(pending WHO endorsement)
Major criteria
Minor criteria
a Or, hemoglobin (Hb) or hematocrit (Hct) >99th percentile of reference range for age, sex, and altitude of residence, or Hb >17 g/dL in men, >15 g/dL in women, if associated with a documented and sustained increase of at least ≥2 g/dL from a person’s baseline value that cannot be attributed to correction of iron deficiency, or red cell mass >25% above mean normal predicted level.
As a matter of fact, while overt PV in a patient with extensive erythrocytosis, associated with leukocytosis and/or thrombocytosis and/or typical disease-associated manifestations (pruritus, splenomegaly, microvessel disturbances, rubeosis, to name a few), usually presents no diagnostic difficulties, early stages of the disease may be difficult to establish, eventually resulting in delayed diagnosis and appropriate treatment.3,4 A basic hint to diagnosis derives from the knowledge that the JAK2 mutation is associated with almost any case of PV: about 95% of patients carry the V617F mutation and an additional 3–4% harbor abnormalities in JAK2 exon 12. The JAK2 V617F mutation is harbored also by 60% of patients with essential thrombocythemia (ET) and primary myelofibrosis (PMF). Therefore, finding a JAK2 mutation undoubtedly establishes an underlying MPN, although it does not help in differentiating PV from the other two classical MPN, ET and PMF.5 Furthermore, a patient with a JAK2 V617F-mutated ET may have higher levels of hemoglobin/hematocrit than another one presenting a calreticulin (CALR) or thrombopoietin receptor (MPL) mutation or no mutations at all, supporting the hypothesis of a phenotypic continuum between JAK2 V617F-mutated ET and PV.6,7 The best way to demonstrate an increased RCM, usually accompanied by an expanded plasma volume, in a patient suspected of having PV is to use a radioisotope technique with isotope-labeled autologous erythrocytes.8 However, the test has largely been abandoned in most hematology centers because of the need for dedicated facilities for handling radioisotopes, the cost, and also difficulties in standardization. It is debated whether the best surrogate of an absolute increase of RCM is the hemoglobin rather than hematocrit value. There is a consensus that a sustained hematocrit value of >60% and >56% in males and females, respectively, indicates an absolute erythrocytosis. The British Committee for Standards in Haematology has proposed a very practical approach to the diagnosis of PV in a subject with erythrocytosis that relies on the presence of the JAK2 V617F mutation and an increased hematocrit value (>52% in men and >48% in women) (Table 8.2).9 In the absence of the JAK2 V617F mutation, a more stringent definition of increased RCM is needed, including the radioisotope assessment, together with a number of major and accessory diagnostic criteria (Table 8.2).
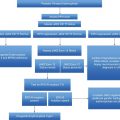
| JAK2-positive polycythemia vera |
| A1 High hematocrit (>0.52 in men, >0.48 in women) or raised red cell mass (>25% above predicted) |
| A2 Mutation in JAK2 |
| Diagnosis requires both criteria to be present |
| JAK2-negative polycythemia vera |
| A1 Raised red cell mass (>25% above predicted) or hematocrit >0.60 in men, >0.56 in women. |
| A2 Absence of mutation in JAK2 |
| A3 No cause of secondary erythrocytosis |
| A4 Palpable splenomegaly |
| A5 Presence of an acquired genetic abnormality (excluding BCR-ABL) in the hematopoietic cells |
| B1 Thrombocytosis (platelet count >450 × 109/L) |
| B2 Neutrophil leukocytosis (neutrophil count > 10 × 109/L in non-smokers; >12.5 × 109/L in smokers) |
| B3 Radiological evidence of splenomegaly |
| B4 Endogenous erythroid colonies or low serum erythropoietin |
| Diagnosis requires A1 + A2 + A3 + either another A or two B criteria |
A novel set of criteria for making the diagnosis of PV has been developed in a proposal of modification of the 2008 WHO classification, expected to be revised in 2015 (Table 8.1).10 The main change concerns the role of bone marrow biopsy that now represents a major diagnostic criterion, based on studies supporting the validity of histology in recognizing a typical PV-associated pattern 3,11 and in discriminating JAK2-mutated “masked” or early PV from ET.12 Also, the threshold level of hemoglobin required to diagnose PV was lowered to 165 g/L and 160 g/L in men and women, respectively, and that of hematocrit to 49% and 48%.10 Finally, due to the intrinsic variability and the difficulty to standardize, the test of endogenous erythroid colonies has been abandoned.
Soon after the discovery of the JAK2 V617F mutation, phlebotomies were initiated in the ICU, and eight 250-mL phlebotomies were required in the subsequent 3 weeks in order to reach and steadily maintain a hematocrit value at 43–45%. The patient was discharged from the ICU 10 days later and transferred to a neurologic/rehabilitation unit, where he spent the next 40 days. At that time, a bone marrow biopsy was performed, that showed typical PV-associated features. Age-adjusted cellularity was markedly increased (80–90%), and found to expand also to the subcortical bone areas; panmyelosis was accounted for by markedly increased erythroid and megakaryocyte lineages. Erythropoiesis was morphologically normal while megakaryocytes showed a significant degree of pleomorphism with different sizes and often hyperlobulated nuclei; they tend to form loose clusters, but atypical forms and/or tight clusters, as seen in myelofibrosis, were absent. Granulopoiesis was slightly increased and morphologically normal. Reticulin stain showed a normal reticulin fiber network and stainable iron was absent. The pathologist’s diagnosis was “polycythemia vera, WHO-defined.” The karyotype was 46,XY. Overall, the findings confirmed the initial diagnosis of an early form of PV.
The clinical presentation
Arterial and venous thrombosis are considered as the main disease-associated complications in patients with PV, occurring at diagnosis and/or during the disease course, and represent the leading cause of morbidity and mortality.4 In a recent survey of 1,545 patients with strictly WHO-defined PV, the proportion of patients with a history of arterial and venous thrombosis before or at diagnosis was 16% and 7.4%, respectively.13 Interestingly, those frequencies were lower than those recorded in the European Collaboration on Low-dose Aspirin in Polycythemia Vera (ECLAP) study (dating from the 1990s; 27% and 11%, respectively) but comparable to those reported in the recent Cytoreductive Therapy in PV (Cyto-PV) study (arterial 17%, venous 12%).13 As regards thrombosis recurrence after diagnosis, the rate was 2.62% of patients per year, again lower than that reported in the ECLAP trial (4.4% of patients per year) but comparable to the rate in the Cyto-PV study (2.7% of patients per year). There is a discrete preference for patients with first arterial or venous thrombosis to recur in the same area, but in the above-cited study 25% of prior arterial and 39% of prior venous thrombosis had recurrence in the venous and arterial area, respectively.13 Major hemorrhages may occur before or at diagnosis in 4–8% of the patients. Important information is derived from these findings: first, PV is less likely to be diagnosed in concomitance with a cardiovascular event in contemporary patients, possibly as the consequence of early diagnosis, mainly thanks to the presence of JAK2 mutations; second, even if decreasing, there is still considerable morbidity from recurrence of thrombosis, suggesting that treatment is still suboptimal for some patients; third, we can expected that in coming years hematological progression or secondary neoplasia will conversely become the leading cause of mortality in PV patients.
Venous thrombosis in PV as well as in ET and PMF patients is mostly represented by deep-vein thrombosis in common sites such as the deep circulation of the lower limbs and pulmonary embolism, but they also occur in unusual sites at rates higher than expected in the general population. These unusual events include thrombosis in the splanchnic circulation (hepatic, portal, mesenteric, and splenic veins), and occur particularly commonly in young women and, to a lesser extent, in the cerebral circulation. CVT is a rare life-threatening disease in the general population (3–4 cases in every million adults), preferentially occurring in women of fertile age because of its association with oral contraceptives or pregnancy. In patients with MPN the prevalence of CVT is 0.3–1% and an MPN is concomitantly diagnosed in 3–7% of CVT cases.14 Compared to MPN patients suffering from venous thrombosis in usual sites, those displaying CVT have a doubled risk of recurrence.14
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree