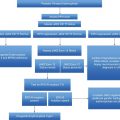
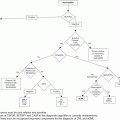
Flow chart of recommendations for the management of patients with essential thrombocythemia (ET). CV, cardiovascular; HU, hydroxyurea; WHO, World Health Organization.
Intervention on lifestyle
Thrombosis is a multifactorial process and its pathogenesis results from an interplay of various factors other than the myeloproliferative disease. The identification and appropriate management of cardiovascular risk factors and the promotion of a healthy lifestyle in ET patients, as in the general population, should be considered a cornerstone of vascular prevention. Particular attention has to be given to smoking habit, which has an important effect on vascular risk of ET patients, as demonstrated in a multivariate analysis of a randomized clinical trial assessing the role of hydroxyurea (HU) in preventing thrombosis in a high-risk population.5 Therefore, smoking cessation is absolutely recommended.
Low-dose aspirin
In ET, low-dose aspirin has been found to control microvascular symptoms, such as erythromelalgia, and transient neurological and ocular disturbances, including dysarthria, hemiparesis, scintillating scotomas, amaurosis fugax, migraine, and seizures. Higher doses, up to 500 mg daily, may be necessary in the acute phase of erythromelalgia.
The use of low-dose aspirin as primary prophylaxis for vascular events is a matter of discussion since formal clinical trials addressing this issue are not available in ET. In a retrospective study, the incidence rates of arterial and venous thrombosis were evaluated in 300 low-risk ET patients treated with either antiplatelet drugs as monotherapy or observation only.8 While overall rates of thrombotic events did not differ between these patient groups, an increased risk of major bleeding was observed in patients with platelet count >1,000 × 109/L treated with antiplatelet therapy (incidence rate ratio (IRR): 5.4; 95% CI: 1.7–17.2; p = 0.004). Subgroup analysis showed that two types of patient did worse with observation only: JAK2V617F-positive patients had an increased risk of venous thrombosis (IRR: 4.0; 95% CI: 1.2–12.9; p = 0.02); and patients with cardiovascular risk factors had increased rates of arterial thrombosis (IRR: 2.5; 95% CI: 1.02–6.1; p = 0.047).
Taking into account all these data, aspirin as primary antithrombotic prophylaxis in ET is indicated in patients with microvascular symptoms or cardiovascular risk factors or carrying the JAK2 V617F mutation, whereas it should be avoided in those with very high platelet count or previous history of major bleeding (Figure 7.1).
Alternative antiplatelet agents, such as the thienopyridines (ticlopidine, clopidogrel, prasugrel), or other reversible adenosine diphosphate receptor antagonists (ticagrelor), have been shown to have an important role in the management of atherosclerotic (cerebral, cardiac, and peripheral) vascular disease in the general population. However, there is a paucity of data on the use of these agents in prevention or therapy of thrombosis in MPN, and the limited available data are not sufficient upon which to base specific management recommendation.
Case 1: part 3
The index patient was classified at low risk for thrombotic complications, but the presence of arterial hypertension and the absence of risk factors for bleeding suggested that he should be given low-dose aspirin daily.
The “intermediate” risk of thrombosis
Whether some ET patients may be classified as at “intermediate risk” of thrombosis is a matter of debate. The rationale for assigning this risk category is the different incidence of thrombotic events in different age ranges (e.g., <40 vs. 40–60 vs. > 60 years) and the uncertainty over the weighting that might be ascribed to weaker or more controversial risk factors. Variable definitions of intermediate risk have been proposed, up to the recent IPSET score that also considers molecular risk factors (Table 7.1). However, prospective clinical trials are needed to establish whether patients with an intermediate risk of thrombosis deserve a different treatment as compared to the other risk categories.
How to manage “high-risk” patients
The cytoreductive drugs most commonly used for the treatment of high-risk ET include HU, anagrelide, and interferon-alpha (IFN-alpha). Practical recommendations for their management and dosing are reported in Figure 7.1 and Table 7.2 and the main results from randomized clinical trials comparing these drugs are summarized in Table 7.3.
| Hydroxyurea The starting dose of hydroxyurea is 1,000–1,500 mg daily until response is obtained. Thereafter, a maintenance dose should be administered to keep the response without reducing white blood cell count values below 2,500 × 109/L. Complete hemogram should be recorded every 2 weeks during the first 2 months, then every month, and, in steady state in responding patients, every 3 months |
| Interferon-alpha Interferon-alpha is contraindicated in patients with thyroid and/or mental disorders: for this reason, an accurate evaluation of thyroid function and inquiry of previous or present mental disorders in candidate patients are recommended. Interferon should be administered at the dose of 3 MU s.c. three times a week until a response is reached; then, therapy has to be adjusted at the lowest weekly doses which maintain the response. Complete hemogram must be recorded every week during the first month of therapy, every 2 weeks during the second month, then every month and, in steady state in responding patients, every 3–4 months. Pegylated interferon-alpha is given at a starting dose of 45 μg weekly. In patients who failed to achieve a response after 12 weeks, the dose is increased up to 90 μg weekly. Then, therapy has to be adjusted at the lowest dose that maintains the response and monitored as above |
| Anagrelide The recommended starting dosage of oral anagrelide for adults is 0.5 mg twice daily; this dosage should be maintained for at least 1 week and thereafter titrated individually to achieve a platelet count <600 × 109/L and ideally 150–400 × 109/L. Dosages should be increased by no more than 0.5 mg/day in a single week, and should not exceed 10 mg/day. The recommended maximum single dose is 2.5 mg. Patients with cardiovascular disease or hepatic dysfunction should be monitored closely. The drug is contraindicated in patients with severe cardiac insufficiency as well as hepatic (Child–Pugh classification C) or renal (creatinine clearance <30 mL/min) impairment |
| Study | Patients and follow-up | Treatments and main results | p | |
|---|---|---|---|---|
| Cortelazzo et al.5 | 114 patients; 27 months | HU | No cytoreduction | |
Thrombosis | ||||
| 2 | 14 | 0.003 | ||
| Harrison et al.4 | 809 patients; 39 months | HU + ASA | Anagrelide + ASA | |
Arterial thrombosis | ||||
| 17 | 34 | 0.004 | ||
Venous thrombosis | ||||
| 14 | 3 | 0.006 | ||
Major bleeding | ||||
| 8 | 22 | 0.008 | ||
Transformation to myelofibrosis | ||||
| 5 | 16 | 0.01 | ||
Death from any cause | ||||
| 27 | 31 | n.s. | ||
| Gisslinger et al.17 | 258 patientsa; 36 months | HU | Anagrelide | |
Arterial thrombosis | ||||
| 8 | 7 | n.s. | ||
Venous thrombosis | ||||
| 6 | 2 | n.s. | ||
Major bleeding | ||||
| 2 | 5 | n.s. | ||
Transformation to myelofibrosis | ||||
| 0 | 0 | n.s. | ||
HU, hydroxyurea; ASA, aspirin; n.s., not significant.
a ET strictly diagnosed according to WHO 2008 criteria.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree