Key points
- •
The pathologic endpoint for percutaneous thermal or ethanol treatment of unresectable tumors is coagulation necrosis. Similarly, the goals of transarterial therapy are cell necrosis and apoptosis.
- •
Although specific follow-up imaging protocols differ among practitioners and institutions, contrast-enhanced computed tomography (CT), positron emission tomography (PET), and magnetic resonance imaging (MRI) are the primary imaging modalities used.
- •
Because tumors can recur months or years after treatment, long-term imaging follow-up should be instituted.
- •
Suspicious findings for residual or recurrent tumor include nodular, scattered, eccentric enhancement about the ablation zone, ablation zone that does not completely encompass the tumor, heterogeneous or focally increased 18-F fluorodeoxyglucose (FDG) uptake, and increasing or new marked FDG uptake.
Introduction
Histopathology is the gold standard for assessing the impact of locoregional treatment of tumors. However, this is impractical except in cases such as treatment preceding organ transplantation or excision. Generally, one must assess treatment response using imaging, in spite of its limitations. Each imaging modality has its own particular advantages and disadvantages. In this section, we will review the mechanisms of tissue injury, the classic pathologic findings, and the imaging correlates that are seen with percutaneous thermal ablation and transcatheter arterial ablation of tumors in various solid organs.
Mechanisms of tissue injury and histopatholic assessment
Radiofrequency ablation
Much of the literature on locoregional tumor treatment focuses on the effects of radiofrequency ablation (RFA) on liver tumors. The concepts are largely applicable to other thermal ablative treatments and to other organ systems. In essence, successful targeted treatment of tumor entails the induction of cell necrosis or programmed cell death (apoptosis) using extreme heat.
Gross pathologic evaluation of the thermally ablated tissue typically reveals a localized hardening of the parenchyma, with a central “white zone” of coagulation and a surrounding “red zone” of hyperemia ( Figure 5-1 ). The zone of coagulation mostly consists of coagulative necrosis, wherein intact nuclei are absent within cells, a finding that is consistent with cell death. Capsular retraction can be seen if the tumor is subcapsular. In the chronic stage, the necrotic tissue hardens, undergoes volume loss, and is often surrounded by a thick fibrous capsule.
RFA functions by imparting frictional energy to a tissue causing destruction (coagulative necrosis) through heat energy. In the most common system, a monopolar system, this energy is created by a rapidly alternating voltage current between the electrode placed within the target lesion (active electrode) and dispersive pads (reference electrode) placed on the patient’s skin. This alternating electrical current, with frequencies in the range of 375–500 kHz, results in agitation of the surrounding tissue ions, resulting in tissue heating. , The density of this electrical current is greatest immediately surrounding the electrode, and diminishes with distance. Coagulation necrosis will occur if the tissue temperature is elevated and maintained at 60°C for several seconds. Therapeutic RFA strives to heat tissues in the range of 60–100°C.
Immediately following RFA, conventional hematoxylin and eosin stain will not demonstrate significant change to the tissue other than subtle distortion of sinusoidal architecture. This apparent false negative can be mediated by the use of specialized staining agents, such as the nicotinamide adenine dinucleotide stain, which will highlight devitalized tissue. , In the chronic phase, cytolysis and obscuration of the nucleus will result in an amorphous eosinophilic hepatocyte. Infiltration by fibroblasts and mononuclear cells are also seen. Eventually, a capsule of granulation and fibrous tissue forms around the ablation zone. ,
Heat sink phenomenon
The “heat sink” phenomenon has been most often described in the setting of RFA. It refers to the reduction in tissue temperature due to the conductive effects of adjacent vessels, typically described as 3 mm or greater in diameter. It is one explanation for suboptimal treatment results and distortion of the ablation zone.
Techniques to overcome the heat sink effect include pharmacologically decreasing blood flow, temporary vascular balloon occlusion, intraarterial embolization, and the Pringle maneuver. The latter entails temporarily occluding the hepatic artery and the portal vein by compression during RFA at laparotomy.
Cryoablation
The mechanism of tissue injury in percutaneous cryoablation is variable and depends on several factors, including the rate and extent of freezing. Fast freezing rates result in direct tissue injury, whereby ice crystals form within cells. Slower rates result in ice crystals forming in the extracellular spaces resulting in cellular dehydration, leading to membrane damage. , Another mechanism of cryoablation-induced tissue injury is expansion of the microvasculature, leading to endothelial damage and subsequent thrombosis. , Additionally, sublethal freezing temperatures can lead to apoptosis (programmed cell death), a finding often seen in the periphery of the ablation zone. ,
Like other thermal ablative technologies, coagulation necrosis is the desired histopathologic endpoint. Lethal temperature is considered to be within −40° to −50°C, although tumor cell survival has been reported after freezing to less than 50°C. Most cryoablation protocols entail two freeze–thaw cycles, which compared to a single cycle, have been shown to result in a larger area of coagulation necrosis on pathologic specimens and in a larger ice ball on imaging.
Conceptually similar to the heat sink phenomenon described in RFA, cryoablation is limited by the delivery of warm blood by vessels adjacent to tumors, resulting in higher than desired temperatures. This is sometimes referred to as the “cold sink,” and it can likewise result in variable size of the ablation zone and extent of the coagulation necrosis. ,
Microwave ablation
A relatively new treatment option for locoregional control of unresectable tumor is microwave ablation, which employs electromagnetic wavelengths in the frequency range of 900–2450 MHz. Rapid directional current changes in the microwave electrode causes surrounding water dipoles to oscillate, resulting in heat generation leading to cellular coagulative necrosis. Unlike RFA, microwave ablation is not limited by the conductive properties of tissues, and therefore temperatures higher than 100°C can be more easily achieved, theoretically resulting not only in a larger area of coagulation necrosis but also in a more uniform ablation zone as the heat sink effect by regional vessels is less a factor. ,
Percutaneous ethanol injection
Owing to its ease of use and cost-effectiveness, percutaneous ethanol injection (PEI) remains a viable option in many parts of the world, particularly for the treatment of hepatocellular carcinoma (HCC) in cirrhotic livers. Coagulation necrosis is achieved by both direct and indirect mechanisms. Alcohol instillation will result in immediate cytoplasmic dehydration and protein denaturation. Indirectly, tumor death results following alcohol-induced vascular endothelial necrosis and subsequent thrombosis. Although it is not affected by heat sink, its success is largely limited by the difficulty in obtaining a uniform distillation of ethanol over a large tumor volume. As such, PEI is often not used in tumors with large size, heterogenous or dense composition, and in those without a capsule or pseudocapsule (the latter theoretically would aid in increasing intratumor concentration of alcohol). , Given the effectiveness and safety profile of RFA and other thermal ablative technologies, PEI has largely been replaced by these modalities in the treatment of HCC. ,
Transcatheter embolization
Exploiting the dual blood supply of the liver, transarterial therapy offers a unique adjunct to surgery and systemic chemotherapy. Most often described for nonresectable primary and secondary malignancies in the liver, transcatheter embolization can be further divided into three categories: arterial embolization, chemoembolization, and radioembolization. Extensive discussion of these techniques and their respective indications, risk profiles, and success rates is beyond the scope of this section. Detailed peer-reviewed literature abounds for the interested interventionalist. For our purposes, a short review of their mechanisms and imaging findings will be discussed.
Arterial embolization
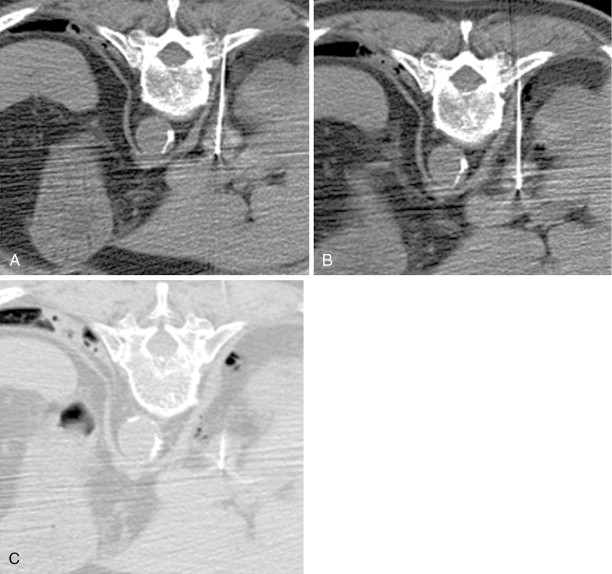
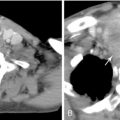
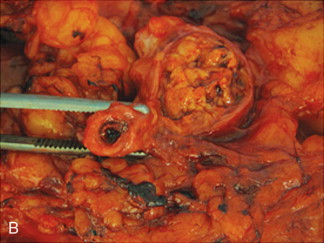
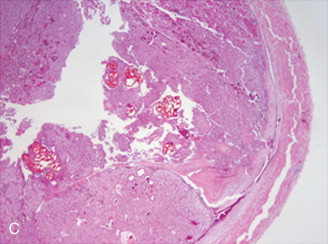
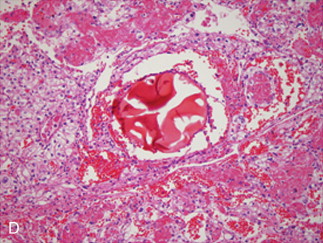
Akin to percutaneous thermoablative treatments, the goal of arterial embolization is cell necrosis. In this modality, occlusion of the terminal vasculature feeding the tumor results in ischemia and subsequent coagulation necrosis. Various agents have been used, including gelatin sponge, polyvinyl alcohol, or spherical embolic particles. Extensive (>95%) coagulation necrosis can be seen within 3 days after complete embolization of the feeding artery ( Figure 5-2 ).
Chemoembolization
Technically similar to arterial embolization, chemoembolization also includes the injection of anticancer drugs with or without lipiodol (ethiodized oil) directly into the hepatic artery. In theory, the targeted delivery of chemotherapeutic agents would result in high local concentrations, thereby limiting the systemic toxicity associated with these drugs. However, there remains much debate as to the optimal chemotherapeutic to use, confounded by some authorities debating that bland embolization is equally as effective. , Special care is taken with patients with poor hepatic reserve as the toxicity of such high concentrations within the liver could result in irreversible hepatic failure if not properly controlled.
Radioembolization
Radioembolization represents a potential option in patients with limited hepatic reserve. To counter the potentially destructive effects of high concentrations of intrahepatic chemotherapy in the treatment of unresectable liver malignancies, radioembolization with yttrium-90 has been considered. Yttrium-90, a radioisotope with a half-life of approximately 64 hours, is a beta emitter with a maximum tissue penetration of about 1 cm. This limited tissue penetration allows extremely high levels of radiation (goal range of 125–150 Gy) to be selectively delivered to the tumors while regional hepatic and systemic toxicities are kept at acceptable levels. Particles are delivered transarterially to the intended target in a fashion similar to other transarterial therapies. Appropriate dosing calculations need to be performed and pretherapy embolization of certain collateral vessels must be performed, which adds to the complexity of this therapy.
Imaging assessment of treatment response
The primary modalities utilized to assess tumor and tissue response to locoregional treatment are computed tomography (CT), positron emission tomography (PET), magnetic resonance imaging (MRI), and ultrasonography. Each has its own advantages and limitations. With the increased utilization of ablative techniques, there is growing interest in identifying the optimal protocol for imaging follow-up.
At some centers, contrast-enhanced imaging is obtained immediately following completion of the procedure, especially for tumors with preprocedure enhancement. Otherwise, a common follow-up protocol is to obtain contrast-enhanced CT or MRI 1–6 weeks after the initial ablation and every 3–4 months thereafter. Some practitioners advocate more frequent follow-up in the first year following treatment, the time during which most local recurrence would manifest.
Regardless of the imaging modality used, the margins, shape, and if applicable, enhancement pattern of the ablative zone should be described and the absence or presence of complications should be assessed. Because percutaneous treatments will inevitably include the ablated perilesional tissue, comparison of ablation zone size should be made to the cross-sectional measurements obtained postablation, rather than preablation.
As with any technologically based treatment, newer-generation equipment as well as tuning and experience of current equipment will continue to improve. Thus, it is important to understand certain elements that are fundamental to ensuring an adequate ablation: (1) The imaging appearance and the amount of perilesional normal parenchyma to ablate to obtain an “A0” ablation; (2) The various thermal maps supplied with each electrode/cryoprobe in addition to changes of these zones when more than a single probe is utilized in unison; (3) Properly identify on postablation imaging both expected findings as well as what constitutes local tumor recurrence allowing prompt retreatment when necessary.
Much of this session will focus on CT, which is the most commonly used modality, although CT descriptors are often translatable to both MRI and ultrasonography.
Computed tomography
At most centers, CT is the primary modality used for immediate postprocedure imaging and follow-up after percutaneous ablation or transarterial embolization. This is largely due to its wide availability, relatively low cost, and greater user experience. Depending on the target organ treated and pretreatment tumor imaging characteristics, the phase and protocol used when performing posttreatment follow-up will vary. For instance, when CT is performed following treatment of hepatic tumors, a common imaging protocol includes a triphasic CT that includes arterial, portal venous, and delayed phases allowing optimal comparison to pretreatment images.
Ablation zone
In the immediate postablation setting, most practitioners obtain a scan to establish a new imaging baseline and to assess for treatment adequacy.
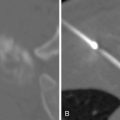
Following microwave ablation or RFA in the lung, ground-glass opacity surrounding the treated lesion is correlated with treatment success ( Figure 5-3 ). A circumferential margin of at least 5 mm is ideal to ensure complete tumor kill. Conversely, areas lacking this opacity are associated with recurrence. , In solid organs, thermally ablated lesions generally appear as increased density in the center with poorly marginated peripheral hypoattenuation on immediate postprocedural noncontrast CT. Secondary to this poor margination, intravenous contrast administration is often used to more readily identify residual viable tumor, which can manifest as regions of enhancement, and therefore guide further therapy.
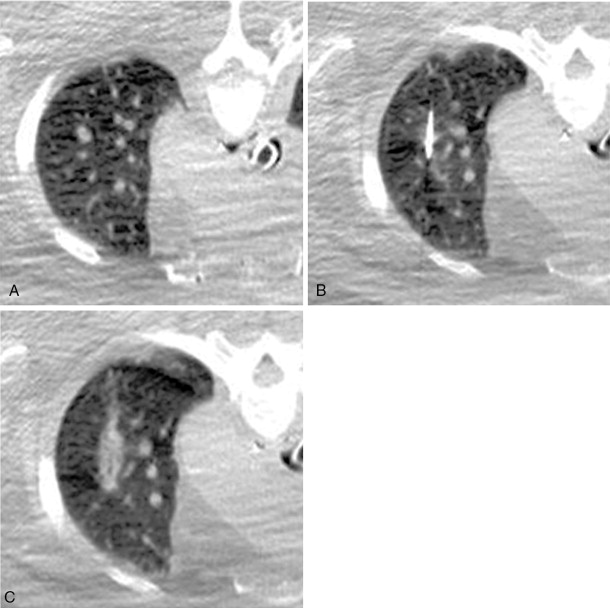
When cryoablation is utilized in solid organs such as liver or kidney, the ice ball formed is often well depicted on intraprocedural CT as an area of low attenuation. It is important to understand that the entire circumference of this ice ball does not often constitute “lethal” ice, or temperatures required to kill all viable tumor. This has been confirmed in a porcine model in liver, kidney, and lung. The ice ball is often not well visualized in lung tissue secondary to the already low attenuation of the surrounding normal air-filled alveoli, making confirmation of complete treatment difficult. Therefore, it is of extreme importance to study and understand the isotherm maps for each cryoprobe used to both optimally treat the tumor and obtain adequate peritumoral margins.
Although most often spherical in shape, the RFA zone can also appear slender or irregular. This can be attributed to several etiologies, including the aforementioned heat sink effect, deformation by large hepatic vessels, insufficient radiofrequency power output, or movement of the electrode with patient breathing. Additionally, parenchymal infarction abutting the RFA zone can have the same attenuation as the ablated tissue, thus giving the impression of abnormal shape.
If factors preclude obtaining an adequate treatment margin, such as juxtaposition to large portal or hepatic vessels in the liver, the patient should be closely observed with contrast-enhanced imaging for evidence of residual tumor.
Contrast enhancement
Contrast enhancement following treatment does not always correspond to residual tumor. In fact, enhancement of the periablation parenchyma seen on contrast-enhanced imaging is a pathologically proven benign physiologic response to thermal injury. Postulated etiologies for this phenomenon include psuedoenhancement, such as seen with renal cysts, or contrast escaping into surrounding tissues secondary to leaky capillaries secondary to treatment. Initially, the response is reactive hyperthermia. This is followed by fibrosis and giant cell reaction. This benign periablational enhancement, seen immediately following ablation, most commonly resolves within 1 month of treatment; however, it can be seen months afterwards owing to variability in imaging protocols, such as the injection rate and the scanning delay as well as perilesional inflammation. This widespread variability complicates recommendations for appropriate timing of imaging follow-up.
In the kidney, minimal contrast enhancement (<20 HU) that is seen soon after ablation represents devitalized tissue on pathology, compatible with treatment success.
Findings on contrast-enhanced CT has also been used to evaluate treatment outcome following RFA of unresectable pulmonary malignancies. Abtin et al. showed that imaging findings concerning for treatment recurrence include enhancement greater than pretreatment levels, nodular or central enhancement greater than 10 mm or 15 HU, or progressive enhancement (beyond 180 seconds) on multiphasic imaging. As a corollary to this finding, Suh et al. showed that mean contrast enhancement dramatically decreases as soon as 1 month following treatment, which the authors attributed to injury to the local vasculature. Although this reduction in enhancement “recovered” somewhat on subsequent scans, the level of enhancement remained less than that seen on pretreatment scans, suggesting positive treatment outcome. This led the authors to conclude that CT densitometry can potentially play an important role in the noninvasive evaluation of thermally ablated pulmonary tumors.
In the liver, an area of benign enhancement can be seen adjacent to the ablation zone. This is thought to be secondary to arterioportal shunting, which is often wedge-shaped or nodular in appearance. The pathogenesis is likely related to mechanical injury to the hepatic microvasculature from the treatment probe, which is seen both following biopsy and percutaneous ethanol ablation. , These shunts are typically small and resolve spontaneously, conferring little clinical significance.
Benign periablational enhancement should be differentiated from the irregular peripheral enhancement that signals the presence of residual or recurrent tumor at the treatment margin. This pattern of enhancement is often described as scattered, nodular, or eccentric. , On CT, residual tumor is usually of high attenuation during the arterial phase and low attenuation during the portal venous and equilibrium phases, indicating washout. Because many hypovascular tumors exhibit delayed enhancement, portal venous or delayed imaging may be indicated.
Other findings and complications
Other imaging findings following thermal ablative therapy includes inflammatory stranding in the acute setting and fibrosis, scarring, and architectural distortion in the chronic setting. Scarring and fibrosis typically results in volume loss of the ablation zone, inclusive of the index tumor and of the treated parenchymal margin, over time. One study evaluating the CT findings following RFA of hepatocellular carcinoma reported mean volume changes of 79%, 50%, 27%, and 11%, at 1, 4, 16, and 19 months, respectively. It is important to note that the absence of involution is not synonymous with treatment failure.
Air within the immediate postablation cavity or tract is a normal finding ( Figure 5-4 ). This typically resolves within 1 month. Their presence is likely related either to the production of gas during tissue necrosis or to the introduction of air when the probe is advanced into the organ. , This finding often complicates diagnosis of superimposed infection. In one study, 4 of 14 patients with air pockets after microwave ablation was found to have hepatic abscesses, but all four patients presented with fever and pain that persisted for more than 2 weeks. Of note, biliary obstruction has been reported as a predisposing factor for postprocedure hepatic abscess formation when transarterial chemoembolization of the liver is performed.