Introduction
Head and neck cancers are a complex, heterogeneous group of diseases with a variety of treatment options. Surgery, chemotherapy, and radiation all have a role. Outcomes data are often ambiguous, and the same tumor may be treated differently at different institutions, depending on the local expertise and experience. Cross-sectional imaging plays a central role in assessing the extent of disease, and in planning treatment strategies.
Anatomy
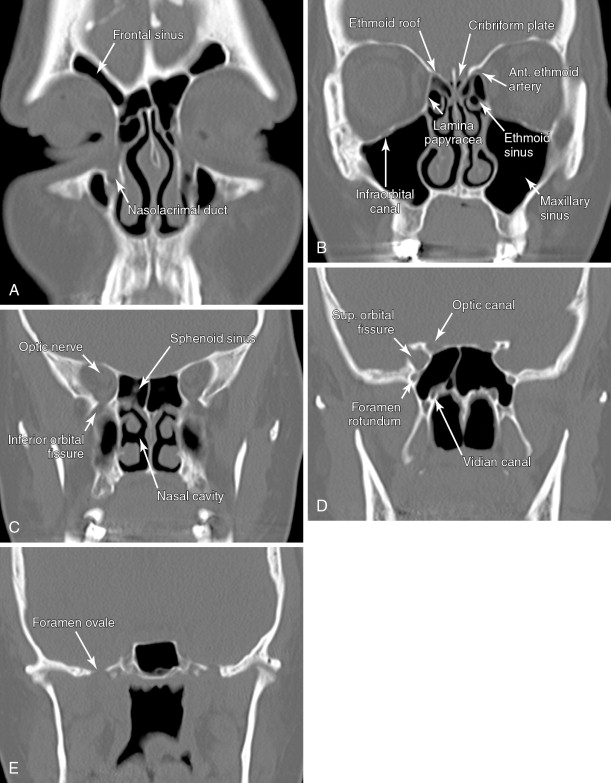
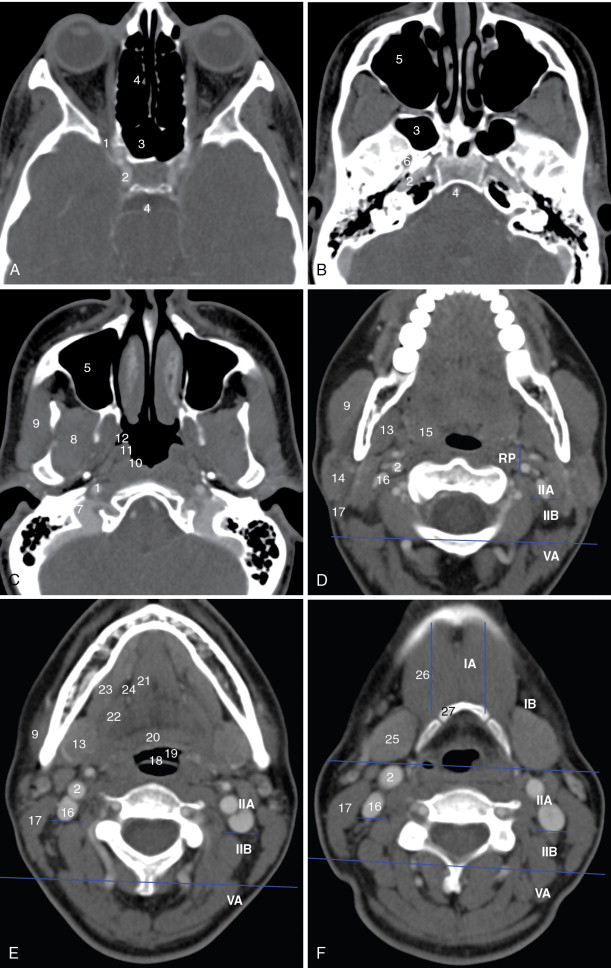
A detailed understanding of cross-sectional anatomy in the head and neck is critical in interpreting pretherapy imaging studies. Figure 21-1 details the anatomy of the paranasal sinuses, nasal cavity, and anterior skull base. Figure 21-2 details the anatomy of the upper aerodigestive tract.

Imaging strategy
Computed tomography (CT), magnetic resonance (MR), and F-fluorodeoxyglucose positron emission tomography (FDG-PET) are complementary imaging modalities, each with unique advantages. CT is widely available, making it a workhorse modality. The very rapid acquisition time of a CT study with modern scanners, on the order of seconds, makes CT an especially useful modality for patients who are unable to lie still for the approximately 45 minutes required for an MR examination. CT also depicts bony anatomy and cortical bone erosion better than any other modality.
MR provides superior soft tissue contrast, and distinguishes between tumor, edema, and muscle with greatest accuracy. MR has the highest sensitivity for bone marrow infiltration and perineural tumor spread, and is superior for assessing the extent of disease in the oral cavity, where artifact from dental amalgam often obscures the soft tissues. In the sinonasal region, MR readily distinguishes among tumor, edema, and retained secretions, where CT is often unable to do so. In the larynx, MR is more sensitive for laryngeal cartilage invasion, and probably equally specific, depending on the criteria that are applied.
FDG-PET, when combined with CT, is the best exam for nodal staging, and is the most effective way to detect disease recurrence in the posttreatment neck. Limitations include false positives due to physiologic uptake, inflammation, and infection. False negatives include small tumors, tumors that are obscured by areas of normal physiologic uptake, and malignant tumors that are not FDG avid.
Assessing local extent of disease
Local extent of disease is in many cases the most important factor that determines the initial treatment plan. Radiologic imaging and clinical examination are complementary in assessing the local extent of disease. Superficial mucosal tumor is better seen by direct inspection or endoscopy than by cross-sectional imaging. It may be impossible to pass an endoscope beyond a bulky mass to see the full extent of a mucosal mass. Although fixation to deep tissues on clinical examination can provide some information on the deep extent of disease, this finding is confirmed and often better detected with cross-sectional imaging. Invasion of bone, intracranial extension of disease, and perineural spread of tumor are findings that are typically discovered by cross-sectional imaging.
Nodal staging
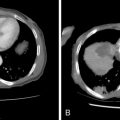
For any head and neck tumor, the size and extent of the primary tumor must be determined, and the regional lymph nodes must also be assessed. Determination of lymph node involvement may be based on morphologic features or on size criteria. Lymph node size is determined in the axial plane, which typically bisects the long axis of the lymph node, as most cervical lymph nodes have a craniocaudal orientation. One may measure either the longest dimension in the axial plane, or the shortest dimension in the axial plane. For either measurement, 10 mm is considered the upper limit of normal. The exception to this is the highest level IIA lymph node, that is, the jugulodigastric node. A normal jugulodigastric lymph node may measure up to 11 mm in the shortest dimension in the axial plane, or up to 15 mm in the longest dimension in the axial plane. Regardless of size, a lymph node is abnormal if it demonstrates necrosis or extracapsular tumor extension ( Figure 21-3 ).
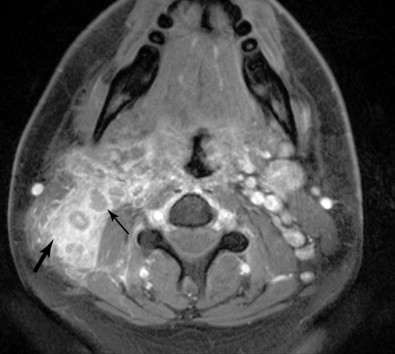
Lymph nodes are grouped by region. Superficial lymph nodes, such as facial chain, periparotid, intraparotid, preauricular, and occipital lymph nodes, are described by location. Retropharyngeal lymph nodes, which lie medial to the internal carotid arteries near the skull base, are grouped into medial retropharyngeal lymph nodes (uncommonly seen) and the more common lateral retropharyngeal lymph nodes (nodes of Rouviere). Cervical chain lymph nodes are grouped into seven levels, determined by anatomic landmarks (see Figure 21-2 ). If a lymph node overlaps two regions, it is assigned to the level that contains the majority of its bulk. Level I lymph nodes are anterior to the posterior edge of the submandibular gland. These may be further subcategorized into Level IA (medial to the anterior belly of the digastric muscle) and Level IB (lateral to the anterior belly of the digastric muscle). Level II lymph nodes are found posterior to the posterior edge of the submandibular gland, lateral to the internal carotid artery, anterior to the posterior edge of the sternocleidomastoid muscle, and superior the inferior edge of the hyoid bone. These may be divided into Level IIA (anterior to the internal jugular vein or touching the internal jugular vein), and Level IIB (posterior to the internal jugular vein). Level III lymph nodes are bordered superiorly by the inferior edge of the hyoid bone, medially by the medial edge of the common carotid artery, inferiorly by the inferior edge of the cricoid cartilage, and posteriorly by the posterior edge of the sternocleidomastoid muscle. Level IV lymph nodes are bordered superiorly by the inferior edge of the cricoid cartilage, medially by the medial edge of the common carotid artery, posterolaterally by a line drawn through the lateral edge of the sternocleidomastoid and the lateral edge of the anterior scalene, and inferiorly by the clavicle. If the clavicle is visible in the axial plane, then a lymph node is said to be in the supraclavicular station. Because of varying anatomy between individuals, the level IV station may be quite small or even nonexistent. Level VA lymph nodes are located posterior to the posterior edge of the sternocleidomastoid muscle, from the skull base to the inferior edge of the cricoid. Level VB lymph nodes are located posterolateral to a line drawn from the lateral edge of the sternocleidomastoids to the lateral edge of the anterior scalene muscles, below the inferior edge of the cricoid, and above the clavicle. Level VI lymph nodes are below the inferior edge of the hyoid bone, medial to the common carotid arteries, and superior to the manubrium of the sternum. Level VII lymph nodes are in the superior mediastinum, medial to the common carotid arteries, below the top of the manubrium of the sternum, and above the brachiocephalic vein.
Perineural spread
Perineural spread is a specific pattern of local disease extension that warrants separate discussion, because of the complexity of the involved anatomy. The cranial nerves and their branches may be conduits for spread of disease. Although a variety of benign lesions such as nerve sheath tumors, meningioma, infection, and granulomatous disease may spread along nerves, this pattern of disease is most commonly seen with malignancy. Care should be taken to distinguish between perineural invasion and perineural spread . Perineural invasion is not detectable by imaging; it is a feature of the primary tumor that is seen on histopathology, and is associated with worse prognosis. Perineural spread refers to the phenomenon of tumor using the nerve as a pathway to spread away from the primary site. Perineural spread can happen early in the disease course, can be a pattern of recurrence, and can be responsible for the only presenting symptoms. It is the responsibility of the radiologist to detect perineural spread so that an appropriate treatment plan can be selected. Adenoid cystic carcinoma and squamous cell carcinoma are the tumors that are most commonly associated with perineural spread. Perineural spread may also be seen with other minor salivary gland tumors, lymphoma, melanoma (particularly the desmoplastic subtype), and sarcomas. Pain, paresthesia, and motor denervation (when V3 and/or facial nerve are involved) are the most common complaints, though up to 40% of patients may be asymptomatic or have nonspecific complaints. , Furthermore, presence of symptoms is not specific for perineural spread.
Pathways of spread
V1 : Perineural spread along V1 typically relates to cutaneous malignancy. The supraorbital and supratrochlear nerves, branches of the frontal nerve, are most commonly involved. Tumor can then spread back along the main V1 trunk to the superior orbital fissure, cavernous sinus, and Meckel’s cave.
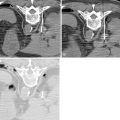
V2 ( Figure 21-4 ): The main trunk of V2, the maxillary nerve, runs through foramen rotundum, through the cavernous sinus, and continues to Meckel’s cave. Tumor may reach the maxillary nerve through several pathways. Minor salivary gland tumors arising from the palatal mucosa may extend through the greater and lesser palatine foramina, to the pterygopalatine fossa, and then to the maxillary nerve. Cutaneous carcinomas arising from the cheek or temporal region may extend along the zygomaticofacial and zygomaticotemporal nerves and then to the pterygopalatine fossa. Tumors of the maxillary sinus may extend along the anterior and posterior superior alveolar nerves to reach V2, and tumors of the face or maxillary sinus may spread along the infraorbital nerve.
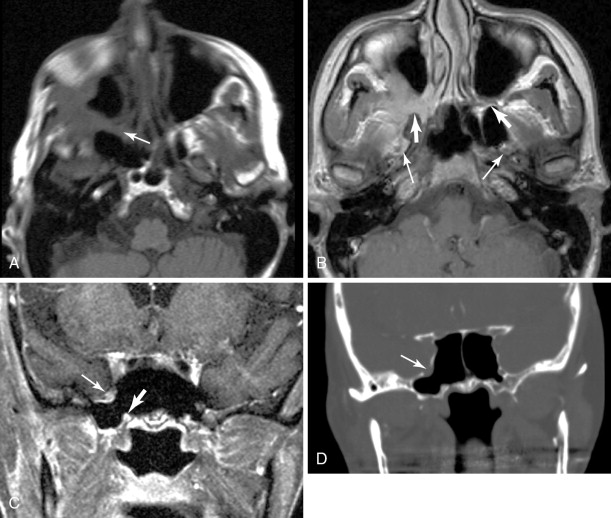
Figure 21-4
Perineural tumor—V2, vidian, V3
Vidian nerve (see Figure 21-4 ): Tumor that reaches the pterygopalatine fossa, through the pathways described above, may extend along the Vidian nerve and progress intracranially. Tumor may then extend further along the greater superficial petrosal nerve (GSPN) to the geniculate ganglion and to other branches of the facial nerve. Tumor that has reached Meckel’s cave may also extend down to GSPN and then anteriorly along Vidian nerve.
V3 (see Figure 21-4 , Figure 21-5 ): Tumor arising in the masticator space or prestyloid parapharyngeal space may extend along V3, through foramen ovale and to Meckel’s cave. Tumor that reaches Meckel’s cave by spreading along V2 may spread antegrade along V3. Parotid gland tumors that involve the facial nerve may also spread to V3 via the auriculotemporal nerve. The auriculotemporal nerve passes immediately posterior to the mandibular condyle and splits around the middle meningeal artery before passing through the otic ganglion and connecting with V3. Tumor that spreads retrograde along V2 to reach Meckel’s cave may also then spread antegrade along V3. When tumor involves V3, denervation change may be seen in the muscles of mastication.
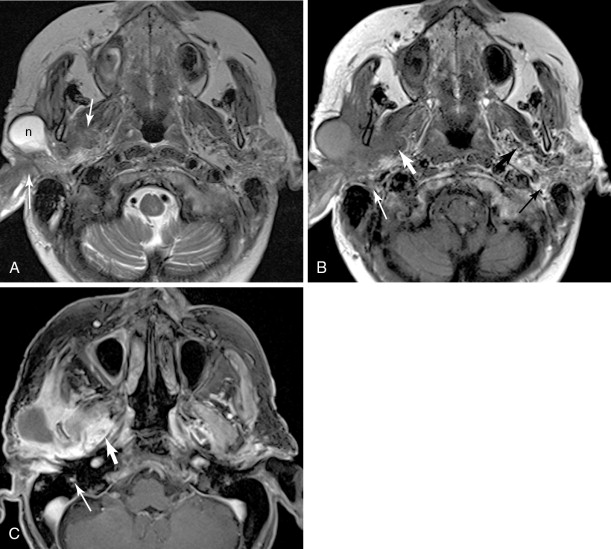
Figure 21-5
Parotid squamous cell carcinoma, perineural spread and intracranial
Facial nerve (see Figure 21-5 ): Primary salivary gland tumors and malignancies that involve the parotid gland secondarily may extend along the facial nerve, through the stylomastoid foramen, and along the facial nerve as it courses through the temporal bone. Tumor involving the Vidian nerve may also extend along the GSPN to the geniculate ganglion and main facial nerve, and tumor involving Meckel’s cave may extend inferiorly to involve the adjacent GSPN. Tumor involving V3 may extend to the facial nerve along the auriculotemporal nerve. The chorda tympani, a branch of the facial nerve, is also connected to the lingual nerve branch of V3, though this is not generally considered an important pathway of perineural spread of tumor.
Hypoglossal nerve : Tongue cancers can spread retrograde along the course of the hypoglossal nerve, and secondarily involve cranial nerves IX, X, and XI.
Imaging of perineural spread
MRI is the preferred modality for detecting perineural spread. According to most reports, CT is 80%–90% sensitive and specific for perineural spread. , MRI is considered much more sensitive than CT, with >95% sensitivity, and equal or greater specificity. According to an early report, MRI does not detect the full extent of perineural spread with high sensitivity, though current performance is probably much better with modern equipment.
Direct signs of perineural spread on MRI include obliteration of the fat around the involved nerve, enhancement of the nerve, or thickening of the nerve. Any single one of these features should be considered evidence of disease, with one important caveat: the mastoid, tympanic, and geniculate segments of the facial nerve frequently enhance in normal subjects, because of enhancement of perineural and epineural venous plexus. , Thus, to be considered involved by disease, the facial nerve should show abnormal thickening and enhancement, a marked asymmetry in enhancement, or denervation change in affected muscles. Obliteration of fat at the stylomastoid foramen is also helpful in confirming extension along the facial nerve from a parotid tumor (see Figure 21-5 ). CT is sometimes able to demonstrate perineural spread by showing enlargement of the involved foramen, or obliteration of fat surrounding the involved nerves. A standard MR imaging protocol tailored for detection of perineural spread should include axial and coronal T1-weighted images, axial and coronal T2-weighted images (including at least one plane with fat suppression), and axial and coronal contrast-enhanced T1-weighted images with fat suppression.
Indirect signs of perineural spread include denervation change in the target muscles of motor nerves. There are several stages of denervation change. Initially, muscle volume is slightly increased, T2 is prolonged, and the affected muscle enhances diffusely. There may be subtle loss of the normal T1-bright fat interspersed among the muscle fibers. Next, the muscle volume normalizes, and some fatty infiltration is seen, evidenced by intrinsic T1 hyperintensity. Enhancement persists, and is best detected on fat-suppressed postcontrast images. In the next stage of denervation, T2 hyperintensity and enhancement resolve, and fatty replacement continues. In the final stage of denervation, the muscle volume is reduced and there is diffuse fatty infiltration of the muscle. In general, these stages are seen in muscles innervated by the trigeminal and hypoglossal nerves. Small muscles innervated by other nerves (such as the facial nerve) typically progress directly to fatty atrophy without clearly defined intermediate stages by current imaging techniques. It is important to be aware of these changes so as not to confuse denervation edema with direct tumor extension or an inflammatory process. In addition, reflex sympathetic dystrophy can involve the muscles of mastication following skull fracture or craniotomy. Localized edema and inflammation can also be associated with contusion, laceration, or temporomandibular joint inflammation. Identifying denervation change also sometimes improves confidence in detecting perineural spread.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree