Key points
- •
Imaging plays an important role in diagnosis
- •
Thermal ablation is becoming a treatment option for select patients with small tumors
- •
techniques include radiofrequency and cryoablation
- •
long-term follow-up with imaging is necessary
- •
- •
Embolization can be used in the treatment of angiomyolipomas, and in select patients with renal cell carcinoma
Introduction
Imaging has evolved to play a significant and integral role in both the diagnosis and treatment of renal tumors. Over the past decade, image-guided procedures have increasingly been used in the management of renal tumors including angiography and embolization, targeted biopsy, and thermal ablation. The use of thermal ablation to treat renal tumors has increased, particularly in older patients with small (T1a) tumors. This chapter first reviews both benign and malignant renal tumor imaging characteristics and then focuses on their percutaneous management of renal tumors. A brief review of the surgical options is included to give a multidisciplinary understanding to the management of renal tumors.
Renal tumors
There has been an increase in the incidence of renal cell carcinoma over the past several decades, with an estimated number of 58,240 new cases being diagnosed in 2010. , This is in part due to the increased use of cross-sectional scans; however, this cannot be fully explained by imaging use alone. , The classic triad presentation of abdominal pain, mass, and hematuria is no longer typical, with the majority of tumors now being found incidentally. These tumors are typically lower grade and smaller when compared to those identified because of symptoms.
The imaging characteristics of renal tumors can, for the most part, predict the histology of the visualized renal lesion. Benign renal tumors include oncocytoma and angiomyolipoma. Although oncocytomas may have the classic appearance of a central stellate scar and a “spoke and wheel” on angiography, they rarely present this way. The majority of oncocytomas are extremely difficult to differentiate from clear cell renal carcinoma both on imaging and with biopsy. Unlike oncocytomas, angiomyolipomas can be reliably diagnosed on imaging, by identifying the presence of macroscopic fat. Sporadic cases of angiomyolipoma tend to occur in women in their fifth decade, whereas cases associated with tuberous sclerosis complex are likely to be multiple and occur in patients 2 decades earlier.
Malignant tumors include primary renal tumors, transitional cell carcinoma of the collecting system, and more rarely, lymphoma and collecting duct carcinoma. Primary renal tumors can be further divided according to the Heidelberg Classification into clear cell, medullary, chromophobe, and papillary carcinoma. Pathologic subtypes largely determine prognosis. Clear cell renal carcinoma generally has a worse prognosis, with a 10-year cancer specific survival rate of 60%, versus 80% for both chromophobe and papillary types. Pathologic subtypes also suggest a patient’s response to chemotherapy with clear cell subtypes responding favorably to tyrosine kinase inhibitors. The classification of these tumors is important as these different subtypes portend different prognosis, and can often be suggested by imaging studies based on enhancement characteristics. ,
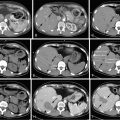
Ultrasonography, computed tomography (CT), and magnetic resonance (MR) are the main imaging modalities for the assessment of renal lesions. Ultrasound’s use is limited to the diagnosis of simple cysts, which demonstrate a well-circumscribed anechoic lesion within the renal parenchyma. CT and MRI diagnosis depend on enhancement and fat detection to characterize renal lesions. A lesion that contains gross fat, less than −20 HU on CT or by chemical characteristics on MRI, is diagnostic of renal angiomyolipoma. The vast majority of enhancing renal masses are renal cell carcinoma, unless they are less than 3 cm, in which case 25% are benign. The enhancement pattern of these lesions helps classify them as clear cell, chromophobe, or papillary renal carcinoma. This difference is best seen in the corticomedullary (late arterial) phase, with clear cell subtypes enhancing avidly, chromophobe subtypes enhancing intermediately, and papillary subtypes enhancing poorly. In fact, using an 84% change in enhancement when comparing the lesion to the cortex in the corticomedullary phase can reliably differentiate clear cell from papillary renal cell carcinomas.
Treatment options (for malignancy)
Surgery
The historic standard of reference in the management of renal cancer is the radical nephrectomy first described by Robson. This includes resection of the entire kidney, Gerota’s fascia, ipsilateral adrenal gland, and lymph nodes from the crus of the diaphragm to the aortic bifurcation. The past two decades have seen a dramatic change in the management of renal tumors: initially with partial nephrectomy (removing only the tumor), then with laparoscopic nephrectomy, and finally with laparoscopic partial nephrectomy. These innovations in surgical technique have lead to improved convalescence, preservation of renal function, and overall survival, without compromising oncologic outcomes when compared to radical nephrectomy for tumors less than 4 cm. , Focally ablative techniques, which are truly minimally invasive and offer excellent renal preservation, are becoming viable treatment options in the appropriate patient population. Cryoablation of renal tumors was first reported in 1995 and radiofrequency ablation of a renal tumor was subsequently reported in 1999. , Since this time, there has been a trend toward nephron sparing techniques with a decrease in radical nephrectomies and increase in partial nephrectomy and ablative therapies. The indications for focal ablation are described in a dedicated section.
Targeted renal biopsy
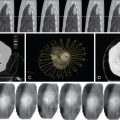
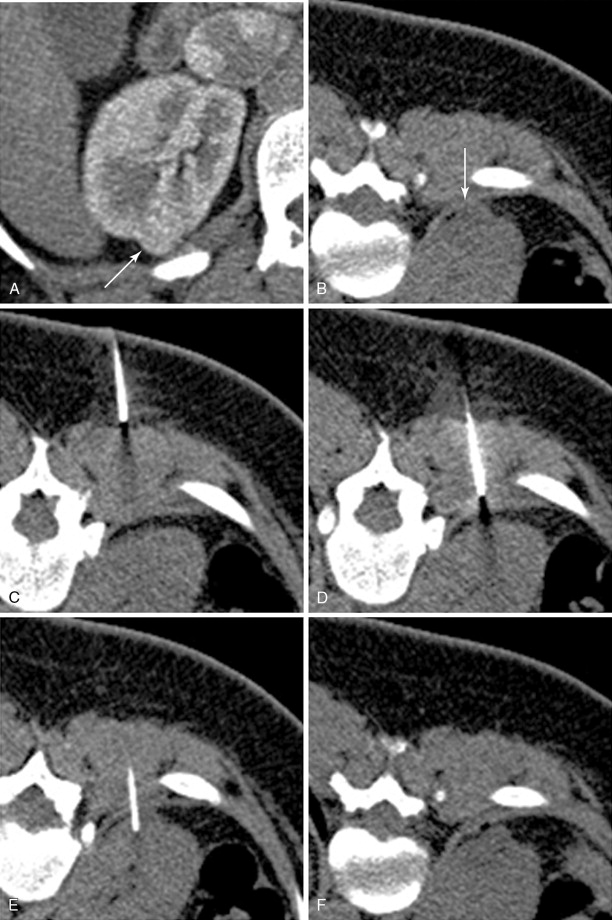
Biopsy can play an important role in patient management of patients with metastatic disease (e.g., melanoma, lymphoma, lung cancer, or breast cancer). When performing a biopsy in a lesion for which ablation is planned, it is important to perform the biopsy prior to the ablation as the underlying tumor pathology impacts patients’ prognosis and postablative samples will only demonstrate postablative histologic changes, not initial pathology. Needle biopsy has a detection rate for malignancy of 80%–92% and a sensitivity of 83%–100%. The biopsy is generally performed immediately before the ablation. The biopsy sample is optimally taken with a 17/18-gauge coaxial system after the probes have been placed. The reason for this is, when using ultrasound guidance of needle placement, the sampling process can introduce artifacts, making imaging with ultrasound difficult for subsequent probe placement. A 17/18-gauge system is used because an 18-gauge needle core biopsy has been shown to provide adequate sample for tissue diagnosis. After samples have been taken, the ablation is performed, and the coaxial biopsy system is removed at the termination of the procedure, just prior to probe removal ( Figure 17-1 ). The risk of track seeding is extremely rare, with only a single case report of tract seeding.
Active surveillance
Bosniak first suggested active surveillance in lesions less than 3 cm, as approximately 25% of these tumors are benign. Other authors have reiterated this finding and suggested active surveillance in the proper patient population. A large meta-analysis of observed renal masses found that the overall growth rate is 0.28 cm/year for small renal masses and that the chance of metastasis is less than 1%. However, having no or low measurable growth in renal lesions does not completely reassure against malignancy. Given that there is a real and significant risk for malignancy, active surveillance is generally reserved for lesions of less than 1 cm diameter in patients with significant comorbidities and limited life expectancy. For patients in whom active surveillance is chosen, the patients are observed with imaging and surgery or ablation will occur when lesion growth rate is greater than 0.5 cm/year. ,
Radiofrequency ablation
The setup for radiofrequency ablation includes an electrical generator, a needle electrode, and grounding pad. There are three radiofrequency ablation devices that are currently U.S. Food and Drug Administration approved in the United States—Starburst probe (Angiodynamics, Latham, NY), LeVeen Probe (Boston Scientific, Natick MA), and Cool-Tip (Covidien, Boulder, CO)—and their probe sizes range from 14 to 17 gauge. The RFA generator produces a high-frequency alternating current with a wavelength of 460–500 kHz and a power setting of 150–200 W. A fluctuating current produces ion agitation and frictional heat deposition at the tip of the needle and causes an increase in temperature. This increase in temperature leads to coagulation necrosis—cell membrane and cytoplasm damage and protein degeneration. Cell death occurs at temperatures higher than 45°C. Complete tumor necrosis occurs at temperatures of 60°–100°C. Current, and thus energy delivery to the target tissue, is inversely proportional to the square of the distance from the electrode. The maximum zone of ablation of a 17-gauge needle electrode is approximately 1.6 cm. To increase the ablation zone, there has been development of multi-tined electrodes, cluster arrangement of multiple electrodes, pulsing electrical current, internal cooling of the electrode, and interstitial saline infusion of electrodes. These have been developed to decrease the risk of vaporization and charring, thereby allowing the spread of heat and subsequently increasing the zone of ablation.
Cryoablation
Cryoablation is based on the Joule-Thomson effect that converts argon gas to cold low-pressure liquid. The cryoablation system is composed of gas distribution apparatus, needle-like cyroprobes, and a thermocouple. Two cryoablation devices are available: ICerod (Oncura, Plymouth Meeting, PA) and Cyrocare device (Endocare, Irvine, CA).
Cell death occurs directly by cytotoxic effects from ice crystallization and indirectly by ischemia associated with local microvascular occlusion leading to coagulative necrosis at temperatures between −19.4°C and −40°C. Histologic pathology shows vascular congestion, nuclear pyknosis, mitochondrial damage, and coagulative necrosis. The temperature at the edge of the ice ball is 0°C, which is nonlethal; therefore, the ice ball must extend 3.1 mm beyond the tumor margin. Double freeze–thaw cycles can be used to increase the size of the ice ball.
Embolization
Embolization of renal tumors was first described in the 1970s and is used for three main indications. Embolization is used in the management of large renal tumors with caval thrombus to help decrease overall tumor size, improve surgical technique by inducing edema (which helps surgical dissection), and decrease perioperative blood loss. Embolization has also been shown to improve survival in patients with unresectable renal cancer, with one study demonstrating a 1-year survival rate of 29% in patients undergoing embolization versus 13% in nonembolized patients. Some authors also argue that embolization may improve paraneoplastic syndromes and hematuria. The combination of both embolization and RFA to treat renal cell cancer by decreasing the vascular heat sink affect has been shown to be feasible by improving the efficacy of RFA. Embolization is performed using three embolic agents: liquids such as absolute ethanol, particles such as microspheres, and metallic coils. The interventionalist’s selection of embolic agent depends on the vascular composition of the tumor and kidney. Initially reported complication rates were as high as 10%, but this has decreased significantly with more recent groups reporting complication rates as low as 0%. , Almost all patients will experience some form of postembolization syndrome, which includes flank pain, fever, nausea, and vomiting within the first 24–96 hours. The postembolization syndrome is self-limiting. Renal embolization has demonstrated utility in the preoperative management of large resectable tumors and in the management of unresectable disease. The combination of RFA and tumor embolization is feasible to help reduce heat sink effects, resulting in larger zones of ablation.
Treatment considerations
Initial workup
The initial evaluation of a patient with a renal mass should include contrast-enhanced cross-sectional imaging (CT or MRI) and a chest x-ray. Both an interventional oncologist and urologist should be consulted prior to tumor ablation if this is a consideration. Laboratory analysis should include complete blood count (CBC), coagulation profile including international normalized ratio (INR), partial thromboplastin time (PTT), and a chemistry panel. A bone scan can be ordered if there is concern for bony metastatic disease as evidenced by bone pain, elevated calcium, or an elevated alkaline phosphatase level. Neurologic symptoms reported by a patient warrant a contrast-enhanced brain scan to rule out metastatic disease. This workup determines the TNM staging, which is important as it correlates with patient prognosis and, therefore, management considerations. Survival at 5 years is 95% with stage I disease, 88% with stage II, 59% with stage III, and 20% with stage IV.
Indications for nonsurgical management
General indications are less than 10-year but greater than 1-year life expectancy, multiple renal tumors, solitary kidney, limited renal function, and comorbid conditions precluding surgery. Special considerations should be applied to patients with genetic predisposition to renal cell carcinoma (RCC), including von Hippel-Lindau (VHL), hereditary papillary RCC (HPRCC), and Birt-Hogg-Dubé syndrome. In this cohort of patients, lesions greater than 3 cm have been historically treated surgically . However, focal thermal ablation may be optimal in the management of these patients in order to preserve renal function.
The American Urologic Association’s recommendation on the management of Stage I tumors (renal lesions confined to the kidney ≤7 cm) with regard to thermal ablation is that it should be considered in patients with T1a lesions (renal lesions confined to the kidney ≤4 cm) with major comorbidities and increased surgical risk.
A comprehensive discussion on the oncologic efficacy for informed consent is discussed after the section on technique.
Contraindications
Healthy patients who do not have increased surgical risk or major comorbidities should be counseled on the limited long-term data that is available regarding thermal ablation. Generally speaking, lesions >4 cm (T1b) should be advised to undergo surgical management. Bleeding diatheses (including platelets fewer than 70,000/µL and INR greater than 1.5) and inability to remain in position for an extended period of time are established contraindications.
Technique
Anesthesia
The choice of general anesthesia, monitored anesthesia care, or conscious sedation is variable and depends largely on both the interventionalist and patient. General anesthesia provides excellent control over patient breathing, allowing for better control in assessing difficult-to-target lesions. When considering the type of anesthesia, radiofrequency ablation is generally considered to be more painful than cryoablation.
Patient positioning
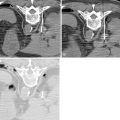
The goal is to achieve an acceptable window for needle placement that avoids critical structures. Positioning is largely operator dependent. however, lesion location tends to predict patient positioning. Upper-pole lesions are best approached with an ipsilateral decubitus position that allows the bowel to fall dependently out of position, minimizes respiratory motion effects on the kidney, and brings the ipsilateral lung down, thereby decreasing the risk of entering the lung and pleural space. Lower-pole tumors are best approached with the patient in the prone position ( Figure 17-2 ). The pleural space extends to the 10th rib, so care must be taken when approaching a lesion intercostally so as to avoid the pleural space and lung. Anterior and lateral lesions can be accessed with the patient in the supine position; however, the liver, spleen, and colon can obstruct access. Anterior lesions can also be approached in the oblique position, with the ipsilateral side up 30°, which allows the bowel to fall dependently out of position.
Probe placement
Contrast-enhanced imaging prior to ablation is integral for ablation planning. When ablating left renal tumors, attention to the large bowel, small bowel, spleen, pancreas, and adrenal gland is important. When ablating a right-sided renal tumor, one must pay close attention to the location of the liver, duodenum, large bowel, gallbladder, and adrenal gland. Once the location of the lesion to be ablated is identified, the need for organ displacement is determined. Lesions that will be within 5 mm of an adjacent organ should undergo organ displacement to protect them during the ablation. If the location of the tumor is within 5 mm of the collecting system and ureter, pyeloperfusion should be performed. This is commonly seen in both central- and lower-pole lesions.
Any cross-sectional modality can be used for probe placement: ultrasonography, MRI, and CT. Probe placement and subsequent ablation has been done entirely with ultrasound, with comparable results to CT- and CT/ultrasound-guided ablation. A limitation with ultrasound is that both the formation of an ice ball with cryoablation and air bubbles with RFA reflects sound waves, thus limiting full evaluation of the tumor when the ablation is ongoing.
When treating large tumors with a single electrode, the probe may be repositioned to ablate the tumor in totality.
Cryoablation allows multiple probes to be used at the same time. A single cryoprobe will produce a 2-cm ice ball. Because a 3.1-mm margin of tissue is generally needed, 2- to 3-cm tumors are usually treated with two to three probes. Multiple freeze–thaw cycles involving a 15-minute freeze and a 10-minute thaw can be used with temperatures reaching −130°C.
Lesion position
The location of tumor is an important predictor of successful ablation. Classifying tumors as either exophytic , central , or mixed can help predict outcomes with regards to treatment and complications. Exophytic tumors are defined as tumors that have at least 25% of the tumor diameter in contact with the perinephric fat, central tumors are defined as lesions that extend into the renal sinus, and mixed tumors have tumor in both the renal sinus and perinephric fat. Exophytic tumors usually have a higher rate of primary success, probably because their distance from the central vessels reduces the overall heat-sink effect. Central tumors are more likely to have not only a lower rate of primary success (heat-sink effect) but also a higher risk for complications compared with exophytic tumors. Tumor size also predicts success with tumors greater than 4 cm having increased rates of recurrence.
Hydrodissection
Hydrodissection with sterile water or 5% dextrose (D5W) has been shown to be effective in protecting surrounding organs during thermal ablation. Carbon dioxide and air have also been used for this purpose. Hydrodissection with fluid usually will require continuous fluid instillation during the ablation. Normal saline or other electrolyte-based, electrically conductive solutions should be avoided when using radiofrequency ablation. If D5W is used for a diabetic patient, serum glucose levels should be monitored.
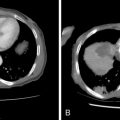
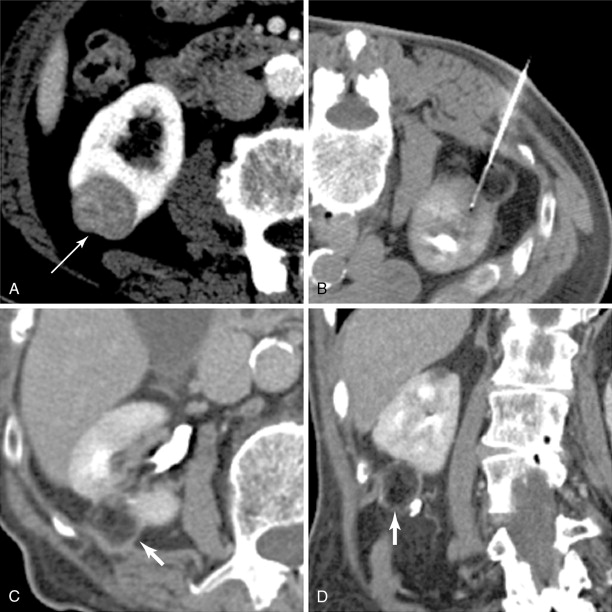
Hydrodissection is performed when a lesion is within 5 mm of the organ of concern. A 5-Fr sheath needle is placed under ultrasound guidance, and CT or MRI can then be performed to ensure appropriate position ( Figure 17-3 ). Once in place, a nonelectrolyte-based solution is instilled. Hydrodissection is monitored with repeat cross-sectional imaging.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree