Key points
- •
Liver metastasis is a major cause of mortality and morbidity in patients with cancers of various origins and presents a therapeutic challenge because only a few patients are candidates for surgical resection, and systemic chemotherapy offers limited benefit.
- •
Locoregional therapies have been shown to be safe and effective in selected patients with liver metastases from melanomas, breast cancer, and other gastrointestinal tumors.
Introduction
The liver is a common site for metastases from various malignancies. In addition to colorectal cancers and neuroendocrine tumors, other cancers that frequently metastasize to liver include melanoma, especially uveal melanoma, breast cancer, and gastrointestinal stromal tumors (GISTs). Metastasis to the liver is common in patients with melanoma. Approximately 30% of patients with ocular melanomas develop systemic metastases, and hepatic metastases are seen in 95% of such patients. Hepatic metastases can be seen in 14%–20% of patients with metastatic cutaneous melanomas. Breast cancer has a tendency to metastasize to the liver, with approximately 25% of patients with metastatic breast carcinoma developing hepatic involvement during the course of their disease. , GISTs are rare but are the most common mesenchymal neoplasms of the gastrointestinal tract. The liver is the most common site of metastasis from GISTs, with a reported incidence of 55%–72% in patients with recurrence. ,
The presence of hepatic metastases is associated with a poor prognosis and is a major cause of morbidity and mortality in patients with malignant tumors of different origins. The presence of hepatic metastases in patients with ocular melanoma is associated with median survival rates of only 6–8 months, and the 1-year survival is approximately 1015%. , For patients with breast cancer metastatic to the liver, median overall survival from the beginning of therapy ranges from 4 to 14 months, and the survival of untreated patients is 0.5–4 months.
Treatment options for patients with liver metastases are limited. Surgery is the only therapy that offers the possibility of cure for patients with liver metastases. Several studies have shown that surgical resection may prolong survival time of patients; however, only a small percentage of patients (10%–15% in various studies) are suitable for curative resection. Systemic chemotherapy, immunotherapy, and hormone therapy are considered mainstays in the treatment of patients with inoperable liver metastases but offer only limited benefit, and trials using systemic therapies have shown disappointing results. Systemic chemotherapy alone or in combination with immunotherapy yields response rates of less than 20% in patients with liver metastases from ocular or cutaneous melanomas, with median response durations of 5–7 months. , , Although systemic treatment can prolong survival of patients with metastatic breast cancer, the mean survival is dismal, ranging from 4 to 17 months. , For GIST, imatinib is a tyrosine kinase inhibitor (TKI) that has been shown to produce a partial tumor response or tumor stabilization in 70%–85% of patients with advanced disease. , With imatinib therapy, the median progression-free survival is in the range of 20–24 months, and the estimated median overall survival duration exceeds 36 months. Unfortunately, approximately 15% of patients have tumors that show no response to this drug, and in a proportion of patients whose tumors do respond, resistance to the drug develops after an average of 2 years of treatment. , For these reasons, local or regional liver-directed treatment options that include ablative therapies and transcatheter intraarterial therapies play a major role in the management of patients with unresectable liver metastases. In this chapter, we will review the current role of these treatment options in patients with liver metastases.
Transcatheter intraarterial tumor therapies
The Society of Interventional Radiology defines “image-guided transcatheter tumor therapy” as the intravascular delivery of therapeutic agents via selective catheter placement with imaging guidance. Various agents such as chemotherapeutic agents, embolic particles, and radioactive materials can be injected via feeding vessels into tumor(s) in an attempt to achieve tumor cell death by enabling more focused delivery or by deposition of higher concentrations within the tumor.
Hepatic arterial chemoinfusion
Direct hepatic arterial infusion of chemotherapy offers pharmacokinetic advantage over systemic administration. Enhanced first-pass extraction of chemotherapy by cancer cells permits relatively high doses of chemotherapy to be delivered with fewer significant systemic side effects. The increased intratumoral drug concentration can potentially lead to an increased therapeutic response.
Hepatic arterial chemoinfusion is generally administered through implantable catheters that are inserted into the hepatic artery and connected to a subcutaneous port to allow easy and repetitive infusion of chemotherapy. This can be done with either surgical laparotomy or percutaneous insertion using minimally invasive interventional techniques. , Various percutaneous access routes, such as subclavian, hypogastric, femoral, and brachial arteries, have been described for placement of arterial catheters and subcutaneous ports. , Alternatively, temporary catheters can be placed multiple times for repeated cycles of chemotherapy.
Safe and optimal drug distribution to liver tumors requires arterial redistribution methods to convert multiple hepatic arteries into a single vascular supply and to reduce extrahepatic cytotoxic side effects. In patients with multiple hepatic arteries, all hepatic arteries except the one to be used for chemotherapy infusion are embolized proximally with coils or liquid embolic agents. This results in the opening of intrahepatic arterial collaterals that allows drug delivery to the entire liver through a single hepatic artery catheter. To prevent nontarget infusion of cytotoxic agents to extrahepatic organs, arteries such as the gastroduodenal artery, the right gastric artery, the accessory left gastric artery, and the replaced posterior superior pancreaticoduodenal artery must be embolized, if they share a common origin with the hepatic artery to be used for infusion of chemotherapy. A number of novel techniques can be used to secure the catheter tip in the hepatic arteries.
Hepatic artery infusion chemotherapy treatment has been for patients with metastatic uveal melanoma ( Table 16-1 ). Cantore et al. reported an overall response rate of 38% and a median survival of 15 months in a series of 8 patients with uveal melanoma metastatic to the liver treated with HAI using a carboplatin-based chemotherapy. Leyvraz et al. reported the use of hepatic arterial infusion in a phase 2 trial of 30 patients treated with intraarterial fotemustine through an implanted hepatic artery catheter. They noted an objective response rate of 40% (four complete responses [CRs] and eight partial responses [PRs]), a median duration of response of 11 months, and a median overall survival of 14 months. A subsequent multicenter study involving 101 patients treated with hepatic arterial infusion of fotemustine reported an objective response rate of 36% (15 CRs and 21 PRs); median overall survival for the whole group was 15 months, with a 1-year survival rate of 67%. Siegel et al. used hepatic arterial infusion of fotemustine to treat 30 patients with metastatic cutaneous or uveal melanomas and found no significant difference in response rates between cutaneous and uveal melanoma patients. In the study by Becker et al., patients treated with hepatic arterial infusion of fotemustine had a higher response rate than those treated with systemic chemotherapy (22% and 8%, respectively); however, the median survival durations were similar in the two treatment groups (12.1 and 11.5 months, respectively).
| Author, year | Tumor type | Number of patients | Chemotherapy used | Response | Overall survival |
|---|---|---|---|---|---|
| Cantore et al., 1994 | Melanoma (uveal) | 8 | Carboplatin | PR, 3; PD, 5 | Median, 15 months |
| Leyvraz et al., 1997 | Melanoma (uveal) | 30 | Fotemustine | CR, 4; PR, 8; SD, 15 | Median, 14 months |
| Becker et al., 2002 | Melanoma (uveal) | 23 | Fotemustine; subcutaneous interleukin 2 and interferon alpha 2 | 22% | Median, 12 months |
| Peters et al., 2006 | Melanoma (uveal) | 101 | Fotemustine | CR, 15; PR, 21; SD, 48 | Median, 15 months; 1-year, 67% |
| Siegel et al., 2007 | Melanoma (uveal or cutaneous) | 36 | Fotemustine | PR, 9; SD, 10, PD, 11 | Median,14 months |
| Fraschini et al., 1987 | Breast | 26 | Vinblastine | PR, 9 | Median, 11 months |
| Fraschini et al., 1988 | Breast | 34 | Cisplatin, vinblastine | PR, 11 | Median, 11 months |
| Arai et al., 1994 | Breast | 56 | Doxorubicin, 5-fluorouracil, mitomycin | 81% | Median, 12.5 months |
| Ikeda et al., 1999 | Breast | 26 | Doxorubicin, 5-fluorouracil | 63% | Median, 25.3 months |
| Camacho et al., 2007 | Breast | 10 | Paclitaxel | PR, 3; SD, 5, PD, 2 | N/A |
| Tsimberidou et al., 2010 | Breast | 8 | Cisplatinum and systemic liposomal doxorubicin | PR, 3; SD, 5 | Median, 8.5 months |
Hepatic arterial infusion chemotherapy is also an attractive alternative to systemic approaches for breast cancer patients who have unresectable liver metastasis and no or minimal extrahepatic tumor burden (see Table 16-1 ). In a pilot study involving 26 patients with breast carcinoma metastatic to the liver and refractory to prior chemotherapy treated with sequential hepatic arterial infusion of vinblastine, Fraschini et al. reported a partial response in 9 and a minor response in 4 of the 25 patients evaluable for response. In another study, 34 patients with metastatic breast carcinoma were treated with hepatic arterial infusion of cisplatin and vinblastine; partial response was observed in 33% of evaluable patients. Fraschini et al. reported a partial response in 11 (33%) and a minor response in 8 (24%) of the 33 patients evaluable for response. Median time to progression for responding patients was 31 weeks (range, 6+ to 74 weeks), and median survival was 11 months (range, 5–19 months). However, many patients developed adverse events, and seven failures were related to treatment intolerance or major toxicity. Arai et al. reported a response rate of 81% and a median survival of 12.5 months in a study involving 56 patients with unresectable liver metastases from breast cancer treated by repeated hepatic arterial infusion chemotherapy. In a phase 1/2 study of continuous intraarterial infusion chemotherapy using doxorubicin and 5-fluorouracil in 26 patients with metastatic breast cancer, the median duration of response was 5.8 months (range, 1–23+) and median survival was 25.3 months (range, 6.2–54.7+). In a more recent study, Camacho et al. used repeated hepatic arterial infusion of paclitaxel to treat 10 patients with breast carcinoma and dominant liver metastases. Fifty-six courses of paclitaxel were delivered. No procedure-related complications were observed. Three patients (30%) attained partial responses that lasted for 6, 7, and 48 months; 4 other patients had stable disease for 5–9 months. One patient underwent liver resection after receiving hepatic arterial infusions of paclitaxel and remained disease free for 48 months. Hepatic arterial chemoinfusion can also be combined with systemic treatment in patients with both hepatic and extrahepatic metastatic disease. A recently reported phase 1 clinical trial of hepatic arterial infusion of cisplatin in combination with intravenous liposomal doxorubicin in patients with advanced cancer and dominant liver involvement included 11 patients with breast cancer. The regimen was well tolerated, and partial response was observed in 3 of the 11 breast cancer patients.
Hepatic artery embolization and chemoembolization
Liver metastases derive 90% of their blood supply from the hepatic artery. This feature, combined with the facts that the liver has a dual-blood supply and a healthy liver receives 80% of its blood supply from the portal vein, provides a good rationale for using either hepatic arterial embolization or chemoembolization to treat these metastases. Chemoembolization involves infusion of a mixture of chemotherapeutic agents with or without iodized oil into the hepatic arteries supplying the tumor, followed by embolization with particles. The purpose of embolization is to prevent washout of the drug from the tumor and to induce ischemic necrosis. The resulting tumor drug concentrations are reported to be 10–25 times higher than those achieved by infusion alone and the dwell time of the agents is markedly prolonged.
A preliminary arteriography of the celiac axis and superior mesenteric artery is performed to evaluate the arterial supply to the liver and to ensure the patency of the portal vein. After the initial diagnostic arteriogram, the selected hepatic artery is catheterized with a 5-F catheter or a coaxially advanced microcatheter. Currently, there is no consensus on the best chemoembolization protocol in terms of patient selection criteria, choice of the embolic agents used, choice and dose of the anticancer agents, the embolization end points, and time interval between treatments.
In patients with bilobar disease, embolization of the whole liver in a single treatment session is generally avoided because of the risk of prolonged postembolization syndrome or liver failure. Only one lobe of the liver is subjected to embolization during each treatment session; generally, the hepatic lobe with the greatest tumor burden is treated first. To avoid liver failure in patients with extensive (>75% liver parenchyma replaced with tumor) liver involvement, only a small portion of the liver lobe is subjected to embolization during each session. Additional hepatic artery chemoembolization procedures are performed with the goal of treating the entire liver.
The timing of subsequent chemoembolization procedures is generally determined by the patient’s physical condition, tumor status, response to initial embolization, and ability to tolerate the procedure. Extensive liver involvement and mildly abnormal liver functions (serum albumin level <3 g/dL, aspartate transaminase level >100 U/L, lactate dehydrogenase level >425 IU/mL, and serum bilirubin level >2 mg/dL) indicate an increased risk of liver failure after chemoembolization. If several of these factors are combined, the risk of liver failure may be unacceptable.
Chemoembolization has become an accepted locoregional therapy for uveal melanoma metastatic to the liver ( Table 16-2 ). Studies have shown some survival benefit in patients with metastatic uveal melanoma who had responses to chemoembolization. The use of chemoembolization for liver metastases of uveal melanoma was first reported by Carrasco et al, who reported tumor regressions in two patients after one or two chemoembolization sessions using polyvinyl sponge material and cisplatin. A study at the University of Texas M.D. Anderson Cancer Center involving 30 patients with uveal melanoma metastatic to the liver treated with chemoembolization using cisplatin and polyvinyl alcohol particles reported a response rate of 46% and a median overall survival of 11 months. A more recent study by the same group involving a larger number of patients (n = 64) reported a response rate of 36% for first-line and 25% for subsequent chemoembolization treatments, and a median survival of 14.5 months in responding patients. In fact, more recent studies have shown even lower radiologic response rates, ranging from 0% to 25%.
| Author, year | Tumor type | Number of patients | Chemoembolization regimen | Response | Liver- PFS/TTP | Overall survival |
|---|---|---|---|---|---|---|
| Mavligit et al., 1988 | Melanoma (uveal) | 30 | Cisplatin; PVA | CR, 1; PR, 13; SD, 7; PD, 9 | NR | Median, 11 months |
| Bedikian et al., 1995 | Melanoma (uveal) | 44 | Cisplatin ± other drugs; PVA | Response rate, 36% | NR | 14.5 |
| Huppert et al., 2009 | Melanoma (uveal) | 14 | Cisplatin/carboplatin; PVA | PR, 8; SD, 4; PD, 2 | Median TTP, 8.5 months | Median, 11.5 months |
| Patel et al., 2010 | Melanoma (uveal) | 30 | BCNU; Gelfoam | 24 evaluable patients – CR, 1; PR, 4; SD, 13; PD, 6 | Median TTP, 6.7 months (patients with CR,PR, SD) | Median, 5.2 months |
| Sharma et al., 2008 | Melanoma (uveal and cutaneous) | 20 | Cisplatin, doxorubicin, mitomycin; ethiodized oil, PVA or Gelfoam | PR, 0; SD, 13; PD, 7 | Median PFS, 185 days | Median, 271 days |
| Vogl et al., 2007 | Melanoma (uveal) | 12 | Mitomycin C; lipiodol, and resorbable microspheres | PR, 3; SD, 5; PD, 4 | NR | Mean, 19.5 months |
| Gupta et al., 2010 | Melanoma (uveal) | 125 | Cisplatin ± other drugs; PVA or Gelfoam | 105 evaluable patients—PR, 12; MR, 17; SD, 68; PD, 8 | Median PFS, 3.8 months | Median, 6.7 months |
| Fiorentini et al., 2009 | Melanoma (uveal) | 10 | Drug-eluting beads (irinotecan) | PR, 10 | NR | Median, 6.5 months |
| Ahrar et al., 2011 | Melanoma (cutaneous) | 42 | Cisplatin ± other drugs; PVA or Gelfoam | 36 evaluable patients; PR, 5; MR, 9; SD, 17; PD, 5 | Median TTP, 6 months | Median, 7.7 months |
| Brown et al., 2011 | Melanoma (uveal) | 6 | Drug-eluting beads (Doxorubicin) | Response rate, 100% | NR | Median, 12.3 months |
| Maluccio et al., 2006 | Sarcomas (16 GIST) | 24 | PVA or trisacryl microspheres | Response rate, 60% | NR | Median, 24 months |
| Mavligit et al., 1995 | GIST | 14 | Cisplatin, vinblastine; PVA | Response rate, 70% | NR | Median, 12 months |
| Rajan et al., 2001 | Sarcomas (11 GIST) | 16 | Cisplatin, doxorubicin, mitomycin C; ethiodized oil and PVA | Response rate, 13% | NR | Median, 13 months |
| Kobayashi et al., 2006 | GIST | 85 | Cisplatin; PVA or Gelfoam | PR, 13%; SD, 69% | Median PFS, 8.2 months | Median, 17.2 months |
| Giroux et al., 2004 | Breast | 8 | Cisplatin, doxorubicin, mitomycin; ethiodized oil with PVA | Response, 5; SD, 1; PD, 2 | NR | Mean, 6 months |
| Li et al., 2005 | Breast | 28 | 5-FUDR, cisplatin, doxorubicin; ethiodized oil or Gelfoam | PR, 10; SD, 13; PD, 5 | NR | Median, 28 months |
| Vogl et al., 2010 | Breast | 208 | Mitomycin C only , Mitomycin C with gemcitabine , gemcitabine only; ethiodized oil and starch microspheres | PR, 27; SD, 105; PD, 76 | NR | Median, 18.5 months; Mean, 30.7 months |
| Martin et al., 2011 | Breast | 40 | Drug-eluting beads (doxorubicin) | Response rate, 50% | Median PFS, 26 months | Median, 47 months |
In another report, Huppert et al. performed 34 chemoembolization procedures with cisplatin or carboplatin in 14 patients with uveal melanoma metastatic to the liver. Partial response was seen in eight patients (57%) and stable disease in four patients (29%). Median time to progression was 8.5 months (range, 5–35 months). Median survival after first chemoembolization was 14.5 months in responders compared with 10 months in nonresponders. Patients with less than 25% liver involvement had a higher incidence of PR (86% vs. 29%) and a longer median survival (17 vs. 11 months; P = 0.02) compared with those patients with more than 25% liver involvement.
In a phase 2 trial involving 29 patients, Patel et al. used 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) in combination with ethiodized oil (lipiodol) followed by embolization with gelatin sponge particles. Among 24 evaluable patients, one patient achieved a CR, four patients achieved a PR, and 13 patients had stable disease (SD). Median overall survival of the entire intent-to-treat group of patients was 5.2 months (range, 0.1–27.6 months); median overall survival was 21.9 months (range, 7.4–27.6 months) for patients with CR or PR, 8.7 months (range, 2.9–14.4 months) for those with SD, and 3.3 months (range, 1.6–5.6) for those with progressive disease. Patients who had less than 20% of liver replaced with tumor had a better survival than those with 20%–50% tumor in the liver (median, 19.0 vs. 5.6 months, respectively).
Vogl et al. used the super-selective chemoembolization technique in 12 uveal melanoma patients with solitary ( n = 6) or oligonodular hepatic metastases ( n = 6). The embolization consisted of infusion of a maximum of 10 mg/m 2 mitomycin C and a maximum of 15 mL of iodized oil, followed by embolization with 200–450 mg of resorbable microspheres. Three patients achieved a PR and five had SD without significant side effects. The median survival rates of patients with SD and PR after chemoembolization were significantly longer at 15.7 months and 21.0 months, respectively, whereas patients with progressive disease survived only 8.3 months ( P = 0.01). Vogl et al. also found that retention of ethiodized oil in the tumors after chemoembolization is a predictive marker for good radiologic response. In their series, the patients who showed significant accumulation of ethiodized oil in their hepatic metastases achieved CR or PR, whereas patients with a lower degree of ethiodized oil enhancement had progressive disease.
Sharma et al. treated 21 patients with chemoembolization using a mixture of 50 mg of cisplatin, 50 mg of doxorubicin, and 10 mg of mitomycin C emulsified with ethiodized oil followed by embolization with either absorbable gelatin sponge or 300–500 µm polyvinyl alcohol (PVA) particles. Of 20 evaluable patients, there were 13 with SD but no patients with CR or PR. The median overall survival of patients was 271 days. In their study, the angiographic pattern of liver metastasis was strongly predictive of survival after chemoembolization. Patients with lesions that had a nodular angiographic appearance had longer overall survival than patients with lesions that had an infiltrative appearance (median overall survival, 750 vs. 109 days; P = 0.0002).
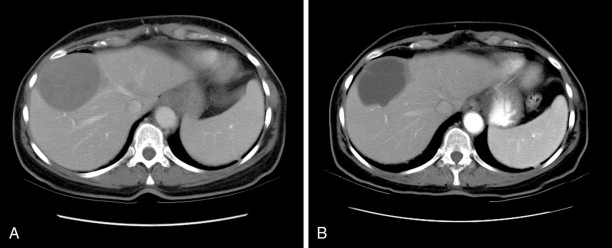
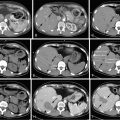
In a recently published study, we reported our institution’s experience with chemoembolization in 125 patients with uveal melanoma metastatic to the liver. We found an overall response rate of 28.6%, which was not significantly different from that reported in most previous studies. Of 105 patients in whom radiologic responses could be evaluated, 12 (11%) had partial responses, 17 (16%) had minor responses, 68 (65%) had stable disease, and 8 (8%) had progressive disease ( Figure 16-1 ). The median overall survival and progression-free survival were 6.7 and 3.8 months, respectively. Multivariate analysis showed that >75% liver involvement and high lactate dehydrogenase levels were associated with short overall survival. Patients who had radiologic responses to chemoembolization had a longer median overall survival duration than did patients who did not (15.8 vs. 6.1 months; P = 0.0005). Patients with >75% liver involvement had a median overall survival duration of only 2.4 months.