Key points
- •
In general, the higher the cholangiocarcinoma (type 3 and type 4) and the more multifocal the disease/obstruction, the more likely the patient is to require percutaneous therapy/drainage rather than endoscopic drainage.
- •
The two chief indications for preoperative biliary drainage are (1) the presence of preoperative cholangitis and (2) estimated FLR <30%. The goal of percutaneous biliary drainage is hepatic reserve optimization by improving coagulopathy and resolving any hepatorenal syndrome, thereby improving surgical outcome.
- •
The disadvantages of performing drainage prior to resection include introduction of polymicrobial organisms to the biliary tree.
- •
Ultimately, the possible advantages of covered stents should also be weighed against their increased expense, especially in light of the fact that many patients will not outlive a bare metal stent. This may explain why recent randomized trials (bare stents vs. covered stents) have not shown a clear and significant patency advantage of covered stents when compared to bare stents.
- •
A randomized trial of photodynamic therapy versus conventional biliary drainage demonstrated a survival benefit so significant (98 vs. 493 days, P < 0.0001) that the trial was terminated early.
- •
Transarterial catheter therapy (whether chemoembolization or radioembolization) have shown promising initial results in the management of cholangiocarcinoma.
- •
Percutaneous tumor ablation is feasible, with good results; however, its role in the overall management of cholangiocarcinoma is yet to be determined.
Introduction
Cholangiocarcinoma is the second most common primary liver malignancy, after hepatocellular carcinoma, and its incidence appears to be increasing. Approximately 2000 cases are reported annually in the United States. Nearly two thirds of cases occur in patients aged 50–70, with a male predominance. The presenting symptoms of cholangiocarcinoma include jaundice, weight loss, pruritis, pain, and liver failure. Because symptoms typically occur late in the disease, staging at diagnosis tends to be advanced and prognosis for the disease is poor overall, with certain subgroups having extremely poor survival. Overall, patients with biliary duct malignancies have a reported median survival of 3–10 months. It should also be noted that gallbladder carcinoma, central intrahepatic metastasis, and metastatic lymphadenopathy may also result in malignant biliary obstruction, which may present similar to cholangiocarcinoma. Although this chapter attempts to primarily reflect the scientific literature on cholangiocarcinoma, some of the principles will also apply to these other etiologies of biliary obstruction. Moreover, on review of the literature, all etiologies of malignant biliary obstruction are commonly amalgamated and, as a result, it is difficult to ascertain outcomes that are specific to cholangiocarcinoma.
The current chapter focuses on the percutaneous biliary drainage and management of cholangiocarcinoma. Cholangiocarcinomas are a heterogenous group of tumors in terms of their size, location, symptoms, and survival ( Table 11-1 ). The complexity of this multifaceted subject, with its numerous variables, is compounded by the fact that varying techniques and methods are often amalgamated from an outcomes standpoint ( Table 11-1 ). This goes as far as amalgamating the outcomes of endoscopic and percutaneous management together, and thus in many studies it is commonly difficult to ascertain specific outcomes of percutaneous management overall, let alone evaluate outcomes that are specific to particular percutaneous techniques/stent. In this chapter, we will first discuss biliary drainage for cholangiocarcinoma in terms of its indications, technique, and method of drainage (endoscopic vs. percutaneous). We will also discuss the various types and configurations of biliary catheters and stents available, and the rationale for their use. In addition, the impact of the obstructing lesion (distal vs. hilar, right vs. left) on the treatment approach will be discussed ( Table 11-1 ). The preliminary results of covered versus bare stents will also be discussed along with locoregional transluminal therapies.
| Variables in Describing and Managing Cholangiocarcinoma | |
|---|---|
| Indications | Preoperative vs. palliative management |
| Location of Cholangiocarcinoma | Hilar vs. distal cholangiocarcinoma ( Figure 11-1 ) |
| Modality of management | Endoscopic vs. percutaneous management |
| Laterality of approach | Right-sided vs. left-sided PTC and percutaneous biliary drainage |
| Degree of drainage | Unilobar vs. bilobar hepatic biliary drainage |
| Endoprosthesis (stents) | Plastic vs. metallic endoprosthesis (stents) |
| Covered metallic vs. bare metallic stents | |
| Y-configured vs. T-configured metallic stents | |
Management overview
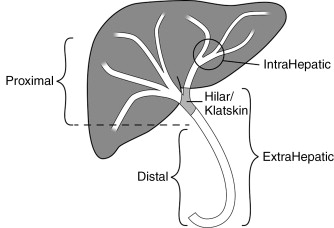
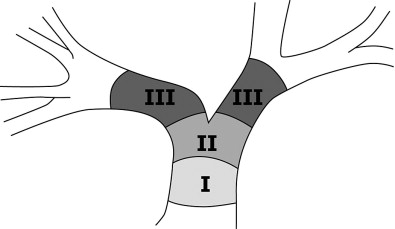
Cholangiocarcinomas are a heterogenous group of tumors in terms of their size, location, symptoms, and survival. Figure 11-1 demonstrates the terminology of cholangiocarcinoma based on location. Management needs to be individually tailored for each patient. In terms of location, multiple classification schemes exist. Cholangiocarcinoma may be described as proximal/distal or, using the Bismuth-Corlette classification system as types 1, 2, 3a, 3b and 4 according to their intrahepatic/hilar/extrahepatic distribution ( Figure 11-2 ). Type 1 lesions generally have the best prognosis, while type 4 lesions have the poorest prognosis.
Overall, patients with biliary duct malignancies have a reported median survival of 3–10 months, particularly when the presenting symptoms are associated with biliary obstruction, which is usually the case. Medical therapy is very limited for cholangiocarcinoma and no standard chemotherapy regimen exists, because of minimal survival benefit. Radiation therapy is controversial because of limited efficacy and increased risk of complications (cholangitis, gastroduodenitis, and increased hospitalization). Decompressive surgery is the traditional treatment for obstructing cholangiocarcinoma. The majority of surgeries are performed for palliation, with less than 30% of patients with cholangiocarcinoma having a chance for a curative resection. Moreover, many patients at the time of presentation are not surgical candidates, with up to 70%–80% of patients being nonsurgical candidates. As a result, most nonsurgical patients are managed by minimally invasive means (endoscopic or percutaneous biliary drainage). These minimally invasive approaches are now considered standard of care for many patients along with systemic or new loco-regional therapies.
Endoscopic versus percutaneous biliary drainage
Studies have shown that the method of biliary drainage has no impact on survival. However, endoscopic retrograde cholangiopancreatography (ERCP)–guided internal biliary drainage is often the preferred method of drainage and is generally thought to be less invasive than percutaneous drainage, because it does not require creation of an invasive transhepatic/transcapsular (Glisson capsule) tract. As a result, ERCP is the most definitely preferred procedure in patients with severe coagulopathy or ascites. However, there are several scenarios in which endoscopic drainage may fail or may not be feasible. In terms of disease location, ERCP generally can adequately drain the liver when a distal lesion is present, but may be more problematic in hilar lesions or in cases with multiple strictures. In general, the higher the cholangiocarcinoma (type 3 and type 4) and the more multifocal the disease/obstruction, the more likely the patient is to require percutaneous therapy/drainage rather than endoscopic drainage.
Additionally, previous upper gastrointestinal tract surgery (Bilroth II or gastric bypass, to name two) may make endoscopic stent placement difficult or impossible. In these cases, percutaneous drainage may succeed where endoscopic drainage has failed. In cases of proximal lesions or in patients with multiple strictures (numerous intrahepatic isolated biliary segments), percutaneous drainage may be superior because it can specifically target the area of abnormality or biliary tract dilation. However, it is important to note that cholangiocarcinomas with more advanced Bismuth class (corresponding to more diffuse involvement) tend to have fewer dilated intrahepatic ducts on imaging studies and that hilar cholangiocarcinoma accounts for 40%–60% of all cholangiocarcinomas.
Percutanous transhepatic management
Patients undergoing percutaneous transhepatic cholangiography (PTC) for purely diagnostic purposes is largely a thing of the past and the vast majority of patients are now diagnosed on the basis of noninvasive imaging such as magnetic resonance cholangiopancreatography (MRCP), computed tomography (CT), or ultrasonography prior to any minimally invasive procedure (ERCP or PTC) (please see Preprocedural Imaging section). Percutaneous transhepatic management can be classified into (1) PTC and percutaneous transhepatic biliary drainage, (2) internalization of drainage by stent placement, and (3) new loco-regional therapies such as brachytherapy and photodynamic therapy.
Initial PTC and percutaneous drainage
Preoperative versus palliative biliary drainage
Percutaneous biliary drainage is typically indicated in one of two broad-based scenarios, preoperative (for surgical candidates) or palliative (in patients who will not have surgery). Surgery for cholangiocarcinoma can be performed with either the goal of a curative resection or for palliative decompression. In either of these scenarios, preoperative drainage can be entertained. Complete surgical resection, often necessitating major hepatic resection, remains the only possibility for cure in patients with cholangiocarcinoma. The role of orthotopic liver transplantation in this population is not well defined at present. As many as 90% of patients with cholangiocarcinoma are not candidates for curative resection. For patients who are not surgical candidates or who do not elect to undergo surgery, percutaneous biliary drainage may be performed as a palliative measure.
Currently, performing the drainage procedure prior to surgery is controversial and has been debated for years. As a result, practice varies between institutions. Many Asian centers have advocated preoperative drainage routinely, while Western centers have tended to be more conservative. The advantages of preoperative drainage include resolution of any preexisting cholangitis and optimization of the hepatic reserve. , The goal of hepatic reserve optimization is to improve coagulopathy and to resolve any hepatorenal syndrome, thereby improving the patient’s surgical outcome. The disadvantages of performing drainage prior to resection include introduction of polymicrobial organisms to the biliary tree. , Another potential disadvantage is increased morbidity both from the drainage procedure itself and from the associated increase in hospital stay. Moreover, a percutaneous biliary drainage catheter may hinder the ability to assess the full extent of the tumor both on preoperative imaging and intraoperatively.
In terms of hepatic reserve, the concept of future liver remnant (FLR) is important to understand. Future liver remnant, as the name implies, is defined as the difference between the total liver volume and the volume of resected liver. The FLR cannot be truly known until after the surgery; however, it can be accurately predicted preoperatively on CT or magnetic resonance imaging (MRI) using volumetric analysis. , If the FLR is expected to be less than 30%, patients tend to have worse surgical outcomes. Therefore, optimization of the hepatic reserve becomes more important in this group as it will improve the liver’s capacity for postoperative hypertrophy. , Two studies demonstrated that without percutaneous biliary drainage, postoperative hepatic dysfunction with an FLR less than 30% and greater than 30% was 24% vs. 0%, respectively. Similarly, the postoperative death with an FLR less than 30% and greater than 30% was 19% versus 5%, respectively. ,
Therefore, the two chief indications for preoperative biliary drainage are (1) the presence of preoperative cholangitis and (2) estimated FLR <30%. These indications apply to both curative and palliative resections. These indications should be weighed against the significant risk of introducing polymicrobial infection from the procedure. Incidence of cholangitis following percutaneous drainage is reported at 20%–60%. Review of five Level 1 clinical trials demonstrates that 65% of patients who received preoperative drainage had biliary aspirates positive for polymicrobial organisms compared to 8% of patients who did not receive preoperative drainage. , In terms of preoperative management, it is important to obtain high-quality abdominal imaging prior to drainage in order to avoid the catheter compromising the preoperative imaging.
Indications
Patients undergoing PTC for purely diagnostic purposes is largely a thing of the past. Drainage for simply the relief of asymptomatic obstruction or visualization of dilated/obstructed biliary radicals is not an indication for PTC and subsequent percutaneous biliary drainage. , The clinical indications for PTC and subsequent biliary drainage is pruritis (particularly refractory to medical therapy), cholangitis, biliary sepsis, and hyperbilirubinemia in patients who will undergo systemic chemotherapy with agents that require hepatic metabolism and/or biliary excretion. , In a study by Robson and coworkers, the proportions of patients with the primary indication of pain/pruritis, jaundice precluding chemotherapy, and cholangitis were 18%, 53%, and 10%, respectively. These chemotherapeutic agents include 5-flourouracil (5-FU), irinotecan, and gemcitabine. The degree of reduction in serum bilirubin is the chief indicator of successful drainage. The target total bilirubin after percutaneous drainage for the administration of chemotherapeutic regimens is usually less than 2 mg/dL. ,
Preprocedural workup and clinical evaluation
Preprocedure workup should include a detailed history (assessing indications and contraindications), physical examination, laboratory data, and noninvasive imaging. Because of the risk of bleeding from the procedure, coagulation parameters and platelets should be optimized. There are no set coagulation parameters; however, an international normalized ratio (INR) of less than 1.5 and a platelet count greater than 50,000–75,000/mm are common thresholds. The presence of preprocedure cholangitis should be carefully assessed for, as percutaneous drainage may worsen/disseminate any underlying infection. Prophylactic antibiotics are recommended. Common prophylactic antibiotics used include intravenous piperacillin and tazobactam combination or intravenous ceftriaxone. Preprocedural sedation and/or anesthesia evaluation is also performed, as PTC with subsequent drainage can be performed under moderate sedation, deep sedation, or general anesthesia.
Preprocedural imaging evaluation from a technical safety standpoint
Preprocedural imaging is vital for planning of effective percutaneous transhepatic biliary drainage. Preprocedural imaging is used to evaluate for intrahepatic anatomy and pathology such as portal vein thrombosis, hepatic segmental atrophy and hypertrophy, intrahepatic masses and tumors, and obviously, levels of biliary obstruction, degree of biliary obstruction, and isolated biliary segments. Preprocedural imaging is also required for evaluating extrahepatic anatomy and pathology such as ascites, bowel, subcostal access routes, and chest anomalies. Bowel and distorted hepatic anatomy is particularly prevalent in chronic obstruction, prior therapy, and prior hepatic resections.
Ultrasonography is effective in determining the degree of biliary dilation and the level of biliary obstruction. However, its effectiveness varies with operators and there is considerable interoperator variability. More importantly, it may overlook or underestimate extrahepatic anatomic aspects such as bowel and it may not reliably and accurately assess intrahepatic tumors that may need to be avoided. MRI is very effective for determining affected segments and determining intrahepatic tumors and extrahepatic structures. However, it is not effective in identifying calcifications and metal objects such as surgical clips and prior stents (metal or plastic) that are used as landmarks during PTC by experienced operators. Thus, it is particularly less suitable in patients with recurrence who commonly have complicated obstructions and prior stents. Moreover, the quality of MRI may vary from one patient to another depending on body habitus, patient cooperation, and respiratory artifacts. CT, especially contrast-enhanced CT, is the most reliable, most available, and most reproducible imaging modality when it comes to prebiliary drainage planning. It shows the pertinent intra- and extrahepatic anatomy and pathology as well as dense landmarks such as calcifications, stents, and clips. It also identifies the most dilated biliary segments, degree of dilation, and isolated segments. In the authors’ opinion, as in the case with prior authors, the most ideal imaging modality for preoperative planning is CT. Needless to say, ultrasonography is a more important imaging modality for image guidance for drain placement (see below) but less important for predrainage planning.
Preprocedural imaging evaluation from a drainage efficacy standpoint
Drainage efficacy depends on the indication of drainage (intended/desired clinical endpoint). If the primary clinical indication is cholangitis with or without biliary sepsis or pruritis (a biliary stagnation issue leading to systemic dissemination of bile/bacterial infection), then the target biliary segments should be the most dilated segments regardless of hepatic parenchymal volume and portal vein thrombosis (see next section). In this respect, all cross-sectional imaging modalities (including ultrasonography) are effective in preoperatively identifying the most dilated biliary segment(s). Obviously, no operator can safely say which biliary segment is the source of infection; however, targeting the most dilated segments is “statistically” prudent. The most dilated segments are probably the most chronically obstructed and have the largest stagnant biliary volume; both of which are higher probabilities of the presence of infected bile. It should be mentioned that cholangitis with or without biliary sepsis is not a frequent presenting symptom/indication in a “virgin biliary system” that has not been instrumented. Moreover, it is not uncommon to find contamination of isolated biliary segments that are not drained (by endoscopy or percutaneously) but are adjacent to drained biliary segments. These adjacent undrained isolated biliary segments are a source of recurrent cholangitis and possibly sepsis that translates into increased patient morbidity. This should be avoided at all costs and it emphasizes the strict adherence to the clinical indications of endoscopic and/or percutaneous biliary drainage.
If the primary indication is hyperbilirubinemia (a biliary stagnation issue leading to poor function) with or without the need to optimize hepatic function, then the target biliary/hepatic segments are not necessarily those with the most dilated biliary radicals. The ideal target hepatic segments for drainage are the largest functional hepatic segments exhibiting biliary obstruction and displaying patent portal venous supply and not replaced by tumor (the size of the healthy and portal-perfused parenchymal and not the size/degree of dilation of the biliary radicals). The patency of the portal venous supply is paramount because the portal vein provides the trophic blood supply of the liver. , Portal vein occlusion, by thrombosis and/or tumor invasion, particularly when accompanied by same-segment(s) biliary obstruction leads to hepatic parenchymal atrophy.
PTC and percutaneous drainage technique
PTC and subsequent percutaneous biliary drainage can usually be performed with the patient undergoing moderate sedation. This can be complemented with intercostal nerve blocks. Less than 10% of cases require deep sedation or general anesthesia. However, some operators/institutions routinely utilize deep sedation or general anesthesia. Anecdotally, patients undergoing PTC under general anesthesia may have a larger incidence of pleural complications because respiratory splinting in patients undergoing PTC with moderate sedation may reduce the extent of the cephalad (downward) extent of the pleural reflections (this applies to right-sided PTC only). Regardless of the degree of sedation/anesthesia, all patients should be well hydrated and have adequate venous access in case aggressive fluid resuscitation is required. This is because it is not uncommon to have worsening cholangitis and/or biliary sepsis during or after percutaneous biliary drainage.
The approach of the biliary tract (target biliary segment) should be governed by indications and imaging findings and planning (see above). If all things are considered, a right-sided (right midaxillary) versus left-sided (subxiphoid/epigastric) approach varies between operators from a preference standpoint ( Figure 11-3 ). This choice almost always occurs in cases with central extrahepatic biliary obstruction and has become less commonly encountered by interventional radiologists because many of these cases are drained endoscopically. The advantages and disadvantages of left-sided and right-sided approaches are listed in Table 11-2 .
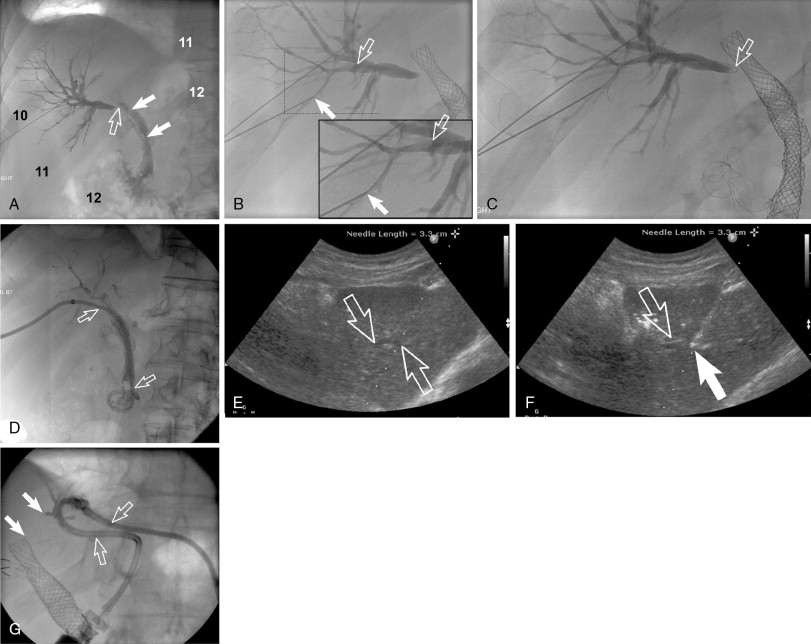
| Right-sided PTC / PBD | Right-sided PTC / PBD | |
|---|---|---|
| Advantages | Usually more peripheral duct access | More convenient for patient (less painful) |
| Usually a greater hepatic territory drained | More convenient for patient care of drain (better self-care) | |
| Favorable angles for metallic stent placement | Less morbidity with ascites | |
| More convenient for operator and less radiation exposure to operator | Less pleural complications | |
| Disadvantages | More painful to patient | Usually less hepatic territory drained |
| Higher pleural complications | Usually more central access | |
| Poor self-care | Less favorable angles for stent placement | |
| Higher morbidity in the presence of ascites | More radiation to operator hands |
PTC and percutaneous biliary drainage is commonly performed using fluoroscopic guidance. , , Grayscale ultrasound guidance can be used for real-time bile duct access followed by fluoroscopic guidance for catheter wire manipulation and drain placement. Ultrasound guidance is commonly used for left-sided approaches ( Figure 11-3 , E and F ); however, it can be used for right-sided approaches as well ( Figure 11-4 ). Very detailed technical aspects are beyond the scope of this chapter and could be reviewed in technical articles in “Techniques of Vascular Interventional Radiology: Biliary Interventions—Part 1,” Tech Vasc Interv Radiol. 2008;11(1):14-50. , ,
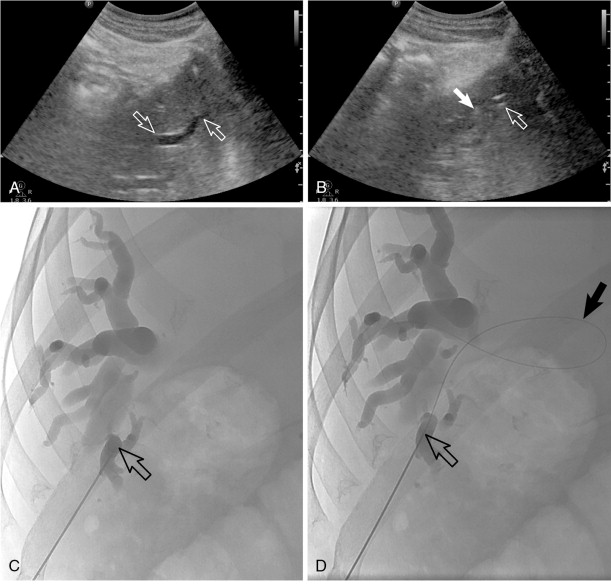
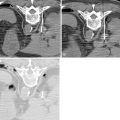
Local anesthesia should be generously administered because of the likely need for multiple needle passes. The two primary initial imaging guidance modalities for biliary access are (1) fluoroscopic guidance, (2) ultrasound and fluoroscopic guidance. For the fluoroscopic method, a site is marked on the right lateral abdomen in the midaxillary line usually at the 10th or 11th intercostal space just above the rib(s) to avoid the intercostal neurovascular bundle (see Figure 11-3 , A ). The goal of the initial access site is to avoid pleural puncture, which could lead to biliary drainage into the right pleural space. After a small skin incision is made, a 22-gauge Chiba needle (which can accept an 0.018-in. guide wire) is advanced into the liver usually toward the 9th or 10th costovertebral junction (see Figure 11-3 , A ). The stylet is then removed and the needle is slowly withdrawn while injecting small amounts of contrast to opacify the biliary tree. It is critical to not overinject contrast as this will lead to obscuration of the liver and biliary anatomy. Bile ducts can usually be differentiated from hepatic arteries, portal veins, and hepatic veins because contrast will tend to remain stagnant rather than washing out. Moreover, there are structural and anatomic feature differences between the three vessels. Once the Chiba needle (Cook Corp, Bloomington, IN) is confirmed to be within a biliary duct, a limited cholangiogram is obtained. The cholangiogram should be limited to confirm biliary access and to opacify and confirm the target biliary radicals based on the preprocedural imaging planning (see above). Too much contrast may lead to compression of the biliary tree and bacterial vascular escape, which may translate to worsening cholangitis and biliary sepsis.
If the initial needle access is not ideal, a second Chiba needle (intended to be the definitive biliary drainage access) is used to target an appropriate third- or fourth-order (more peripheral) bile duct. The rationale for accessing a peripheral bile duct is to decrease the risk of vascular injury, as the larger vessels are located close to the hilum. Oblique views are helpful in targeting the second needle access sites. Under fluoroscopy, a characteristic pop may be seen as the needle deforms/effaces and ultimately punctures the bile duct. Once the needle is within the duct (which can be confirmed with contrast injection), a 0.018 wire is then advanced into the biliary tree (see Figure 11-3 , B and C ). Hydrophilic wires may be useful because of their steerability and ability to buckle within the biliary tree (see Figure 11-3 , B and C ). Although there is an increased risk of wire shearing with hydrophilic wires, a retained wire fragment in the liver does not carry the consequences it would in the vascular system. The ideal intended definitive access (1) should be in the target biliary segment planned by preoperative imaging, (2) should enter the target biliary segment from a peripheral biliary branch, and (3) should take into consideration angulation issues for more central drainage and interventions (stent placement).
Once the definitive biliary access is obtained with adequate 0.018-in. guide-wire access, a telescoped transitional dilator (tapered to 0.018 in. and allows a 0.035-in. wire placement) is then placed over the 0.018-in. wire and used to upsize the guide-wire platform to a 0.035-in. guide wire. At this point, depending on the patient’s symptoms and degree of hemodynamic stability, the operator may elect to leave an external biliary drainage catheter connected to bag drainage. A particular type of an external drain is a U-tube (see Figure 11-3 , G ). A U-configured tube is the most secure tube from a dislodgment standpoint. If the patient is hemodynamically stable, the operator should attempt to advance the 0.035-in. wire into the small bowel and place an internal/external biliary drainage catheter. If this is unsuccessful, the patient can be brought back within the next 48–72 hours for internalization, after cholangitis/biliary sepsis has resolved. For the internalization procedure, hydrophilic steerable guide-wires are often useful for crossing the occlusion. It is essential when placing the multi-side-hole drainage catheter to confirm that side holes are present above and below the obstruction. Additional side holes may need to be placed. It is also important to make sure that the most proximal side hole (closest to the hepatic capsule access point) is within the biliary tree and not the raw transhepatic biliary tract. If this is overlooked, bile may leak along the tract and into the peritoneum or a portal venous bleed may occur.
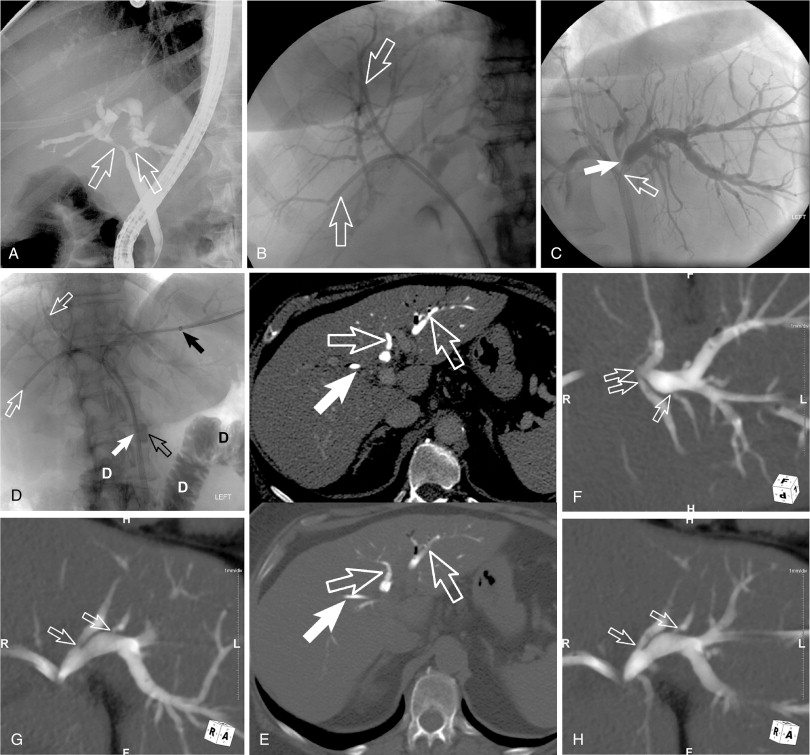
Invasive cholangiography (direct CT cholangiography)
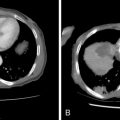
A rarely described imaging technique is direct CT cholangiography or transhepatic contrast-enhanced CT cholangiography. This is performed by direct injection of diluted contrast (1 part ionic contrast to 5–7 parts saline, 1 part gadolinium to 1–2 parts saline) into the biliary drain and obtaining CT images (either cone-beam CT in the angiography suite or in a CT scanner). Postimaging processing includes three-dimensional reformats and maximum-intensity projections (MIPs) as well as minimal-intensity projections (MinIPs). This technique can be important for identifying poorly dilated isolated biliary segments and it can provide imaging details for identifying the subtle extent of cholangiocarcinoma with “pinching” (subtle stenosis) of biliary radicals ( Figure 11-5 ). Obviously, the most common application for this technique is in hilar and centrally located intrahepatic cholangiocarcinoma.
Technical and clinical outcomes of percutaneous drainage
Percutaneous drainage is a highly effective procedure in the palliative setting, with reported technical success rates of 95%–100% and clinical success rates of 70%–80%. , In one study, 57% of patients had a significant reduction in their serum bilirubin following drainage, with 44% experiencing a reduction to less than 2 mg/dL. The average period for bilirubin to drop to less than 2 mg/dL was 2.8 weeks. Pruritis was reported to improve at 1 and up to 4 weeks. Interestingly, quality of life (QoL) parameters did not improve following percutaneous biliary drainage. Minor complications of drainage occur in up to 31% of patients and are listed, with the most common being pain, catheter leaks, and fever. , Major complications occur in up to 7.3% of patients and are listed, with the most common being cholangitis. Of note, while transient hemobilia is a relatively common finding following percutaneous drainage, less than 2% of patients will experience significant bleeding that requires transfusion (considered a major complication). , Procedure-related mortality is reported at 0%–2.6%. However, mortality at 30 and 60 days is reported to be at 2.3%–11% and 28%, respectively. , In terms of long-term complications, catheter dysfunction is frequent and is seen in up to 47% of patients, with 8%–17% of patients experiencing catheter dislodgement. , These data underline the importance of patient education and of prompt tube evaluation by a physician whenever problems are suspected.
Tsai et al. demonstrated predictors of high biliary output following percutaneous drainage, including higher serum albumin, absence of liver metastases, and lower Bismuth classification. Tumor size and degree of biliary dilation were not found to be related to biliary output. Interestingly, in the same study, a high biliary output from a drainage catheter (defined as 300 mL/day) was not associated with more rapid reduction of serum bilirubin. In terms of drop in serum bilirubin, the significant factors were serum albumin, bilirubin, and prothrombin time, which were closer to normal values. Bismuth classification, tumor size, and presence of liver metastases were not found to correlate with drop in serum bilirubin. , Relief of the biliary obstruction, in addition to providing symptom relief, is also correlated with improved survival overall. Patients with serum bilirubin >4 mg/dL after percutaneous biliary stent placement are reported to have poorer outcomes. Advanced age and Bismuth IV lesions also represent poor prognostic factors. Tsai et al. found that in 74 patients with hilar lesions and unresectable cancers, biliary drainage was not associated with a survival benefit. Indar et al. found that prestenting serum bilirubin had a correlation with survival. It is important to note that regardless of therapy, most of the patients who are nonsurgical candidates will die of cholangitis/biliary sepsis and/or liver failure.
Internalization of biliary drainage (stent placement)
Indication for stent placement
For most patients, the ultimate goal of the percutaneous drainage procedure is usually to either place a definitive internal/external drainage catheter or to provide an access portal for internal metallic stent placement. The only clinical scenario for metal stent placement is palliation. Metallic stents should not be used as a preoperative drainage tool. The rationale for stent internalization in both cases is to limit the amount of fluid and electrolyte loss from external drainage as well as to improve quality of life (reduce patient morbidity). With an internal/external stent, catheter position becomes more critical and catheter malfunction may become more frequent, putting the patient at risk for recurrent episodes of cholangitis, as well increased number of procedures and tube manipulations.
The rationale behind stent placement is that stents improve the patient’s quality of life by limiting their need for an external appliance. Additionally, stents are less prone to dislodgement and malfunction when compared with percutaneous drainage catheters. The benefits of stent placement should be weighted against their potential shortcoming compared to drainage catheters. Stents offer no backup route for biliary drainage if they should be obstructed in the future. Therefore, the decision to place a stent should be carefully considered and not all patients will require stent placement. Again, metallic stents should be palliation only. Moreover, most operators do not place metal stents (without transhepatic access) unless the life expectancy of the patient does not exceed 6–9 months. Even when the life expectancy of the patient is within 6–9 months, the patient should be counseled that if biliary obstruction occurs, he or she may have to undergo another PTC and biliary drain placement that may be more difficult than the initial PTC and drain placement.
The two primary stent options are plastic removable stents (typically placed by gastroenterologists using endoscopy but can be placed percutaneously) and self-expanding metallic biliary stents (which can be placed by either gastroenterologists or interventional radiologists).
Plastic VS. metal stents
In the opinion of the authors, this is a moot point from a percutaneous transhepatic (interventional radiology) standpoint. Internalization in the setting of malignancy should be metallic stents and for palliation. This is because metal stents have consistently shown improved patency over plastic stents (see next section). The debate may still have importance for endoscopists. This is reflected in that most of the debate concerning stent selection has taken place in the gastroenterology endoscopic literature.
Metallic stents have been shown to be superior to plastic stents in terms of patency, quality-of-life, and complication rates. , Metallic stents have high technical and clinical success rates, >95% and 88%–97%, respectively. Retrospective studies demonstrate an average primary patency of 5.6–6.9 months, with an average secondary patency of 8.1 months. Patient survival in this cohort, meanwhile, is 3.1–8.7 months. The take-home point here is that for many (though not all) patients, the patency of a metallic stent will exceed the patient’s life span, and this point must be kept in mind when discussing treatment options with patients and their families in order to choose the appropriate stent.
Raju et al. demonstrated average patency of 5.6 months for metallic stents, in a group of patients who had a median survival of 9.1 months. Plastic stents demonstrated a much shorter primary patency of 1.9 months, leading to an average reintervention rate of 4.6 per patient, compared to metallic stents that had a reintervention rate of 1.5 per patient. Khan et al. proposed that in patients with less than 3–6 months’ life expectancy, plastic stents are more cost-effective and associated with lower morbidity. In patients with more than 3–6 months’ life expectancy, a metallic stent was recommended because of the higher reintervention rates associated with plastic stents in that period. Metallic stent patency is far better in distal lesions than hilar lesions. Predictors of poor survival in patients who receive metallic stents is a postprocedural bilirubin level that remains above 4 mg/dL (>3.5 times mortality) and/or Bismuth IV lesion (>4.5 times mortality).
Unilobar vs. bilobar and hilar vs. distal tumors
For hepatic functional palliation, 25%–50% of the hepatic parenchyma needs to be drained. Therefore, many authors advocate unilobar drainage, as bilobar drainage has not been shown to have superior results and carries a higher complication rate. 3,27,30-32 Placement of more than one stent is highly controversial, as each additional stent placed increases the potential for complications. With that said, we believe that in some cases, multiple drainage catheters are necessary. In a Bismuth-Corlette Type I (distal) cholangiocarcinoma, one drainage catheter is often sufficient. In Bismuth-Corlette Type II and III (both of which involve the hilum), two drainage catheters may be necessary to obtain satisfactory drainage.
Conversely, Bulajic et al., studying 49 patients with Bismuth-Corlette Type II (hilar) tumors, demonstrated that bilateral drainage resulted in lower serum bilirubin levels at 7 days compared to unilateral drainage. The incidence of early complications was similar between the two groups; however, late complications were more frequent in the bilateral group. Survival, however, did not differ between the two groups, with only 18% of patients alive at 1 year. It can be therefore inferred that bilateral drainage may provide better symptomatic control in hilar tumors than unilateral drainage even if it does not prolong survival. , In Bismuth-Corlette Type IV (multifocal or diffuse involvement), more than two may be necessary on occasion. Few authors advocate placing more than three drainage catheters, and the current authors concur with this. However, more than three percutaneous biliary drains have been described in up to 7% of patients with only 54% requiring one percutaneous biliary drain (39% requiring two to three drains).
Y- VS. T-configured stents
For metallic stent placement (internalization) in patients requiring multiple bilobar drainage to achieve sufficient drainage, two primary metallic stent configurations have been described. These are the Y-configured stents ( Figure 11-6 , A ) and T-configured stents. A Y-configured stent placement relies on bilateral percutaneous biliary access or, rarely, a unilateral metal stent placement by endoscopy and a contralateral metal stent placement placed percutaneously. The common (“kissing part,” where the two stents run parallel) inferior limb of the Y configuration is typically central in the common hepatic duct or (if commonly extended) common biliary duct. A T-configured stent placement only requires one percutaneous biliary access whereby the crossbar of the T crosses from right to left (if the access is from the right) or from left to right (if the access is from the right). The central stent is then placed in the common hepatic duct or common bile duct. The “kissing part” of the T-configured stent placement (where the two stents run parallel) is intrahepatic and on the side of the percutaneous access. The advantage of the T-configured stent placement is that it only requires one percutaneous access or can be performed in cases of bilateral percutaneous access where only one side can be internalized. Conversely, the Y-configured stent placement requires bilateral percutaneous placement and bilateral internalization of the percutaneous biliary drains. However, the inability to internalize the second biliary drain should be a thing of the past given the described techniques on internalizing the second biliary access utilizing the internalized biliary access. , This includes the Forced Buckle Technique described by Saad.