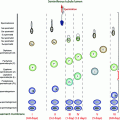
Fig. 10.1
Undescended testes along the pathway of normal testicular descent into the scrotum, and ectopic locations. Illustration by Dr. James Grubola, Professor of Fine Arts, University of Louisville
Congenital cryptorchidism is present since birth. An ascended testis, documented to be descended at birth and during infancy, has subsequently ascended above the scrotal position during childhood. Commonly occurring from about 5 years of age until pubertal onset, ascent may be a consequence of atypically low activity of the hypothalamus–pituitary–testicular axis during childhood. A retractile testis that readily retracts into a supra-scrotal position by the cremasteric reflex is considered a normal testis and usually resides in the scrotum. A continuum may exist so that retractile testis is the less-affected end of the spectrum of the ascended testis. In this review, cryptorchidism that occurs as a complication of inguinal area surgery or trauma is referred to as acquired cryptorchidism [6], although many reports have used the term “acquired” to refer to ascended testes in general, occurring without a previous insult.
A non-palpable testis may also indicate an absent testis, which is felt to be a consequence of agenesis, dysgenesis or an intrauterine accident such as occluded blood supply or testicular torsion (vanishing testis syndrome). Bilateral absence of the testes is referred to anorchia.
An ectopic testis is undescended and located elsewhere than along the normal pathway of descent (Fig. 10.1). The mechanism may be an abnormal primary attachment of the gubernaculum to structures other than the base of the scrotum, such as laterally or medially in the femoral, penile, or perineal areas.
Orchidopexy is the surgical relocation of the testis into the scrotum. It is the most successful therapy for cryptorchidism, while hormonal therapy is not recommended.
Epidemiology
The prevalence of cryptorchidism is 2–9% in full-term infants [7–14]. Among premature infants, the prevalence is as high as 21%, but diminishes with advancing gestational age and increasing birthweight. It is also noteworthy that there is an increased prevalence of both cryptorchidism and hypospadias among those born early and with low birthweights, although these findings may be more likely to be associated with a more pervasive development defect [15]. As a consequence of spontaneous descent after birth in both the term and premature infant, the prevalence of cryptorchidism decreases to approximately 1–2% by 3 months of age (Table 10.1) [14, 16]. Spontaneous descent is more likely among boys who weigh <2500 g at birth or are gestational age <37 weeks [8]. The likelihood of descent in the 3 months after birth is greater for bilateral maldescent and if the scrotum is large and well developed at birth [7, 9].
At birth | |
Overall | 2.7–6.7% |
Bilateral | 3.0% |
Unilateral | 1.9% |
Related to birth and gestational age | |
>2500 grams | 2.7–5.5% |
<2499 grams | 21.0–28.9% |
>37 weeks | 2.1% |
At 3 months of age | |
Overall | 0.9–1.6% |
3 months from EDC | 1.3% |
Based upon birthweight | |
<2000 gm | 7.7% |
2000–2500 gm | 1.7–4.6% |
>2500 gm | 0.9–1.4% |
After the age of one year, the prevalence of non-descent ranges from 0.7 to 2.0% of males with unilateral and bilateral cryptorchid testes [3, 7, 9, 14, 17, 18], similar to the 0.82% found among untreated Nigerian schoolboys aged 5–13 years [19].
The incidence of cryptorchidism and other urogenital malformations has been reported to have increased in recent decades. The estimated prevalence varies considerably in different regions [20, 21], however, perhaps from different levels of exposure to anti-androgenic chemicals, with hormone-disrupting actions, such as pesticides and phthalates [22]. Increasing incidence trends have been reported from Denmark and the United Kingdom [9, 10, 14, 23, 24]. These studies used rather similar diagnostic criteria for the ascertainment of cryptorchidism, the children having been prospectively examined by trained researchers.
Normal Development and Descent of the Testis
Testicular development involves a cascade of sequential steps under the control of numerous genes [25]. Several transcription factors are known to contribute to the initial development of the adrenogenital primordium [26, 27] including EMX2 (Empty Spiracles 2), LHX1 (Lim Homeobox Gene 1), WT1 (Wilms Tumor 1), and WNT4 (Wingless–type MMTV Integration Family Member 4). Mutations in these genes cause agenesis or dysgenesis of the gonads, adrenal glands, and the urogenital system. Further development to the bipotential gonad requires LHX9 (Lim Homeobox Gene 9), NR5A (Nuclear Receptor Subfamily 5 Group A Member; previously called SF1, Steroidogenic Factor 1), GATA4 (GATA Binding Protein 4), DMRT1/2 (Doublesex– and Mab3–related transcription factor), and CBX2 (Chromobox Homolog 2).
The primordial germ cells (PGCS) migrate from the epiblast to the developing gonadal ridge under the guidance of Kit ligand and c-Kit receptor interaction [28]. Soon thereafter, testicular differentiation of the gonad is initiated by the SRY gene product (Sex determining region of the Y–chromosome) [29]. Expression starts in the somatic cells that differentiate into Sertoli cells forming the seminiferous cords [30]. Peritubular myoid cells surround the cords, and Leydig cells develop in the interstitial space. The Sertoli cells regulate testicular development. SRY regulates SOX9 (SRY–box Containing Gene 9) in the Sertoli cells which determines the gonadal structure [31]. If SRY is missing (as in 46, XX), WNT4 and RSPO1 (R–spondin 1) lead the gonadal development toward the ovary [32]. FOXL2 (Forkhead box L2) is necessary for the maintenance of the ovary. SOX9 regulates the production of fibroblast growth factor 9 (FGF9) and prostaglandin D2 (PGD2) in Sertoli cells, and these reciprocally stimulate SOX9 expression. SOX9 and FGF9 together inhibit the ovarian genes WNT4, RSPO1 and FOXL2 that, in turn, suppress SOX9. Thus, there is a complex gene interaction network in the gonads securing normal development, and if this fails, gonads may become dysgenetic [32].
Sertoli cells secrete growth factors that influence Leydig and peritubular myoid cell differentiation, such as Desert hedgehog (DHH) and platelet-derived growth factors (PDGF). After differentiation, Leydig cells start to secrete testosterone and insulin-like peptide 3 (INSL3) which masculinize the genital structures and regulate testicular descent into the scrotum. Placental human chorionic gonadotropin and pituitary luteinizing hormone stimulate Leydig cell differentiation, proliferation, and hormone production.
Sertoli cells also secrete anti-Müllerian hormone (AMH) from the 7th week of gestation [33], which leads to the involution of Müllerian ducts. AMH is an excellent marker of the presence of testicular tissue in an infant.
Other developmental events that relate to testicular descent involve the formation of associated ducts, the gubernaculum, and the tunica vaginalis (the serous membrane covering a portion of the testes and epididymis). The gubernaculum develops from mesenchyme, becoming a ligamentous band of tissue that connects the lower end of the epididymis and the base of the scrotum. The processus vaginalis, a peritoneal out-pocketing next to the internal inguinal ring adjacent to the gubernaculum, elongates progressively through the inguinal canal. The testicular ductal system develops from the paired Wolffian ducts under testosterone stimulation, with the portion adjacent to the testes becoming rete testes with ejaculatory ducts at the distal end. Concomitantly, dihydrotestosterone produced in target tissues, such as fetal genital skin, by 5-alpha reductase type 2, SRD5A2, stimulates male external genital differentiation.
Testicular descent [34, 35] occurs after a morphologic reorientation repositions the testis caudally at the level of the lower pole of the metanephric kidney. Then, trans-abdominal migration relocates the testis from the posterior abdominal wall to the inguinal region, accomplished by 15 weeks of fetal life, followed by trans-scrotal migration. Cranial suspensory ligament regression allows both relative intra-abdominal testicular repositioning and trans-inguinal migration. Androgen control of this process is implied by the persistence of intra-abdominal testes in patients with androgen insensitivity syndrome [36], failure of regression in animals treated with an anti-androgen [37], and cranial ligament regression in female animals treated in utero with androgens [38].
Trans-inguinal migration through the inguinal canal to the base of the scrotum occurs after 26 weeks of fetal life [39]. Descent is usually accomplished by the 32nd week but may not be fully complete until post-natal life. Concomitantly, the testis carries the processus vaginalis into the scrotum. Leydig cell-derived insulin-like peptide 3 (INSL3) regulates gubernacular development and regression together with androgens [40]. This androgen-dependent phase involves the gubernaculum, cremaster muscles, the genitofemoral nerve, neurotransmitters, cytokines, intra-abdominal pressure, and epididymal maturation. Some patients with maldescent fail to have attachment of the epididymis to the testes [41]. The gubernaculum, which is intertwined with cremaster muscle, plays a role [42, 43], regressing, perhaps creating a relative negative pressure toward the inguinal canal, concomitant with trans-inguinal migration [42].
It is recognized that the factors causing testicular descent are complex and incompletely understood. Androgen stimulation involves paracrine factors and catabolic enzymes such as acid phosphatase that stimulate gubernacular regression [44]. Animal studies suggest a role for the genitofemoral nerve [45]. In spite of these potential genetic regulators, identifiable genetic causes of cryptorchidism are rare. While INSL3 plays a role in establishing gonadal position in animals [46], mutations in the coding region of the INSL3 gene do not appear to commonly cause human cryptorchidism [47]. It has been shown that RXFP2 (relaxin family peptide receptor 2), a receptor of INSL3, is required for gubernacular development and is necessary for testicular descent [48]. Polymorphisms in the RXFP2 gene may play a role in undescent [49]. Systemic factors may be more frequent with bilateral cryptorchidism while paracrine regulators may be disrupted when only one testis is undescended. Anatomic problems precluding descent could include a detached epididymis [40], testicular-splenic, or testicular hepatic fusion [50]. Inguinal hernia is a common finding with cryptorchidism and is more likely a result than a cause of maldescent.
A hormonal role (gonadotropin-stimulated testosterone production) in testicular descent has been postulated for many years, [51] although factors controlling testicular descent are clearly more complicated. Animal data are consistent with time-specific androgen stimulation of both the abdominal and inguinal phases of descent [52, 53]. Orchidectomy will halt gubernacular regression, and estrogen treatment prevents testicular descent in animals, perhaps via down-regulation of INSL3 expression [54–57].
The testis secretes AMH by the 8–9th fetal week, followed within a week by Leydig cell testosterone production, and by week 12, the hypothalamic-pituitary-testicular (HPT) axis is functional to play a crucial role in final testicular descent [58]. After a decline from about week 17 to term, the HPT axis is reactivated in the first few months after birth, with near adult circulating levels of testosterone at one to three months of age although much of the circulating testosterone is bound to sex hormone binding globulin, with values approaching those seen in men, at this stage of development [59]. This may stimulate spontaneous descent of undescended testes occurring at this age. Failure of spontaneous descent after 6 months of age may be related to diminished hormone levels.
There are contradictory reports of hormonal levels in boys with cryptorchid testes. Early studies reported decreased testosterone levels in cryptorchid infants [60] or preterm cryptorchid infants [61] compared with babies without cryptorchidism, possibly a result of diminished LH secretion [62, 63]. However, more recent research using more sensitive assays has not confirmed that result [64]. A prospective study that compared hormone levels at 3 months of age during “minipuberty” in cryptorchid and control boys reported different results between Danish and Finnish boys. The Finnish group of cryptorchid boys had slightly elevated LH and FSH levels but lower inhibin B levels as compared with non-cryptorchid boys, while among the Danish neonates with cryptorchidism, only FSH levels were significantly higher than in non-cryptorchid boys. Inhibin B levels were lower in both Danish cryptorchid and control newborns than in boys in Finland [65] while testosterone levels did not differ between the groups. When androgen bioactivity was measured in a recombinant cell bioassay, however, all 3-month-old boys with measurable bioactivity had fully descended testes, while all 16 boys with testicular position in the inguinal canal or higher had unmeasurable androgen bioactivity [66]. The explanation for these provocative findings is unclear but may relate to the sensitivity of the testosterone radioimminoassay or differences between populations, and deserves further study. AMH levels have also been reported to be diminished among boys with cryptorchidism [67], suggesting a role for AMH in testicular descent or a consequence of dysfunctional testis. During childhood after the completion of minipuberty, physiologically low LH, FSH, and testosterone levels among cryptorchid boys are not different from values in boys without cryptorchidism [64].
An older report of diminished LH responses to GnRH stimulation among prepubertal boys with undescended testes [68] has led to the unsubstantiated suggestion of mild hypogonadotropic hypogonadism (HH) among cryptorchid boys during childhood. Although patients with congenital HH often do have cryptorchidism, HH is rare among adults with a history of cryptorchidism [69]. Elevated FSH levels during the peripubertal period in some cryptorchid boys suggest instead partial gonadal failure [70], perhaps a consequence of maldescent during childhood. Inhibin B levels vary considerably among boys, and those with cryptorchidism generally have levels within the reference range [71].
Germ Cell Development and the Congenitally Cryptorchid Testis
At birth, the numbers of germ cells in cryptorchid testes has been reported to be similar to those in descended testes [72, 73]. However, after the transformation of gonocytes into adult dark (AD) spermatogonia which begins between 3 and 9 months of age, the undescended testis has fewer AD spermatogonia. This apparent diminished transformation resulting in diminished germ cells provides the primary rationale for early surgical intervention [74]. Further, regarding the risk of tumor development, intra-tubular germ cell neoplasia in situ (GNIS) in the second and third decades contain enzymatic markers similar to neonatal gonocytes, suggesting that failure of transformation into AD spermatogonia may result in testicular tumors. Testes that remain cryptorchid beyond infancy acquire a progressively more abnormal microscopic appearance [75–79] with progression with increasing age [80]. Comparison of findings at 9 months and 3 years (see below) confirm these differences and document that orchidopexy at the earlier date reverses the process [81]. The poorer growth of the cryptorchid testis that results in diminished volume compared with descended testes is also reversed by spontaneous or surgical descent [82].
The study comparing germ cells in testes with orchidopexy at 9 versus 36 months [81, 82] is the most compelling evidence for the age of recommended surgery for the congenital cryptorchid testis, even though this study could not be fully controlled since cryptorchidism is an outcome of a set of heterogenous disorders [83]. The recommendation for orchidopexy is between 6 and 12 months of age because spontaneous descent is unlikely to occur after 6 months of age while there is progressive loss of germ cells after 12 months of age. The dramatic reduction in germ cell number between 9 and 36 months of age (Fig. 10.2) provides a clear indication for earlier surgery that is beneficial for testicular growth and germ cell development [84, 85]. Other studies have verified these results [86–88]. An assessment of hormones and semen in adult men born with cryptorchidism who had bilateral biopsies at orchidopexy found that more severe histopathology tended to be associated with lower sperm density and higher FSH levels [89], although the association was not statistically significant perhaps because of the small sample size and wide age range at surgery. In clinical practice, biopsy at orchidopexy has not been shown to have value in predicting future fertility.
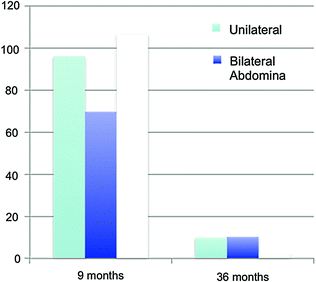

Fig. 10.2
Mean germ cell numbers in testicular biopsy specimens among boys having unilateral, bilateral, and intra-abdominal testes at surgery at 9 months and 3 years. Data (mean + SD at 9 months respectively) 96.1 + 68.7, 69.7 + 49.2, 106.6 + 79.0 and at 3 years 9.8 + 21.3, 10.2 + 13.1, 2.2 + 3.4
It has been theorized for some time that diminished germ cells of the undescended testis might be a consequence of relative gonadotropin deficiency, and hence, GnRH might be a beneficial adjunct therapy after orchidopexy. Pooled effect estimates in a meta-analysis of comparative clinical trials indicated that cryptorchid children treated with GnRH had a higher number of germ cells per seminiferous tubule at various prepubertal ages after biopsy when compared with controls [90]. This suggests that some boys with cryptorchidism may benefit from GnRH treatment as adjunctive to orchidopexy in improving the fertility index. However, longer outcome follow-up is necessary to determine if fertility is increased.
Ascended Testis
Ascent during childhood of a previously normally descended testis (acquired undescended testis) occurs when the testis becomes tethered above the scrotum in an unacceptable position. Acquired UDT is seen in 1–3% of boys during childhood. This may occur among some boys with a retractile testis due to spasticity of the cremaster muscle, or as a consequence of improper elongation of the spermatic cord due to fibrous remnant of the processus vaginalis [23, 91–96]. It is now widely accepted that testes may ascend from the full scrotal position during early- or mid-childhood years inasmuch as boys with undescended testes have been reported whose medical records clearly documented descended or retractile testes that become persistently above the scrotal location [97–100]. The age distribution at orchidopexy is bimodal, with an initial and larger peak within the first five years of life, and a second peak between 8 and 11 years of age. This distribution could be a consequence of acquired cryptorchidism or ascending testes [101]. The pathogenesis of the acquired undescended testis is unclear. It has been suggested that ascending testis may occur more frequently among boys born with undescended testes that undergo spontaneous descent in early infancy by 3 months of age [102]. There is also a suggestion that altered feeding patterns (decreased breast feeding and use of soy formulas) may increase the incidence of ascending cryptorchidism [103], although this has not been confirmed.
Previously, it was felt that ascending testes had the potential for normal function. Since most boys with ascending cryptorchidism have spontaneous testicular descent during puberty [104, 105], surgery was traditionally deferred and performed only in those boys in whom spontaneous descent failed to occur at puberty [106]. One retrospective study involved 2 groups of adult men with a history of ascending testes: group 1 had orchidopexy for lack of spontaneous descent at ages 4.75–17.8 years (n = 26) while group 2 represented men with spontaneous testicular descent during puberty (n = 32) [107]. Overall, men who had unilateral ascent had smaller testes, lower sperm concentration and progressive sperm motility, and softer testicular consistency than control men. No significant differences in fertility parameters were found between men with spontaneous descent versus those with orchidopexy. Those who had bilateral ascent also had smaller mean testicular volume and softer testicular consistency, but also a lower paternity rates, higher LH and FSH and lower inhibin B levels, and reduced sperm concentrations and progressive motility than control men. This latter group was not different from men who had bilateral congenital cryptorchidism. Testicular volume, reproductive hormones, semen quality (better for unilateral than bilateral), and successful paternity rates did not differ between the groups. Small testicular size for age of ascending testes was noted at surgery [108]. Testicular volumes smaller than normal for age by ultrasonography were observed at follow-up after operation in boys and men who had previously been treated with orchidopexy at diagnosis of uni- or bilateral ascended cryptorchidism (n = 155) [109]. Testicular size of ascended undescended testes estimated by orchidometer after spontaneous descent at puberty (n = 494) or after pubertal orchidopexy (n = 85) found that >90% had volumes within normal limits, while in over 50% of unilateral cases the affected testis was smaller at follow-up than the other testis that had always been descended [110]. An evaluation of men with unilateral cryptorchidism who delayed surgery until age 20–30 years found severe maturation arrest (22%), germinal cell aplasia (70%), or tubular atrophy (8%) in the orchidectomy specimens, and no differences in semen parameters or testosterone levels before or after surgery suggesting that testicular function by this age was essentially from the descended testis [111].
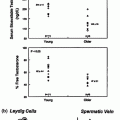
A reduced number of germ cells per tubule have been reported in unilaterally ascended testis and also in the normally descended contralateral testis [112], which is interpreted as an innate defect in testicular development in both testes in unilateral as well as bilateral cryptorchidism. By contrast, when a testis is found to be absent, the contralateral descended testis often has an increased volume, increased germ cell proliferation, and dissimilar maturation patterns compared with the contralateral descended testis of patients with unilateral cryptorchidism. These findings do not suggest an underlying endocrinopathy or other testicular defect, or risk of infertility for patients with a solitary testes [113]. In a systematic follow-up of testicular growth in puberty, testes that had been cryptorchid (either unilateral or bilateral) grew slower and remained smaller than descended testes, and there was no compensatory growth in the contralateral testis as compared with control [114]. In contrast, testes of monorchid boys were larger than control.
The Clinical Evaluation
Physical Examination
If the scrotum is empty, it is fundamental to decide whether the testis is present or not. Repeated physical examinations may be necessary if it is unclear whether a testis is present, or to determine the location of a palpable testis. Incompletely descended testes are not usually fixed in position. A testis may at one examination be just palpable at the internal inguinal ring, and on another occasion be impalpable, or the location may be high scrotal on one occasion and within the inguinal canal on another. Also, the location upon physical examination may differ from that when the patient is under general anesthesia in preparation for surgery. If a testis is not palpable, laparoscopy may be used to determine not only its presence, but also its location [115, 116].
In a prepubertal male, confirmation of an undescended testis often requires two physical examinations because of intra- and inter-observer bias in diagnosing undescended testes [115]. After 6 months of age, the cremasteric reflex is active, and the testes readily retract into the upper scrotum, or even to the external inguinal ring if a boy is anxious, nervous, or cold, all of which are common occurrences during a medical examination. A careful physical examination is essential to determine whether a testis is cryptorchid or retractile [117]. A retractile testis is diagnosed when a testis can be pulled into the scrotum and held there for a brief period, after which it does not immediately retract.
Laboratory Testing
LH, FSH, and T levels are measured in the newborn, during infancy from 6 weeks to 4 months, and at puberty to exclude hypogonadotropic hypogonadism. Inhibin B levels may also be useful [118]. When neither testis is palpable, hormone stimulation, usually with hCG, is also useful since a rise in the serum testosterone level indicates the presence of functional Leydig cells. AMH levels can also be used as an index of Sertoli cell mass [119].
Treatment
An evaluation and treatment guideline for cryptorchidism was recently published by the American Urological Association [120]. Imaging for cryptorchidism is not recommended prior to referral, which should occur by 6 months of age. Orchidopexy is the most successful therapy to relocate the testis into the scrotum, while hormonal therapy is not recommended. Successful scrotal repositioning of the testis may reduce but does not prevent the potential long-term issues of infertility and testis malignancy. Appropriate counseling and follow-up of the patient is essential.
A Nordic consensus on treatment of undescended testes was published in 2007 summarizing current information and making treatment recommendations [119]. These include a recommendation against hormonal treatment based upon poor results and the possibility of long-term adverse effects upon spermatogenesis, and recommend orchidopexy between 6 and 12 months of age, or at diagnosis, if later, to be performed by pediatric urologist/surgeons with pediatric anesthesiologists.
The rationale for treatment of cryptorchidism includes the increased risk of infertility and testicular cancer and the need to correct hernias and decrease the risk of torsion. While bilateral cryptorchidism is associated with infertility if the testes remain undescended beyond puberty, the beneficial effect on fertility of orchidopexy during childhood has not been definitively shown [121]. However, the benefit of reducing the risk of malignancy is assumed. For boys with unilateral cryptorchidism, it has been questioned whether treatment to bring the testis into a scrotal location influences fertility since the percentages of men with azoospermia or oligospermia have been reported to be similar for men with surgical treatments or no treatment [121]. However, the question of whether early surgery is beneficial has not been verified since outcome data are for those whose surgery was generally at ages considerably older than is recommended currently. While semen analysis may be abnormal, evidence (see below) suggests that paternity rates have not been shown to differ from control men [122].
Surgical Treatment
Diagnostic laparoscopy can be used to determine the location of a non-palpable testis and, if needed, to complete orchidopexy [116]. The diagnostic laparoscopy is used to plan the surgical approach, and when no evidence for a testis or associated vasculature is found, it will prevent unnecessary open surgical exploration [116]. Surgical therapy for the palpable undescended testis is commonly orchidopexy using a transverse groin incision over the internal ring, incising the external oblique aponeurosis lateral to the external ring. The testis is located and together with the spermatic cord is freed and placed into the scrotum. Success rates are related to whether the testis was palpable or not, the age at surgery, and the choice of surgical procedure. Success rates are generally greater than 90%, being somewhat lower for abdominal testes [119].
Hormonal Treatment
While human chorionic gonadotropin (hCG) testing is used in bilateral cryptorchid patients with non-palpable testes for diagnostic purposes [123], it is not currently recommended as a treatment modality. When descent occurred with such hormonal therapy, it was seldom permanent [124, 125]. Furthermore, hCG treatment has been associated with inflammatory changes in the testes and long-term adverse effects on germ cell development [126–129]. Hormonal treatment can be considered for patients in whom the testis is in the upper scrotum, or with a high likelihood of retractile testes that cannot be confirmed by routine physical examination or those with a high anesthesia risk. Infants with hypogonadotropic hypogonadism can also be treated with gonadotropins during minipuberty, which may induce testicular descent.
Consequences of Cryptorchidism
Hormone Production and Sexual Development
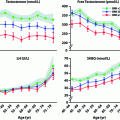
Testosterone levels are generally normal in adult men who were cryptorchid [130, 131, 133]. A minority of formerly unilateral cryptorchid men has elevated FSH and inhibin B levels, however, while inhibin B levels are lower among formerly bilaterally cryptorchid men than controls, and correlate inversely with other markers of fertility, including FSH, LH, total and free testosterone, sperm density, and sperm motility/morphology. FSH levels for men with bilateral and unilateral cryptorchidism are compared with results in control men in Fig. 10.3. Few formerly unilateral cryptorchid men had elevated FSH and low inhibin B levels, while the majority of men who had orchiopexy for bilateral cryptorchidism had elevated FSH and low inhibin B levels. Figure 10.4 shows that FSH levels are inversely related to sperm density, a relationship that has also been reported elsewhere [132]. There is also a positive relationship between inhibin B levels and sperm density for formerly unilaterally cryptorchid men (Fig. 10.5).
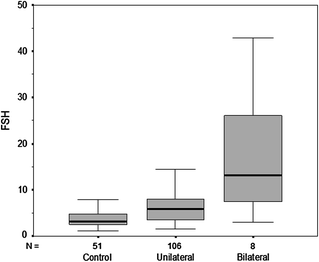
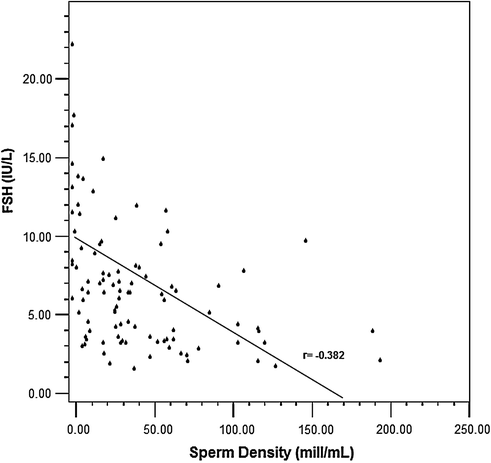
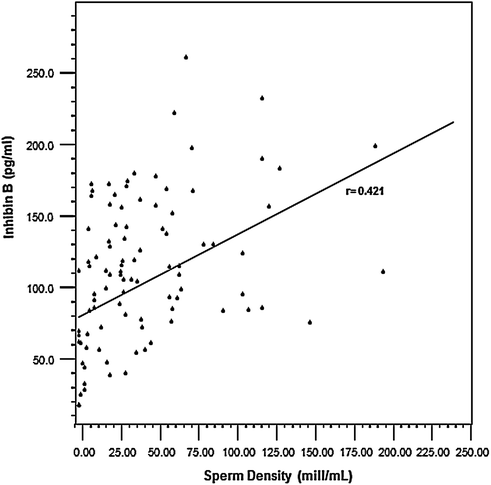

Fig. 10.3
Circulating FSH levels among formerly cryptorchid men compared with control men. The majority of men in the unilateral group have FSH levels within the normal range while almost half of men who had bilateral cryptorchidism had elevated FSH levels

Fig. 10.4
Inverse relationship between FSH levels and sperm density among formerly cryptorchid men

Fig. 10.5
A direct relationship is present between Inhibin B levels and sperm density among formerly unilateral cryptorchid men
Testosterone production in cryptorchid patients results in full stimulation of growth and pubertal maturation of androgen-responsive tissues [133]. Compensatory hypertrophy of the non-affected testis in unilateral cryptorchidism, or in the less-affected testis in bilateral cryptorchidism, may or may not occur, either before or after correction of the cryptorchidism [115, 134]. Catch-up testicular growth may occur after orchidopexy, and mean testicular volume for men with corrected unilateral maldescent is usually within the normal adult range [135] although the undescended testis in unilateral cryptorchid boys remains smaller than their descended testis [115].
Among men with idiopathic infertility, there is a significant positive correlation between testicular volume and indices of spermatogenesis (sperm density and motility), and an inverse relationship with serum FSH levels [137]. On the other hand, no correlation has been found between testicular volume at orchidopexy and paternity, hormone levels, sperm counts, and testicular volume in adulthood in either unilaterally or bilaterally cryptorchid men [89].
Infertility
While the mechanisms remain incompletely understood, the abnormalities in germ cell development in cryptorchidism lead to diminished sperm count and poor sperm motility that increase the risk for infertility.
Germ Cell Counts (Unilateral and Bilateral)
In men with bilateral orchidopexy between 10 and 16 years of age [136, 137], germ cell counts at biopsy at the time of orchidopexy were positively correlated with adult sperm density and combined testicular volume although the percentage of tubular sections containing spermatogonia did not predict sperm density in adulthood [138]. Assessment after orchidopexy when unilateral or bilateral cryptorchidism was performed before 2 years of age [139, 140] found that two-thirds of men, the portion having biopsy evidence for the second stage of maturation to adult dark (Ad) spermatogonia, had normal sperm density. An age relationship was found: all with orchidopexy and biopsy before age 2 years had germ cells, while there was an increased risk for germ cell absence with orchidopexy at older ages [141]. Among boys with good fertility potential (normal numbers of tubular germ cells and normal gonadotropin and inhibin B levels), the mean number of adult dark spermatogonia was significantly greater than in those with low fertility potential [142]. The relationship between sperm density (million/ml) after bilateral cryptorchidism for men who had testicular biopsy at orchidopexy during the first 2 decades of life [83] demonstrating germ cell loss and normal or abnormal adult dark spermatogonia is shown in Table 10.2.
Table 10.2




Median and range of sperm density (million/ml) after bilateral cryptorchidism for men who had testicular biopsy at orchidopexy during the first 2 decades of life [83]
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree