INTRODUCTION
SUMMARY
Microscopic examination of the marrow is a mainstay of hematologic diagnosis. Even with the advent of specialized biochemical and molecular assays that capitalize on advances in our understanding of the cell biology of hematopoiesis, the primary diagnosis of hematologic malignancies and many nonneoplastic hematologic disorders relies upon examination of the cells in the marrow. An aspirate and biopsy of the marrow can be obtained with minimal risk and only minor discomfort and are quickly and easily processed for examination. The marrow should be examined when the clinical history, blood cell counts, blood film, or laboratory test results suggest the possibility of a primary or secondary hematologic disorder for which morphologic analysis or special studies of the marrow would aid in the diagnosis. Leukopenia or thrombocytopenia may require a marrow examination for diagnosis. Nonhemolytic anemia that is not readily diagnosed by blood cell examination and supporting laboratory tests often requires a marrow examination. Abnormal cells in the blood, such as nucleated red cells, white cell precursors, abnormal lymphocytes not explained by concurrent infection, and blast cells, usually require a marrow examination. In addition to determining the cellularity and morphology of precursor cells, or infiltration by nonhematopoietic cells, the study provides marrow cells for immunophenotyping, cytogenetic, molecular and genomic studies, culture of infectious organisms, and storage of marrow cells for further analysis.
HISTORY OF THE MARROW EXAMINATION
The first recorded examinations of marrow in living patients occurred in the first decades of the 20th century, first using the tibia as the source of marrow and then surgical bone biopsies. Neither technique led to routine examination of the marrow, because in the former case the tibia was usually hypocellular in adults, and in the latter case, because of the invasiveness of an open procedure and the discomfort and risk of infection and bleeding. In 1923, Arinkin devised the marrow aspiration technique,1 which was the prototype for our current aspiration procedure. Thirty years passed before the suggestion that the pelvis might be preferable to the sternum gained hold, and another 10 years passed before a practical marrow biopsy instrument was put to use. Regular use of the posterior iliac crest for aspiration and biopsy and regular use of biopsy to complement aspiration did not occur until the 1970s, when staging of lymphoma made biopsy a frequent procedure and new simpler biopsy instruments became readily available.
Acronyms and Abbreviations:
CD, cluster of differentiation; CLL, chronic lymphocytic leukemia; CML, chronic myelogenous leukemia; DMSO, dimethylsulfoxide; EDTA, ethylenediaminetetraacetic acid; FISH, fluorescence in situ hybridization; GPI, glycosylphosphatidylinositol; MDS, myelodysplastic syndrome; M:E, myeloid-to-erythroid cell ratio; MRD, minimal residual disease; PCR, polymerase chain reaction.
INDICATIONS FOR MARROW ASPIRATE OR BIOPSY
The International Council for Standardization in Hematology has published guidelines for marrow aspirate and biopsy to promote consistency in performance and reporting.2 Although marrow aspiration and biopsy techniques are safe, they should be performed with a clear idea as to how the results will help distinguish the differential diagnoses under consideration or provide followup of treatment.3,4,5 In many hematologic disorders, such as most cases of iron-deficiency anemia, thalassemia, and acquired and inherited hemolytic anemia, examination of the blood and specialized laboratory tests usually suffice to make the diagnosis without the need for a marrow examination.
When examination of the marrow is indicated, the decision as to whether an aspirate or an aspirate plus biopsy is desired should be made. Aspiration is always attempted because of the superior morphology offered by examination of the aspirate smear. However, a marrow biopsy is superior to the aspirate in quantifying marrow cellularity and diagnosing infiltrative diseases of the marrow and should be performed when these conditions are part of the differential diagnosis.6,7 Marrow biopsy is useful for diagnosing and following the course of disorders that are commonly associated with reticulin fibrosis, such as megakaryoblastic leukemia, hairy cell leukemia, and the chronic myeloproliferative neoplasms.8 In myelodysplastic syndromes, marrow biopsy is useful for evaluating abnormal localization of immature precursor cells and abnormal megakaryocytes. Marrow necrosis and gelatinous transformation are more readily detected in marrow sections than in aspirate films. Marrow aspirate alone may be appropriate in some clinical settings where the diagnostic question is very targeted, such as diagnosis of childhood immune thrombocytopenia purpura or surveillance followup of leukemia patients in apparent remission.
Depending on the diagnostic question, availability of material, and expected frequency of the abnormal cells, an appropriate selection of specialized diagnostic methods may be needed to support the clinical diagnosis. Morphology of marrow cells is still the gold standard for diagnosis of hematologic malignancy and allows construction of a good differential diagnosis for nonmalignant disorders. Immunocytochemistry provides excellent phenotype–morphology correlation on an individual cell basis, but is limited to epitopes that resist destruction by fixation, decalcification, and paraffin embedding. Flow cytometry allows study of almost any surface or intracellular protein, with the added ability to detect important quantitative changes in cellular proteins and simultaneous determination of multiple proteins within the same cell. However, flow cytometry requires that cells be viable and dissociated from tissue. Gene expression arrays allow analysis of complex patterns of RNA expression by sophisticated mathematical algorithms to discover diagnostic patterns based on gene expression. These studies may point the way to a smaller more practical set of proteins that can be studied by immunocytochemistry or immunofluorescence. Molecular assays target oncogenic DNA sequence alterations from the chromosome to the nucleotide, and include classic metaphase cytogenetics, fluorescence in situ hybridization (FISH), reverse transcriptase polymerase chain reaction (PCR), and targeted or whole-genome sequencing.
MARROW ASPIRATION TECHNIQUE
At birth, all bones contain hematopoietic marrow. Fat cells begin to replace hemopoietic marrow in the extremities in the fifth to seventh year. By adulthood, the hemopoietic marrow is limited to the axial skeleton and the proximal portions of the extremities (Chaps. 5 and 9). Fatty marrow appears yellow, whereas hematopoietic marrow is red. Red marrow contains fat, however, and fat droplets are visible grossly in aspirated marrow specimens. Histologically, yellow marrow consists almost entirely of fat cells and supporting connective tissue. Red marrow contains an abundance of hematopoietic cells, fat cells, and connective tissue. The marrow fills the spaces between the trabeculae of bone in the marrow cavity. Marrow is soft and friable and can be readily aspirated or biopsied with a needle.
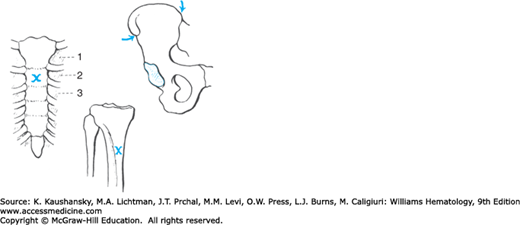
The posterior iliac crest (Fig. 3–1) is the preferred site for marrow aspiration and biopsy. In adults, the anterior iliac crest and rarely the sternum have been used (Fig. 3–2). The sternum should be used for aspiration only. The anterior iliac crest is less preferred than the posterior crest in adults because of its thick cortical bone. The anteromedial surface of the tibia is an option for infants younger than 1 year old (particularly newborns), but the posterior iliac crest is still the preferred site. Serious adverse outcomes after marrow aspiration or biopsy are rare, occurring in less than 0.05 percent. One direct fatality and three episodes of prolonged but not permanent disability were reported in nearly 55,000 marrow biopsies.9 Morbidity most frequently involved hemorrhage, which was associated more with platelet function impairment than thrombocytopenia or coagulation factor defects.9 Infection and reactions to anesthetic agents are other infrequent complications. Penetration of the bone with damage to the underlying structures is possible with all marrow aspirations, but the hazard is greatest in sternal aspirations because the sternum at the second interspace is only approximately 1 cm thick in adults, and the distance from posterior sternal cortex to the ascending aorta varies greatly and may be as little as 4 to 5 mm,10 giving rise to the rare but dramatic consequence of aortal wall tear. To prevent this, a guard should be in place on the needle if a sternal aspirate needs to be done.
Figure 3–1.
A. Jamshidi biopsy instrument. B. Site of marrow biopsy.96 (A, reproduced with permission from Jamshidi K, Swaim WR: Bone marrow biopsy with unaltered architecture: a new biopsy device, J Lab Clin Med 1971 Feb;77(2):335–342.
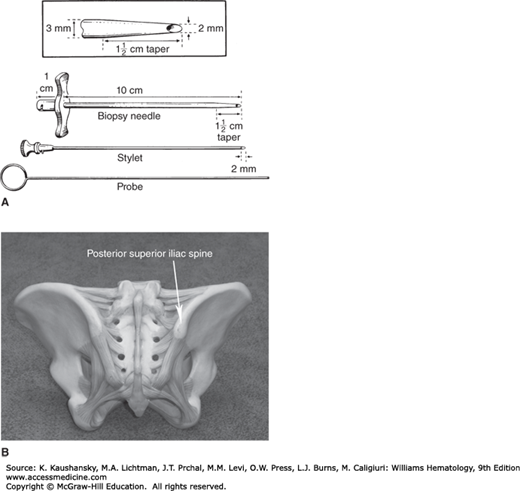
For either a marrow biopsy or aspiration, sedation minimizes anxiety and pain,11 particularly in children,12 for whom propofol, with or without fentanyl, administered under carefully controlled conditions13 with monitoring of oxygen saturation, blood pressure, and vital signs, is frequently used. Midazolam (Versed) is a popular choice for conscious sedation of adult patients, although a variety of other premedications have been used. There is a relative lack of empirical research and consensus guidelines on the subject of pain reduction during adult marrow procedures.14,15 The experience of marrow procedures from the patient’s point of view is worth reading.16 The only significant correlates with severe/unbearable pain (experienced by 4 percent of patients) during marrow examination were quality of the information about the procedure provided before the examination and previous painful experiences.17 Marrow biopsies and aspirations for lymphoma staging purposes often can be performed while the patient is under anesthesia for other procedures. Several different types of needles are available for marrow aspiration.3 For adults, a 16-gauge needle is sufficiently large to permit aspiration of adequate specimens; larger needles are unnecessary. The patient is prone or in the left or right lateral decubitus position. Sterile precautions must be observed. The skin over the puncture site is shaved if necessary and cleansed with a disinfectant solution. The skin, subcutaneous tissues, and periosteum are infiltrated with a local anesthetic solution, such as 1 percent lidocaine. Adequate infiltration of the anesthetic at the periosteal surface is important to minimize severe pain during the procedure, but no more than 20 mL of 1 percent lidocaine should be used in an adult.18 Adequate anesthesia can be achieved with much less lidocaine in virtually all cases. An air gun can be used to anesthetize the skin surface prior to application of anesthetic to the periosteal surface by injection. After the anesthesia has taken effect, usually in 3 to 5 minutes, the marrow needle is inserted through the skin, subcutaneous tissue, and cortex of the bone using a slight twisting motion. In obese patients, the length of the needle must be sufficient to reach the iliac crest. The stylet should be locked into place on the hub of the needle to prevent plugging of the needle with tissue prior to needle entry into the marrow cavity. Penetration of the cortex can be sensed by a slight, rapid forward movement accompanied by a sudden increase in the ease of advancing the needle. The stylet of the needle is removed promptly, the hub is attached to a 10- or 20-mL syringe, and approximately 0.5 to 1.5 mL of fluid is aspirated. The actual aspiration of the marrow causes a transient painful sensation for most patients. If additional specimen volume is required, another syringe is fitted on the marrow needle, the syringe and needle is rotated and an adjacent area is entered and marrow is aspirated. The stylet may be reinserted and the marrow needle slightly repositioned between aspirations. When aspiration is complete, the stylet is reinserted and the needle immediately removed from the bone. Pressure is applied to the skin over the aspiration site for at least 5 minutes to minimize bruising at the site. If platelet number or function is decreased, firm pressure should be applied for at least 10 to 15 minutes. The bloody fluid that is aspirated contains light-colored particles of marrow approximately 0.5 to 1 mm in diameter. They often are readily visible in the syringe, but may not be detected until the syringe contents are discharged on glass slides for film preparation.
If nothing enters the syringe when aspiration is performed, the needle may not be properly placed in the marrow cavity. The needle can be cautiously advanced 1 to 2 mm after reinsertion of the stylet and aspiration attempted again, or the needle can be removed from the bone and reinserted in a nearby site in the anesthetized area. The thickness of the bone must be considered when the needle is being adjusted in the bone. Occasionally the needle must be rotated on its longitudinal axis, or in a larger orbit, in order to loosen the marrow mechanically before the marrow can be aspirated. If a small amount of blood has been aspirated, a new needle should be used because of the probability of clotting the aspirate when it finally is obtained. Aspiration with a 50-mL syringe may succeed if use of a smaller syringe fails. Fibrotic or densely packed leukemic marrow may resist all attempts at aspiration, in which case a biopsy is necessary. The most common cause of failure to obtain marrow is faulty positioning of the needle, and a second attempt at aspiration usually succeeds. A specimen preparation checklist used at the time of procedure to verify presence of spicules, length of biopsy, and other protocol items has been found to increase biopsy specimen length and decrease frequency of non-diagnostic samples.19
NEEDLE BIOPSY TECHNIQUE
Needle biopsy usually is performed with the Jamshidi needle, using the same preparation as described above. The Jamshidi instrument (see Fig. 3–1) consists of a cylindrical needle with constant bore, except for a concentrically tapered distal portion ending in a sharp, beveled cutting tip. The stylet fits precisely inside the opening at the tapered tip, interlocks at the hub of the needle, and extends 1 to 2 mm beyond the end of the needle. An 11-gauge needle is most commonly used in the United States. After the skin and the periosteum of the biopsy site are anesthetized, a 3-mm incision is made in the skin. The needle, with obturator in place, is inserted into the skin incision and through the subcutaneous tissue to the cortex of the bone. The needle is directed toward the posterior iliac spine and advanced with a twisting motion. Penetration of the cortex is sensed by a decreased resistance to forward movement of the needle. The obturator is removed, and the needle is slowly advanced with reciprocal clockwise–counterclockwise twisting motions around the long axis. After sufficient penetration of the bone (up to approximately 3 cm), the needle is rotated several times on its axis and withdrawn approximately 2 to 3 mm. Some needles now come with a “trap” that snares the biopsy so that the needle can be directly removed. The needle is reinserted to the original depth at a slightly different angle, taking care not to bend the needle, and rotated several times to free the specimen from attachments in the marrow cavity. The needle is slowly withdrawn, with the same twisting motion used during insertion. The core of marrow inside the needle is removed by inserting the probe through the cutting tip and extruding the specimen through the hub of the needle. The smaller size of the cutting aperture relative to the bore of the shaft of the Jamshidi instrument yields a specimen that fits loosely inside the needle and therefore is less subject to compression, distortion, or fragmentation. The technique reliably produces good quality biopsy specimens. Marrow biopsy should be performed before marrow aspiration is attempted (or in a slightly different site on the iliac crest) to avoid hemorrhage and distorted marrow architecture in the biopsy core. With the availability of the biopsy needles described in this section, open (surgical) biopsies rarely are necessary but may be performed, for example, for diagnosis of deeply situated bone lesions or at the time of a surgical procedure performed for a related indication (e.g., staging). An FDA-approved battery-powered drill that inserts a biopsy needle into the posterior iliac bone of adult patients provides more consistent and longer biopsy cores and shortens procedure time.20
PREPARATION OF MARROW SPECIMENS FOR STUDY
Several types of preparations can be made from the marrow aspirate to maximize use of the diagnostic material. Most important is the direct film, which is made immediately from a drop of marrow suspension from the unmanipulated aspirate. This preparation is the best for evaluating cellular morphology and differential counts of the marrow. The particle film is best for estimating marrow cellularity and megakaryocyte abundance, but morphology is obscured in the thicker parts of the film. A concentrate film, which is prepared from a concentrate of nucleated cells (marrow buffy coat) achieved by centrifugation of a small volume of anticoagulated marrow, is sometimes used for detecting low-abundance cells when the marrow is hypocellular. The relative proportions of cell lineages are not maintained in the concentrate film preparation (often erythroid precursors are relatively enriched). In addition, this preparation is subject to anticoagulant-induced changes in nuclear morphology or cytoplasmic vacuolization. The touch imprint from the biopsy is quite valuable and sometimes diagnostically necessary for evaluating cellular morphology when the aspirate is hypocellular.21
After aspiration, approximately 0.5 mL of marrow is placed on a glass slide; the rest is mixed into a tube containing ethylenediaminetetraacetic acid (EDTA) solution. The marrow specimen is examined to ensure the presence of “spicules” or particles of marrow containing bony or fatty pieces, indicating successful aspiration of the marrow cavity. Direct marrow films are immediately prepared by transferring drops of the unanticoagulated marrow pool to fresh slides and making push films with coverslips. Sufficient films should be made for special stains. Heparinization of the aspirate is not necessary if the operator works rapidly and should be avoided because heparinization may introduce artifacts. Formalin vapor artifact that can distort morphology can be avoided by making sure formalin containers are not opened until aspirate smears are prepared and put away.
A useful technique is preparing a thick film of marrow by discharging a drop or two of the aspirate on a slide, covering the aspirate with a second slide, gently pressing the slides together to express most of the blood into a gauze sponge, and then pulling the slides apart longitudinally. Such preparations may contain an increased number of broken cells if too much pressure is applied, but they provide a large number of particles from which marrow cellularity can be estimated and which are useful for estimating the amount of hemosiderin present. Broken cells may be minimized using a squash technique with coverslips instead of glass slides, which compared favorably to push (wedge) films in achieving representative distribution of intact cells derived from marrow particles.22
The EDTA-anticoagulated sample may be centrifuged (1500 g for 10 minutes) in a Wintrobe tube to concentrate the cellular elements of the marrow. After centrifugation, the fatty layer and plasma are removed, and the “buffy coat” is mixed with an equal amount of plasma. Multiple films of this preparation are made. All smears should be thoroughly air-dried, which can take longer in a humid environment, before staining to avoid artifacts.
After a biopsy specimen is obtained using the Jamshidi needle, the specimen should be extruded through the hub of the needle and then gently rolled across a glass slide (using an applicator stick to move the specimen) before it is placed in fixative, taking care to avoid crushing. The touch preparations are allowed to dry and are stained in the same manner as films.
It is essential to formulate the diagnostic question before performing a marrow aspiration to ensure an adequate sample is obtained for all the special studies that may be needed to make the correct diagnosis, while avoiding aspiration of more marrow than is needed, which can lead to dilution of the sample.23 A sterile anticoagulated sample containing viable unfixed cells in single-cell suspension is the best substrate for nearly all special studies. Specifically, flow cytometry is best performed on EDTA- or heparin-anticoagulated aspirate specimens, which are stable for at least 24 hours at room temperature. For cytogenetic or cell culture analysis, preservative-free heparin-anticoagulated marrow should be added to tissue culture medium and analyzed as soon as possible to maintain optimal cell viability. Cytogenetic samples are generally not adversely affected by overnight incubation.24 In cases where the marrow aspirate is dry, a duplicate biopsy specimen can be disaggregated to produce a cell suspension for morphology, flow cytometry, and cytogenetic studies.25 FISH for detection of chromosomal deletions, duplications, and translocations can be performed in marrow biopsies when EDTA-based decalcification protocols are used.26
For molecular analysis of fresh specimens, sample storage should be minimized, and storage at 4°C is preferable. EDTA is the preferred anticoagulant because heparin can interfere with some molecular assays. DNA is relatively stable, but RNA has a variable half-life in an intact cell and is degraded rapidly (on the order of seconds to minutes) in a cell lysate by ubiquitous ribonucleases. Sample storage prior to RNA isolation should be minimized.27 Collection tubes have been designed for stabilization of RNA, but for maximal RNA recovery, samples should be transported to the laboratory immediately, where cell suspensions (typically buffy coat or mononuclear cell preparations) can be prepared and nucleic acids extracted under conditions that inhibit ribonucleases. DNA and messenger RNA can be extracted and analyzed from paraffin-embedded tissue sections28 and dried stained films,29 with variable degree of degradation dependent on the length of sequence required.
Archival storage of marrow specimens is important in light of advances in molecular diagnosis that may necessitate validation studies using samples of known origin or retrospective testing of diagnostic material. Isolated DNA or RNA can be stored for long periods at −70°C, whereas viable, intact cells can be reliably preserved only by controlled rate freezing in dimethylsulfoxide (DMSO) and storage in liquid nitrogen.
A variety of techniques for preparing aspirated material for histologic study have been advocated. All of the techniques are designed to collect a sufficient number of marrow particles in a small volume so that adequate sections can be prepared. This goal can be accomplished by discharging the marrow aspirate onto a glass slide, allowing the particles to settle for a few seconds, and then gently tilting the slide so that the excess blood runs off. The particles then are pushed together with an applicator stick, and the remaining blood is allowed to clot. The clot is promptly fixed, typically in buffered formalin, for tissue processing and sectioning. An alternative method using filtration of the anticoagulated aspirate specimen has been described.30
The core marrow biopsy specimen is processed for histologic examination typically by fixation in neutral buffered formalin, followed by decalcification and embedding in paraffin. Decalcification can be accomplished with acid reagents or EDTA, the latter of which is preferred as it provides better preservation of nucleic acid and protein antigens. Sections of high quality cut at 3 μm and stained with hematoxylin and eosin are satisfactory for routine work. Refinements in fixation and embedding techniques have enabled use of many immunologic markers in decalcified paraffin-embedded marrow biopsy specimens. Fixation in neutral-buffered formalin and embedding without decalcification in plastic resin has the advantage of superior morphology,31 but is less frequently used as the potential for immunostaining and molecular assays is more restricted.
MORPHOLOGIC INTERPRETATION OF MARROW PREPARATIONS
The Wright-Giemsa–stained direct marrow aspirate film should be examined as quickly as possible to provide a preliminary assessment of the marrow morphology and allow setup of specialized testing based on this preliminary evaluation while the sample is fresh. Final interpretation of the marrow biopsy and aspirate should be integrated with results from the clinical history, blood film, cell counts, laboratory data, cell marker studies, and molecular or cytogenetic data. No other histologic specimen exists in which a state-of-the-art interpretation is dependent on such an array of supportive data. The challenge for the hematopathologist and hematologist is to understand the advantages and limitations of each diagnostic approach so that results can be reconciled and placed into perspective. Some common pitfalls in preparation and interpretation of marrow aspirates4 and biopsies5 have been reviewed.
The first question in interpreting the marrow is whether the sample is adequate for diagnosis. At the time of the procedure, the presence of marrow particles in the aspirate is the best indicator that the needle entered the medullary cavity and marrow was successfully withdrawn. Marrow particles are bony with a glistening appearance caused by fat in the particles. Specimens containing cortical bone, muscle, or other tissue with little or no medullary bone are inadequate for marrow interpretation. Samples with extensive crush artifact or hemorrhage are suboptimal, underscoring the importance of proper technique in obtaining a useful sample. An unspoken assumption is that the piece of marrow provided for diagnostic evaluation is representative of the marrow as a whole. Based on reproducibility of bilateral biopsies, this more likely is true in leukemia and myeloma than in lymphoma and metastatic tumor.32 A biopsy specimen should contain at least a 0.5-cm length of marrow cavity. However, for detection of lymphoma or metastatic tumor, current recommendations suggest a biopsy length of 1.6 to 2.0 cm.33 A significant proportion of biopsies obtained in routine practice may fall short of this recommended length.34
The marrow cavity was entered if the aspirate contains marrow particles or hematopoietic precursors (e.g., megakaryocytes, nucleated red cells) not found in the blood film. However, this finding does not ensure the specimen is adequate for diagnosis, because the amount of marrow actually aspirated can vary significantly in disease states. Also, some cell types, notably fibroblasts and metastatic tumor cells, are not as readily removed from the marrow space by aspiration as are normal precursors. Lack of particles or precursor cells does not prove the marrow cavity was not entered, because marrow packed with leukemic cells or infiltrated with fibroblasts may yield few cells (“dry tap”).35 Marrow aspirations resulting in a dry tap usually are a consequence of significant pathology (only 7 percent show normal histology on biopsy35) and indicate the need to examine a biopsy specimen, which should include a touch imprint.21
The “gold standard” for overall marrow cellularity is examination of an adequate marrow biopsy specimen.36 The normal cellularity percentage of marrow space occupied by hematopoietic cells as opposed to fatty and nonhematopoietic tissue of iliac crest marrow decreases from a mean of 80 percent in early childhood to 50 percent by age 30 years, with further decreases after age 70 years.37 Consequently, marrow cellularity should be evaluated with reference to normal individuals of the same age as the patient.38 When evaluating cellularity, consider that the marrow spaces directly adjacent to cortical bone frequently are fatty and are not representative of the cellularity of the deeper marrow spaces. A grid can be used to estimate marrow cellularity of a biopsy.39
Cellularity assessment by examination of the direct marrow aspirate film is more difficult because of loss of histologic structure and mixture with blood. The aspirate may suggest the marrow is more hypocellular than indicated by the biopsy.40 Marrow particles (seen in the direct film or a particle preparation) are the best indicators of cellularity. These particles are like “mini-biopsies” and contain sufficient hematopoietic and fatty elements to give some idea of marrow cellularity. Cellularity estimates based on careful examination of particles in the aspirate preparation agree well with cellularity estimated from the marrow biopsy.38
The degree of dilution of marrow aspirate specimens with blood during the aspiration is variable and may affect interpretation of marrow cellularity. Adult marrows with greater than 30 percent lymphocytes plus monocytes likely are substantially admixed with blood, as shown by cytokinetic studies of paired marrow aspirate and biopsy preparations.41 A higher-than-expected proportion of mature neutrophils in the marrow differential is another clue to a hemodilute marrow aspirate. In patients with hematologic disease, from 6 to 93 percent of the nucleated cells were derived from the blood.42 The greatest admixture was observed in patients with leukemia. Substantial dilution with blood may occur in difficult aspirates or when multiple draws were taken from the same puncture site. For instance, contamination of marrow aspirates with blood cells was only 8 percent in the first 1 mL, but 20 percent in subsequent draws.43
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree