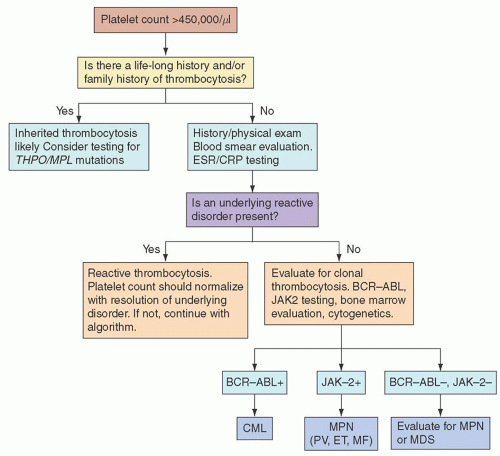
Inherited thrombocytosis is suspected in patients with a lifelong history of asymptomatic thrombocytosis, especially if other family members are also affected. The genetic mutations in inherited thrombocytosis are heterogeneous and may or may not involve mutations in the thrombopoietin gene (
THPO) or its receptor (
MPL).
3 At least four different mutations in
THPO associated with increased TPO production and inherited thrombocytosis have been described in European and Japanese kindreds.
13 Thrombocytosis was inherited in an autosomal dominant manner in all four families. The TPO molecule in these patients is normal, and increased translation appears to explain elevated levels of TPO in these patients.
13 At least three different
MPL mutations have been linked to inherited thrombocytosis; affected kindreds are of European, African-American, Arabic, and Japanese ancestry.
13 Approximately 7% of African-Americans are heterozygous for the MPL-Baltimore mutation.
14 Thrombocytosis in patients with
MPL mutations appears to result from either constitutive receptor activation or reduced receptor binding affinity for TPO.
13Clinical findings in patients with inherited thrombocytosis can be classified by whether the patients have
THPO or
MPL mutations. In general, those with
THPO mutations have elevated TPO levels and mild splenomegaly, but a low incidence of vascular complications and low risk of evolution to acute leukemia and myelofibrosis,
13 although development of bone marrow disease was described in two family members with inherited thrombocytosis due to
THPO mutations.
15 Some patients with
MPL mutations and inherited thrombocytosis have been reported to experience thrombosis, splenomegaly, and myelofibrosis, but these complications have not been reported in African-American patients with MPL-Baltimore.
13