INTRODUCTION
SUMMARY
Therapeutic apheresis refers to several blood processing methods that are used in the treatment of diverse clinical conditions. In most cases, the disorders so treated are characterized by a specific qualitative or quantitative abnormality of the blood. In hematologic practice, apheresis procedures are used to mitigate hyperviscosity in monoclonal protein disorders or remove pathologic autoantibodies and replete important plasma proteins. Red cell apheresis is used to improve the ratio of normal to abnormal red cells in hemoglobinopathies and protozoan disease, and to remove excess red cells, red cell-associated toxins, or excess iron from the body. Leukocyte apheresis is used to reduce the circulating blast count in acute leukemias with hyperleukocytosis and platelet apheresis is used to lower a very elevated platelet count in patients with myeloproliferative neoplasms. Photopheresis is used in the treatment of cutaneous T-cell lymphoma and chronic graft-versus-host disease. Adverse effects of apheresis with current technologies are typically mild and, usually, do not prevent completion of therapy.
Abbreviations and Acronyms:
ADAMTS-13, von Willebrand factor cleaving metalloprotease; ARDS, acute respiratory distress syndrome; ASFA, American Society for Apheresis; CDC, United States Centers for Disease Control and Prevention; ECP, extracorporeal photochemotherapy; FCR, fraction of cells remaining; GRADE, Grading of Recommendations Assessment, Development and Evaluation; HUS, hemolytic uremic syndrome; MHC, major histocompatibility complex; 8-MOP, 8-methoxypsoralen; PUVA, psoralen plus ultraviolet A; TPE, therapeutic plasma exchange; TTP, thrombotic thrombocytopenic purpura; UVA, ultraviolet A light; VR, volume of red blood cells to be removed.
DEFINITION AND HISTORY
The term apheresis emerged in 1914 when John J. Abel, of the Johns Hopkins University Pharmacological Laboratory, demonstrated how large quantities of plasma could be removed from dogs by a process he called “plasmapheresis” (from the Greek apairesos or Roman aphaeresis, meaning take away by force).1 The treatment, by manual plasmapheresis, of hyperviscosity syndrome in patients with Waldenström macroglobulinemia during the 1950s supported the concept that a disease state causally related to a substance in the plasma can be effectively treated by removal of plasma.2,3 Today, a number of automated apheresis, or blood processing, techniques are used in the treatment of a growing list of clinical disorders. The American Society for Apheresis (ASFA) categorizes the indications for apheresis (Table 28–1) according to where apheresis fits into the management strategy for the condition under consideration.4 In addition, ASFA evaluates the individual indications (clinical entities) and issues recommendations regarding the use of apheresis in their treatment according to the GRADE (Grading of Recommendations Assessment, Development and Evaluation) system.4,5 Table 28–2 lists the covered indications most relevant to the practice of hematology. This chapter considers the various apheresis approaches to hematologic disorders.
| Category | Definition of Category |
|---|---|
| I | Apheresis is an accepted first-line therapy for these disorders. |
| II | Apheresis is an accepted second-line therapy for these disorders. |
| III | Individualize decision making. The optimal role of apheresis has not been conclusively determined in these disorders. |
| IV | Published evidence indicates that apheresis is ineffective or harmful in these disorders. Seek institutional review board approval if apheresis is planned. |
| Clinical Disorder | Apheresis Procedure* | Indication Category† | Grade‡ of Recommendation |
|---|---|---|---|
| Amyloidosis, systemic | TPE | IV | 2C |
| Aplastic anemia or pure red cell aplasia | TPE | III | 2C |
| Autoimmune hemolytic anemia (warm) | TPE | III | 2C |
Babesiosis, severe Babesiosis, high-risk population | RBC exchange | I II | 1C 2C |
| Catastrophic antiphospholipid syndrome | TPE | II | 2C |
Coagulation factor inhibitor Alloantibody Alloantibody Autoantibody Autoantibody | TPE IA TPE IA | IV III III III | 2C 2B 2C 1C |
| Cold agglutinin disease | TPE | II | 2C |
| Cryoglobulinemia | TPE IA | I II | 2A 2B |
| Cutaneous T-cell lymphoma; mycosis fungoides; Sézary syndrome (erythrodermic) | ECP | I | 1B |
Erythrocytosis Primary (polycythemia vera) Secondary | Erythrocytapheresis | I III | 1B 1C |
Graft-versus-host disease, skin Chronic Acute Graft-versus-host disease, nonskin (acute/chronic) | ECP | II II III | 1B 1C 2C |
Hemopoietic stem cell transplant, ABO incompatible Major incompatibility, marrow Major incompatibility, apheresis Minor incompatibility, apheresis | TPE TPE RBC exchange | II II III | 1B 2B 2C |
Hemolytic uremic syndrome Atypical Complement gene mutations Factor H antibodies MCP mutations Infection-associated Shiga toxin associated Str. pneumonia-associated | TPE | II I IV IV III | 2C 2C 1C 1C 2C |
Heparin-induced thrombocytopenia Precardiopulmonary bypass Thrombosis | TPE | III III | 2C 2C |
| Hereditary hemochromatosis | Erythrocytapheresis | I | 1B |
Hyperleukocytosis (acute leukemia) Leukostasis Prophylaxis | Leukocytapheresis | I III | 1B 2C |
Hyperviscosity in monoclonal gammopathies Symptomatic Prophylaxis for rituximab | TPE | I I | 1B 1C |
| Immune thrombocytopenia (refractory) | TPE IA | IV III | 2C 2C |
| Myeloma cast nephropathy | TPE | II | 2B |
| Posttransfusion purpura | TPE | III | 2C |
Sickle cell disease Acute stroke Acute chest syndrome Multiorgan failure Preoperative management Priapism Sequestration syndrome (spleen, liver, cholestasis) Stroke prophylaxis Vasoocclusive pain | RBC exchange | I II III III III III II III | 1C 1C 2C 2A 2C 2C 1C 2C |
Thrombocytosis Symptomatic Prophylaxis (or secondary) | Thrombocytapheresis | II III | 2C 2C |
Thrombotic microangiopathy Hemopoietic stem cell transplant-related Drug associated Ticlopidine Clopidogrel Calcineurin inhibitors Gemcitabine Quinine Thrombotic thrombocytopenic purpura | TPE | III I III III IV IV I | 2C 1B 2B 2C 2C 2C 1A |
THERAPEUTIC PLASMA EXCHANGE
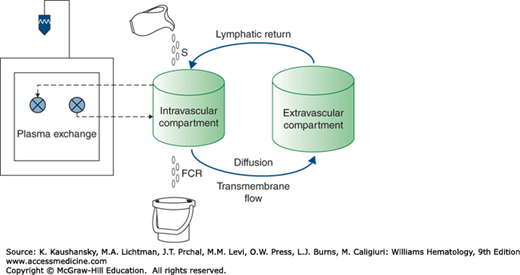
Therapeutic plasma exchange (TPE) is the most commonly performed therapeutic apheresis procedure in the United States and Medicare claims for TPE have doubled over the past 10 years.6 The term plasmapheresis refers to the removal of plasma from the circulation by manual or automated methods; the term plasma exchange refers to a therapeutic procedure in which plasmapheresis is combined with replacement of the removed plasma by a substitute colloid fluid, most commonly a mixture of 5 percent human serum albumin and 0.9 percent saline.4,7 The efficient extraction of the plasma from whole blood, and its replacement with a substitute colloid fluid, is predicated on the hypothesis that the plasma substance targeted for removal (usually an immunoglobulin or other large molecule) does not escape to the extracellular space during the time it takes to perform the plasma exchange procedure.7 This hypothesis underlies the “one compartment model” that forms the basis for our understanding of the physiology of plasma exchange and the depletion of plasma constituents (Fig. 28–1).
Figure 28–1.
Illustration of the “one compartment model” depicting the interaction between intra- and extravascular compartments as relates to plasma exchange. A soluble substance enters the intravascular compartment at synthetic rate (S) and is removed at its fraction catabolic rate (FCR). Movement of the substance of interest between the intravascular and (larger) extravascular compartment takes place by the mechanisms shown. The plasma exchange procedure only removes soluble substances directly from the intravascular compartment. S, FCR, and the intracompartmental movement of soluble substances are in a balanced steady state and proceed much more slowly than the rate of plasma extraction by the apheresis instrument. Thus, for the purpose of the plasma exchange procedure, the intravascular compartment can be considered to be an isolated system which can be depleted of soluble substances by exchanging the plasma for a replacement fluid.
If the “one compartment model” applies, then the intravascular mass of a substance to be removed is a function of its concentration in the plasma (y) and the patient’s plasma volume. Its clearance from the plasma by plasmapheresis depends on the fraction of that plasma volume that is removed per unit of time during the exchange. The fraction of the targeted substance remaining in the intravascular space at any time (t) during the exchange procedure can be expressed as
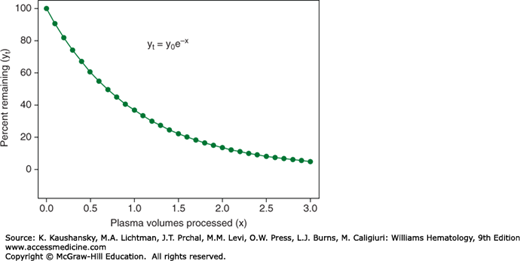
where yt is the concentration of targeted substance remaining in the intravascular space at time t, y0 is the concentration of targeted substance at the start of the procedure (time zero), e is the natural logarithm base (a constant valued at approximately 2.718282) and x represents multiples of the patient’s plasma volume processed by time t. Figure 28–2 is a plot based on this formula that generates an asymptotic curve that predicts the disappearance of the intravascular target substance as a function of plasma volumes processed (i.e., multiples of the patient’s plasma volume). The curve is initially steep, and the processing of one plasma volume removes approximately two-thirds of the substance of interest. Processing of another half plasma volume lowers the remaining substance of interest to approximately 22 percent of its initial level in the blood. But the curve then rapidly flattens, with much less removal of the substance of interest per volume of plasma processed. Thus the “sweet spot” for plasma exchange procedures is the processing of between 1.0 and 1.5 plasma volumes.7 The “one compartment model” is particularly relevant to the removal of large molecules, such as immunoglobulins, that have a predictable rate of synthesis and volume of distribution within the intravascular space. Smaller molecules, that are synthesized and/or metabolized in a less-predictable fashion, or are distributed within total body water, are less predictably removed according to the model.7
Figure 28–2.
Depletion of soluble substances from the intravascular compartment by plasma exchange according to the One Compartment Model. With each incremental volume of plasma removed (and replaced) from the intravascular compartment, a fixed proportion of the remaining intravascular content of a soluble substance of interest is removed. The processing of 1.0 plasma volume depletes the intravascular substance of interest by approximately two thirds. Processing of the next half plasma volume furthers the depletion to almost 80 percent.
As shown in Table 28–2, most indications for plasma exchange in hematologic disorders are weakly recommended based on low-quality evidence. These indications are reviewed in detail elsewhere.8,9 The discussion herein is restricted to situations where plasma exchange has an important impact on hematologic practice.
Hyperviscosity syndrome in the monoclonal gammopathies (Chaps. 106, 107, and 109) is caused by impaired blood flow from an increase in viscosity of blood as a result of immunoglobulin–red cell interactions.10,11,12 It is most common in Waldenström macroglobulinemia because of the highly red-cell-aggregating properties of immunoglobulin (Ig) M and, less often, in IgG or IgA myeloma.13,14,15 Symptoms typically emerge when serum viscosity rises above 4.0 relative viscosity units (normal being 1.4 to 1.8).12,16 Although the relevant variable is blood viscosity, the measurement of serum viscosity is relatively simple and can be used as an indicator of risk of symptomatic hyperviscosity. Specific symptoms may include headache, dizziness, vertigo, nystagmus, hearing loss, visual impairment, somnolence or coma and seizures. In addition, congestive heart failure, impaired respiration, coagulation abnormalities, anemia or peripheral neuropathy may be seen.13 Plasma exchange rapidly relieves symptoms of hyperviscosity by lowering the plasma content of the responsible paraprotein.2,3,16,17
The relationship between monoclonal protein level and serum viscosity is nonlinear, therefore a relatively small (20 percent) decrease in plasma protein can affect a major change in viscosity.10,17 This is noteworthy in that whereas the removal of plasma proteins during plasma exchange from patients without monoclonal proteinemia closely follows the predictions of the “one compartment model” (i.e., yt = y0e−0.94x), removal of plasma proteins from patients with monoclonal proteinemia deviates from the model by as much as 50 percent (i.e., yt = y0e−0.5x).18 The difference likely relates to the underestimated expansion in plasma volume that occurs in monoclonal proteinemias.7 But despite this compromised removal of plasma protein, the nonlinear relationship between serum monoclonal protein level and serum viscosity results in plasma exchange remaining highly effective in alleviating clinical manifestations of hyperviscosity.19
Cryoglobulins are immunoglobulins or complexes of immunoglobulins that reversibly precipitate when exposed to temperatures below 37°C. They can be isolated monoclonal immunoglobulins (type I), a mixture of immunoglobulins including a monoclonal component that exhibits antibody activity toward polyclonal IgG (type II) or mixed polyclonal immunoglobulins of one or more classes (type III). Whereas type I cryoglobulinemia is largely associated with lymphoproliferative disorders, and type III with chronic infections or autoimmune disorders, type II is almost always associated with infection with hepatitis C. Clinical sequelae may include purpura, arthralgia and arthritis, Raynaud phenomenon, peripheral sensory or sensorimotor neuropathy, nephropathy, skin ulcers, or widespread vasculitis.20,21 The removal of cryoglobulins by plasma exchange can be effective in treating the renal, vasomotor, vasculitic, and neurologic manifestations of cryoglobulinemia,22,23,24 but medical treatment of the underlying disorder with which the cryoglobulinemia is associated is also necessary for a persistent good result.
Myeloma cast nephropathy (“myeloma kidney”) results from combination of free light chains with Tamm-Horsfall mucoprotein in the distal nephron and the resultant precipitation of obstructing casts.25 A number of early case reports and small clinical trials suggested that combining plasma exchange with chemotherapy improved the likelihood of recovering renal function in patients with myeloma and renal failure.26,27,28,29,30 The largest trial to date (104 participants) was unable to demonstrate a difference in primary outcome based on the composite measure of death, dialysis dependence, or glomerular filtration rate below 30 mL/min/1.73 m2 at 6 months.31 The effectiveness and rapidity of modern chemotherapy may have subsumed a salutary effect of plasma exchange.32 Plasma exchange is not currently considered to be part of first-line treatment for myeloma with cast nephropathy, but may be a reasonable option when renal function does not rapidly improve with chemotherapy.4
Idiopathic thrombotic thrombocytopenic purpura (TTP) is a medical emergency that presents with microangiopathic hemolytic anemia and thrombocytopenia (Chap. 132). It is typically characterized by central nervous system, cardiac, renal, or other organ impairment as a result of microvascular obstruction by aggregates of platelets and von Willebrand factor.33
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree