-007, developed to allow easier and less toxic parenteral administration of the taxane, selectively by the intraarterial route in various malignancies. Attention focused on squamous cell carcinoma of the head and neck, a historical target of intraarterial chemotherapy. The vehicle used—albumin—seemed to increase the permeability, further raising the drug concentration inside the tumor. Cancer cells develop the molecular transport mechanism of albumin to the maximum, as it is their main energy source, with receptors in the tumor vessel endothelium responsible for this transport. The receptors form caveolae or sacs in which the drug is enveloped and then emptied into the tumor interstitium where free paclitaxel penetrates the tumor cells and causes cell death by interfering with the microtubule system. If antitumoral efficacy depends on the effective dose in the tumor, it follows that regardless of the mechanisms of final transport, the greater local availability ensured by intraarterial administration is a considerable strength of this modality, when practicable.
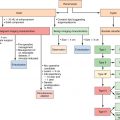
Intraarterial chemotherapy technology and technique for brain and head and neck tumors
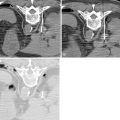
Two main techniques have been developed for transcatheter intraarterial chemotherapy: one with surgical placement and the other with percutaneous placement. Surgical catheter placement is used only for tumors fed by branches of the external carotid artery. The technique entails preparation of the superficial temporal artery anteriorly to the tragus of the ear, arterial cut-down, and insertion of a small-diameter catheter (2-F). The position of the catheter tip can be adjusted by injecting a vital dye (Patent Blue Violet) and checking which cutaneous or mucous region is stained. If the tongue is stained, the catheter tip will be just below the lingual artery; if the palate is stained, it will be just below the internal maxillary artery. Because of the size of the catheter, contrast medium can give rise to inadequate images, even with digital angiography.
Percutaneous catheterization of the neck vessels, generally through the femoral artery, is much more flexible, offering the possibility of treating both sides in a single session and selecting the appropriate region with ready reproducibility and acceptable invasiveness. The procedure is done in serial sessions, normally at 3-week intervals, under local anesthesia and with a minimal hospital stay. The utmost care is recommended for percutaneous access, choosing the smallest-diameter catheters, alternating the side of access and using simple manual compression or dedicated devices for hemostasis.
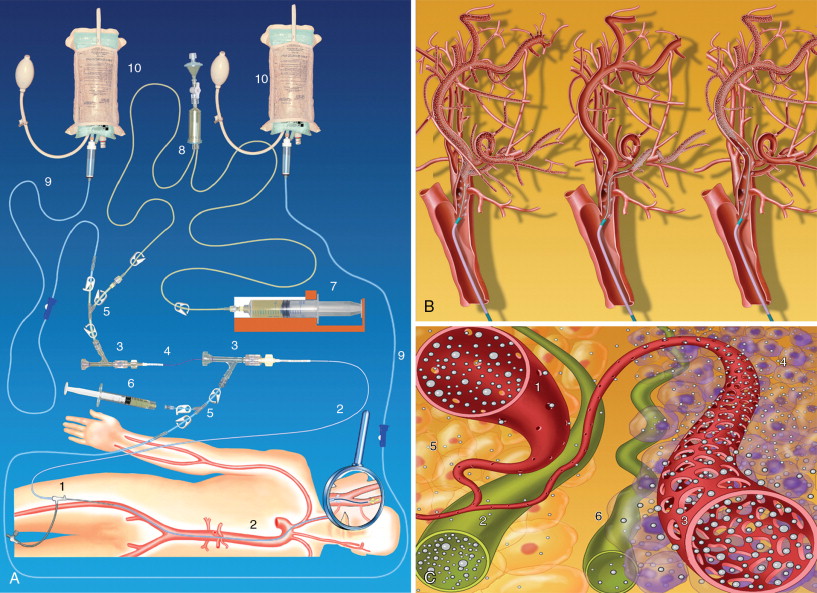
Damascelli and colleagues have developed a disposable coaxial infusion circuit that facilitates the maneuvers of prolonged catheterization. To reduce the risk of thromboembolism, the guide catheter is perfused with heparinized saline throughout the diagnostic stage and the infusion of cytostatic drugs. Superselective catheterization, generally reserved for branches of the external carotid artery, is done with microcatheters and microguides served by an independent flushing line. The drug can be infused using a pump capable of overcoming the resistance offered by the microcatheter and by arterial pressure ( Figure 22-1 ). The drug dose, dilution, and infusion time are chosen on the basis of the characteristics of the molecule used, the actual position of the catheter, and the expected toxicity.
Toxicity
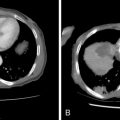
Intraarterial chemotherapy for brain and head and neck tumors began on an absolutely empirical basis with drugs and doses based on systemic treatment. There are no clinical trials defining the maximum tolerated dose and dose-limiting toxicity for intraarterial chemotherapy but only reports of general toxicity or local adverse events reflecting the site of administration. In some cases, optical ischemic neuropathy and also hearing loss have been reported, the latter related to cisplatin treatment. Supraophthalmic infusion seems to involve a greater risk of acute neurologic toxicity such as epileptic fits, confusion, headache, and focal deficits. The drugs implicated are carmustine, cisplatin, and other nitrosoureas. Damascelli et al. conducted a limited study, treating recurrent high-grade glioblastoma and brain metastases from lung cancer with two drugs: liposomal doxorubicin (Myocet) and paclitaxel/albumin (Abraxane). No ophthalmic or neurologic toxicity was observed with infraophthalmic infusion of either drug into the internal carotid artery. The dose of liposomal doxorubicin was 40 mg/m and three out of five patients received respectively five, seven, and eight doses, at 4-week intervals. Abraxane was used up to 250 mg/m , again in a small series of patients, with no central neurologic toxicity or bone marrow depression.
More information is available on intraarterial chemotherapy of tumors of the head and neck. The toxicities were primarily peripheral neurotoxicity, bone marrow depression, and nephrotoxicity. The authors conducted phase 1 and 2 studies with Abraxane, , , in which the dose-limiting toxicity was peripheral paralysis of the facial nerve. This adverse reaction was more frequent at doses above 150 mg/m and with infusion into the external carotid artery (6 cases out of 60), whereas in superselective infusion into the lingual artery no peripheral neurotoxicity was recorded. For the dose of 150 mg/m used in subsequent cycles at 3-week intervals, bone marrow depression and gastrointestinal disturbances were rare, whereas general fatigue, albeit short-lasting, was frequently reported and alopecia occurred in all patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree