Key points
- •
Interventional management of thoracic metastases involves careful consideration not only of parenchymal lesions, but also pleural and chest wall disease.
- •
Management of parenchymal metastases is similar to that of primary neoplasms, with the same technical considerations applying.
- •
Malignant effusion is the most common manifestation of pleural metastases that the interventionalist will be called upon to treat. This is largely accomplished through the use of temporary or indwelling drainage catheters with or without administration of a sclerosing agent to achieve pleurodesis.
- •
Painful osseous metastases may be treated with ablative therapies. Although much of the recent literature supports the use of RFA for this indication, cryoablation offers certain unique advantages, such as direct visualization of the ablation zone. This is especially helpful when ablating near critical structures.
Introduction
The thorax is a common site of metastatic disease, with frequent involvement of the lungs, pleura, osseous structures, and regional lymph nodes. Interventional radiologists are integral to the confirmation of thoracic involvement of malignancy via tissue sampling. There is growing support for the role of image-guided interventions in treating the manifestations of thoracic metastatic disease. This includes thermal ablation of metastatic disease to the lungs and pleura, catheter placement and sclerosis of malignant pleural effusions, and palliative pain management for osseous and soft-tissue metastases.
Techniques for percutaneous biopsy and thermal ablation of lung and bone neoplasms have been discussed in other chapters in this textbook. The first portion of this chapter will focus on patient selection for thermal ablation of discrete lung and pleural metastases. The second section will discuss management of malignant pleural effusion. The chapter will conclude with a section discussing the palliative management of painful osseous and soft tissue metastases with thermal ablation.
Lung metastases
The lung is one of the most common sites of metastatic disease. Lung metastases are present in up to 30% of patients dying from all cancer types ( Figures 25-1 and 25-2 ). The surgical literature has demonstrated that metastasectomy offers improved survival for patients presenting with a limited number of lung metastases. Many of these patients have medical comorbidities, which make them poor surgical candidates. These patients can benefit from alternate forms of therapy, including external beam radiation as well as image-guided thermal ablation.
Rationale for ablation of lung metastases
Patients are referred for thermal ablation of lung metastases with the intent to either cure localized disease or to decrease tumor burden for palliative reasons. The basis for treatment of lung metastases is found in the surgical literature, where a survival benefit of metastasectomy for patients with limited pulmonary tumor burden has been demonstrated. The safety and efficacy of thermal ablation of primary and secondary lung neoplasms has been well established.
General surgical selection criteria necessitate that the primary tumor be controlled; there should be no or few extrathoracic metastasis, with the exception of liver; the metastases are in locations that make them amenable to surgically resection; and the patient’s overall status and pulmonary function must be able to tolerate the procedure. When the above criteria can be met, the survival outcomes are generally good, with a 5-year survival rate that ranges between 30% and 50% in several retrospective studies. Furthermore, Yamamoto et al. showed the potential for complete cure following metastasectomy of pulmonary colorectal carcinoma metastases of 17%. Although the lack of randomized controlled studies makes it difficult to conclude that the survival benefit is due to the surgery itself, the data that have been presented in case series has allowed pulmonary metastasectomy to become well established as a treatment of localized metastatic disease.
The recurrence of disease following metastasectomy is quite high. According to the International Registry of Lung Metastases, the recurrence rate for all patients undergoing complete resection is 53%. Rates of recurrence tend to be higher with certain primaries, such as sarcoma and melanoma. In many series, these patients have been treated with repeat resection. More recently, data have emerged to support nonsurgical management of oligometastatic pulmonary disease, including thermal ablation and stereotactic body radiation therapy. ,
A considerable amount of data has been reported over the past decade to support the safety and efficacy of thermal ablation in the treatment of localized metastatic disease. As in the surgical literature, many of the studies have consisted primarily of colorectal carcinoma, renal cell carcinoma, and sarcomas. The unifying characteristic of each of these malignancies is the occasional presentation of discrete oligometastatic pulmonary disease. The main objective of ablation therapy is eradication of tumor cells within a finite volume of lung so as to not significantly disrupt the overall functional reserve. This is particularly important in patients with limited baseline pulmonary function, such as those with fibrosis or emphysema. In comparison to surgical resection, presumed benefits from thermal ablative therapies include more targeted therapy with greater sparing of functional lung tissue in patients with suboptimal pulmonary function, lower costs, repeatability, and decreased morbidity and mortality ( Figure 25-3 ).
Much of the outcomes data reported have used RFA as the ablative modality. This is not surprising, as RFA was the earliest thermal ablation technique to be adopted for use in the lungs. Studies looking at long-term survival demonstrated 5-year survival rates of approximately 27%, which is comparable to some of the numbers quoted for metastasectomy in the surgical literature. A study that looked at 3-year cancer-specific survival demonstrated percentages of 90.6 for sublobar resection, 87.5 for RFA, and 90.2 for cryoablation.
Some patients with more extensive metastatic disease may be candidates for percutaneous thermal ablation when the bulk of disease is stable, but a select number of metastases display aggressive growth. These lesions may be targeted to prevent complications, particularly airway or vascular compression, which could potentially cause increased symptoms or possibly death. Although cytoreduction is not an indication that is well established in the literature, it is a selection criterion that we have used for treating a select group of patients.
Patient selection
Patients who may benefit from treatment of lung and pleural metastases have a countable number of discrete lesions that are limited to the lung or pleural surfaces. Several cancers that commonly present with oligometastatic thoracic disease include colorectal and renal cell carcinoma, osseous and soft tissue sarcomas, and tracheal adenoid cystic carcinoma. Various numbers have been used to quantify “oligometastatic” disease. In general, we will consider patients with five or fewer metastases, but we have treated patients with a larger number of lesions when the metastases have shown stability for 6 months or longer. The extent of disease should ideally be limited to the thorax, but again, exceptions are sometimes made when discrete treatable lesions are present elsewhere, such as in the liver.
Patients should only be considered after appropriate consultation with a medical oncologist. Thermal ablation is often used concurrently with system chemotherapy. The rationale is that chemotherapy may be more effective for small, microscopic metastatic implants, and may incompletely resolve larger metastases.
Although some groups treat multiple lesions, including those in both lungs, during a single session, we advocate treating a single lesion during each session. This is for several reasons. Each ablation should be performed with ideal patient positioning, whether prone, supine, or oblique. Placement of multiple probes increases the risks of the procedure, including the development of hemorrhage and pneumothorax. This is particularly true when lesions are present in both lungs. Most patients can tolerate a degree of respiratory compromise from pneumothorax or hemorrhage into a single lung, but the possibility of compromise of the function of both lungs must be considered prior to performing a bilateral lung ablation.
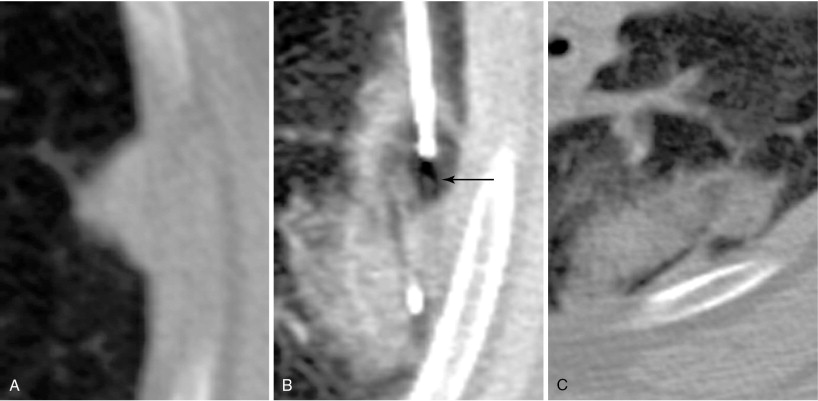
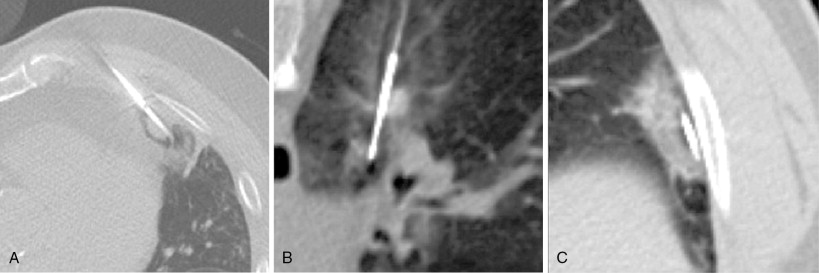
When treating a patient with multiple metastases, a decision as to the order of treatment must be made prior to commencing. Lung ablations are performed with conscious sedation. Patient cooperation with breath holding and remaining stationary is essential. We advise performing a technically simple ablation during the first procedure to allow the patient to get accustomed to the experience. This allows treatment of more difficult lesions during subsequent procedures. It is best not to delay treating lesions that may be more problematic in the future, such as one that is encroaching upon a major vessel that could function as a heat sink ( Figure 25-4 ).
Patients will typically require 1–2 weeks to recover after lung ablation. It is reasonable to schedule a patient for weekly procedures if they are doing well clinically. The goal should be to complete a set of treatments within 2 months. The patient should then undergo active surveillance, both to evaluate for treatment failure and to monitor for disease progression. If disease progression in the form of new lesions is discovered during a set of treatments, it signifies disseminated disease that is unlikely to be controlled via local therapy. Consultation with the patient’s medical oncologist is required.
Procedure and follow-up
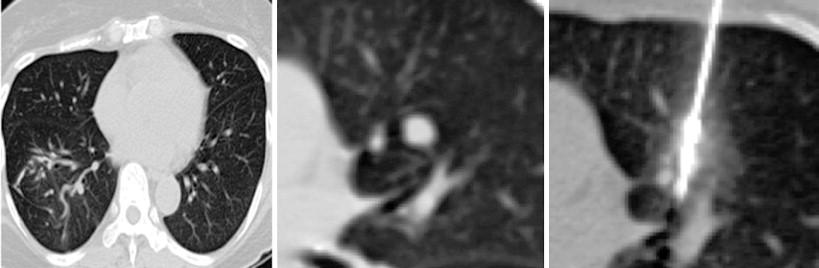
Perioperative management and technical factors for performing a successful percutaneous lung ablation were discussed in the previous chapter. Radiofrequency ablation (RFA), microwave ablation (MWA), and percutaneous cryotherapy (PCT) can be used interchangeably for ablative procedures of lung metastases. The use of one modality over the other is based primarily on operator preference, with the individual modalities having particular strengths and weaknesses ( Figure 25-5 ).
Safety considerations prior to ablation include management of coagulation status, and sedation risk. Most lung ablations are performed with conscious sedation, and therefore, some of the risks of anesthesia are minimized. Additionally, as opposed to ablation of primary lung carcinomas, many patients undergoing ablation of metastatic lung disease have normal pulmonary function.
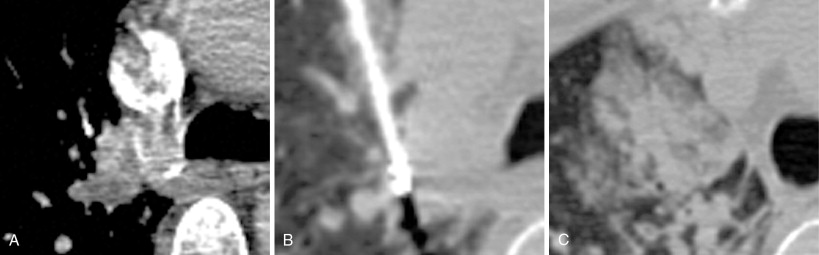
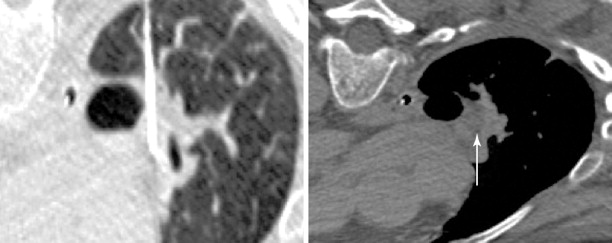
Technical considerations for safe thermal ablation are identical to those used for treatment of primary lung carcinoma. These include use of tangential approach for peripheral lesions ( Figure 25-6 ) and the axial approach for central lesions ( Figure 25-7 ). Care should be taken to not cross major vascular and airway structures during needle placement, as well as avoiding a needle position with the tip pointed toward a major vascular structure. In order to obtain a complete ablation, the interventionalist must be aware of the device-specific expected ablation zone. Multiple probes may be required to fully treat the target lesion. Heat sinks are a major factor that can limit the extent of an ablation.