- Polyuric diseases have been described for over 3500 years. The name “diabetes” comes from the Greek word for a syphon; the sweet taste of diabetic urine was recognized at the beginning of the first millennium, but the adjective “mellitus” (honeyed) was only added by Rollo in the late 18th century.
- The sugar in diabetic urine was identified as glucose by Chevreul in 1815. In the 1840s, Bernard showed that glucose was normally present in blood, and showed that it was stored in the liver (as glycogen) for secretion into the bloodstream during fasting.
- In 1889, Minkowski and von Mering reported that pancreatectomy caused severe diabetes in the dog. In 1893, Laguesse suggested that the pancreatic “islets” described by Langerhans in 1869 produced an internal secretion that regulated glucose metabolism.
- Insulin was discovered in 1921 by Banting, Best, Macleod and Collip in acid-ethanol extracts of pancreas. It was first used for treatment in January 1922.
- Diabetes was subdivided on clinical grounds into diabète maigre (lean subjects) and diabète gras (obese) by Lancereaux in 1880, and during the 1930s by Falta and Himsworth into insulin-sensitive and insulin-insensitive types. These classifications were the forerunners of the etiological classification into type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetes.
- Insulin resistance and (β-cell failure, the fundamental defects of type 2 diabètes, have been investigated by many researchers. The “insulin clamp” method devised by Andres and DeFronzo was the first accurate technique for measuring insulin action. Maturity-onset diabètes of the young was described as a distinct variant of type 2 diabètes by Tattersall in 1974.
- Lymphocytic infiltration of the islets (insulitis) was described as early as 1901 and highlighted in 1965 by Gepts who suggested that it might be a marker of autoimmunity. Islet cell antibodies were discovered by Doniach and Bottazzo in 1979.
- The primary sequence of insulin was reported in 1955 by Sanger and the three-dimensional structure by Hodgkin in 1969. Proinsulin was discovered by Steiner in 1967, and the sequence of the human insulin gene by Bell in 1980. Yalow and Berson invented the radioimmunoassay for insulin in 1956. The presence of insulin receptors was deduced in 1971 by Freychet, and the receptor protein was isolated in 1972 by Cuatrecasas.
- The various types of diabetic retinopathy were described in the second half of the 19th century as were the symptoms of neuropathy. Albuminuria was noted as a common abnormality in patients with diabetes in the 19th century and a unique type of kidney disease was described in 1936 by Kimmelstiel and Wilson. The concept of a specific diabetic angiopathy was developed by Lundbaek in the early 1950s.
- Milestones in insulin pharmacology have included the invention of delayed-action preparations in the 1930s and 1940s; synthetic human insulin in 1979; and in the 1990s novel insulin analogs by recombinant DNA technology.
- The first sulfonylurea carbutamide was introduced in 1955, followed by tolbutamide in 1957 and chlorpropamide in 1960. The biguanide phenformin became available in 1959 and metformin in 1960.
- That improved glucose control in both type 1 and type 2 diabetes was beneficial was proved by the diabetes Control and Complications Trial (1993) and the UK Prospective diabetes Study (1998).
- Landmarks in the treatment of complications include photocoagulation for retinopathy first described by Meyer-Schwickerath; the importance of blood pressure to slow the progression of nephropathy (demonstrated by Mogensen and Parving); the introduction of low-dose insulin in the treatment of diabetic ketoacidosis in the 1970s; and improvements in the care of pregnant women with diabetes pioneered by White and Pedersen.
Ancient times
Diseases with the cardinal features of diabetes mellitus were recognized in antiquity (Table 1.1). A polyuric state was described in an Egyptian papyrus dating fring c. 1550 BC, discovered by Georg Ebers (Figure 1.1), and a clearly recognizable description of what would now be called type 1 diabetes was given by Aretaeus of Cappadocia in the 2nd century AD (Figure 1.2a). Aretaeus was the first to use the term “diabetes,” from the Greek word for a syphon, “because the fluid does not remain in the body, but uses the man’s body as a channel whereby to leave it.” His graphic account of the disease highlighted the incessant flow of urine, unquenchable thirst, the “melting down of the flesh and limbs into urine” and short survival.
Table 1.1 Milestones in the clinical descriptions of diabetes and its complications.
| Clinical features of diabetes | |
| Ebers papyrus (Egypt, 1500 BC ) | Polyuric state |
| Sushrut and Charak (India, 5th century BC) | Sugary urine; thin and obese patients distinguished |
| Aretaeus (Cappadocia, 2nd century AD) | Polyuric state named “diabetes” |
| Chen Chuan (China, 7th century) | Sugary urine |
| Avicenna (Arabia, 10th century AD) | Sugary urine; gangrene and impotence as complications |
| Diabetic ketoacidosis | |
| William Prout (England, 1810–1820) | Diabetic coma |
| Adolf Kussmaul (Germany, 1874) | Acidotic breathing |
| Hyperlipidemia | |
| Albert Heyl (Philadelphia, 1880) | Lipemia retinalis |
| Retinopathy | |
| Eduard von Jaeger (Germany, 1855) | General features |
| Stephen Mackenzie and Edward Nettleship (England, 1879) | Microaneurysms |
| Edward Nettleship (England, 1888) | New vessels, beading of retinal veins |
| Julius Hirschberg (Germany, 1890) | Classification of lesions; specific to diabetes |
| Neuropathy and foot disease | |
| John Rollo (England, 1797) | Neuropathic symptoms |
| Marchal de Calvi (France, 1864) | Neuropathy is a complication of diabetes |
| William Ogle (England, 1866) | Ocular nerve palsies in diabetes |
| Frederick Pavy (England, 1885) | Peripheral neuropathy |
| Julius Althaus (Germany, 1890) | Mononeuropathy |
| Thomas Davies Pryce (England, 1887) | Perforating foot ulcers |
| Nephropathy | |
| Wilhelm Griesinger (Germany, 1859) | Renal disease in patients with diabetes |
| Paul Kimmelstiel and Clifford Wilson (USA, 1936) | Glomerulosclerosis associated with heavy proteinuria |
Figure 1.2 (a) Clinical description of diabetes by Aretaeus of Cappadocia (2nd century AD. Adapted from Papaspyros NS (1952) The History of diabetes Mellitus. (b) Sushrut (Susrata), an Indian physician who wrote medical texts with Charak (Charuka) between 500 BC and 400 BC.

The Hindu physicians, Charak and Sushrut, who wrote between 400 and 500 BC, were probably the first to recognize the sweetness of diabetic urine (Figure 1.2b). Indeed, the diagnosis was made by tasting the urine or noting that ants congregated round it. Charak and Sushrut noted that the disease was most prevalent in those who were indolent, overweight and gluttonous, and who indulged in sweet and fatty foods. Physical exercise and liberal quantities of vegetables were the mainstays of treatment in the obese, while lean people, in whom the disease was regarded as more serious, were given a nourishing diet. The crucial fact that diabetic urine tasted sweet was also emphasized by Arabic medical texts from the 9–11th centuries AD, notably in the medical encyclopedia written by Avicenna (980–1037).
The 17th and 18th centuries
In Europe, diabetes was neglected until Thomas Willis (162–1675) wrote diabetes, or the Pissing Evil [1]. According to him, “diabetes was a disease so rare among the ancients that many famous physicians made no mention of it … but in our age, given to good fellowship and guzzling down of unallayed wine, we meet with examples and instances enough, I may say daily, of this disease.” He described the urine as being “wonderfully sweet like sugar or honey” but did not consider that this might be because it contained sugar.
The first description of hyperglycemia was in a paper published in 1776 by Matthew Dobson (1735–1784) of Liverpool (Figure 1.3) [2]. He found that the serum as well as the urine of his patient Peter Dickonson (who passed 28 pints of urine a day) tasted sweet. Moreover, he evaporated the urine to “a white cake [which] smelled sweet like brown sugar, neither could it by the taste be distinguished from sugar.” Dobson concluded that the kidneys excreted sugar and that it was not “formed in the secretory organ but previously existed in the serum of the blood.”
Figure 1.3 Frontispiece and opening page of the paper by Matthew Dobson (1776), in which he described the sweet taste of both urine and serum from a patient with diabetes [2].

The Edinburgh-trained surgeon, John Rollo (d. 1809) was the first to apply the adjective “mellitus” (from the Latin word meaning “ honey “ ). He also achieved fame with his “animal diet,” which became the standard treatment for most of the 19th century. Rollo thought that sugar was formed in the stomach from vegetables, and concluded that the obvious solution was a diet of animal food. Thus, the regimen described in his 1797 book, An Account of Two Cases of the diabetes Mellitus [3], allowed his patient Captain Meredith to have for dinner “Game or old meats which have been long kept; and as far as the stomach may bear, fat and rancid old meats, as pork.” Rollo was probably the first to note the difficulty that some patients find in adhering to treatment – a difficulty he blamed for the death of his second patient (Figure 1.4).
Figure 1.4 Extract from John Rollo’s account of two cases of diabetes (1797). Rollo was well aware of the problem of non – compliance. Note that “the patient was strongly remonstrated with, and told of the consequences of repeated deviations.” Courtesy of the Wellcome Library, London.

The 19th century
In 1815, the French chemist Michel Chevreul (1786–1889) proved that the sugar in diabetic urine was glucose [4]. In the middle of the century, tasting the urine to make the diagnosis was superseded by chemical tests for reducing agents such as glucose as introduced by Trommer in 1841, Moore in 1844 and – the best known – Fehling in 1848. Measurement of blood glucose could only be done by skilled chemists but needed so much blood that it was rarely used in either clinical care or research. It only became practicable with the introduction in 1913 of a micromethod by the Norwegian-born physician Ivar Christian Bang (1869–1918) and it was the ability to measure glucose repeatedly which led to development of the glucose tolerance test between 1913 and 1915.
Glucose metabolism was clarified by the work of Claude Bernard (1813–1878) [5], the Frenchman whose numerous discoveries have given him a special place in the history of physiology (Figure 1.5). When Bernard began work in 1843, the prevailing theory was that sugar could only be synthesized by plants, and that animal metabolism broke down substances originally made in plants. It was also thought that the blood only contained sugar after meals, or in pathologic states such as diabetes. Between 1846 and 1848, Bernard reported that glucose was present in the blood of normal animals, even when starved. He also found higher concentrations of glucose in the hepatic than in the portal vein, and “enormous quantities” of a starch-like substance in the liver which could be readily converted into sugar. He called this “glycogen” (i.e. sugar-forming) and regarded it as analogous to starch in plants. His hypothesis – the “glycogenic” theory – was that sugar absorbed from the intestine was converted in the liver into glycogen and then constantly released into the blood during fasting.
Another discovery by Bernard made a great impression in an era when the nervous control of bodily functions was a scientifically fashionable concept. He found that a lesion in the floor of the fourth ventricle produced temporary hyperglycemia (piqûre diabetes) [6]. This finding spawned a long period in which nervous influences were thought to be important causes of diabetes; indeed, one piece of “evidence” – cited by J.J.R. Macleod as late as 1914 – was that diabetes was more common among engine drivers than other railway workers because of the mental strain involved [7].
In the first part of the 19th century the cause of diabetes was a mystery, because autopsy usually did not show any specific lesions. A breakthrough came in 1889 when Oskar Minkowski (Figure 1.6) and Josef von Mering (1849–1908) reported that pancreatectomy in the dog caused severe diabetes [8], This was serendipitous, because they were investigating fat metabolism; it is said that the laboratory technician mentioned to Minkowski that the dog, previously house-trained, was now incontinent of urine. Minkowski realized the significance of the polyuria, and tested the dog’s urine.
Possible explanations for the role of the pancreas were that it removed a diabetogenic toxin, or produced an internal secretion that controlled carbohydrate metabolism. The concept of “internal secretions” had been publicized in June 1889, by the well-known physiologist Charles-Edouard Brown-Séquard (1817–1894), who claimed to have rejuvenated himself by injections of testicular extract [9]. It was given further credence in 1891, when Murray reported that myxoedema could be cured by sheep thyroid extract by injection or orally.
In 1893, Gustave Laguesse suggested that the putative internal secretion of the pancreas was produced by the “islands” of cells scattered through the gland ’s parenchyma [10], which had been discovered in 1869 by the 22-year-old Paul Langerhans (18471888) (Figure 1.7). Langerhans had described these clusters of cells, having teased them out from the general pancreatic tissue, but had not speculated about their possible function [11]; it was Laguesse who named them the “islets of Langerhans.” At this time, the glucose-lowering internal secretion of the islets was still hypothetical, but in 1909 the Belgian Jean de Meyer named it insuline (from the Latin for “island”) [12].
It would be wrong to give the impression that Minkowski ’s experiments immediately established the pancreatic origin of diabetes. In fact, during the next two decades, it was widely agreed that diabetes was a heterogeneous disorder with various subtypes, and that its pathogenesis involved at least three organs: the brain, pancreas and liver [13]. The discovery by Blum in 1901 that injection of an adrenal extract caused glycosuria implicated other glands, and led to the “polyglandular theory” of Carl von Noorden (Vienna), who proposed that the thyroid, pancreas, adrenals and parathyroids controlled carbohydrate metabolism.
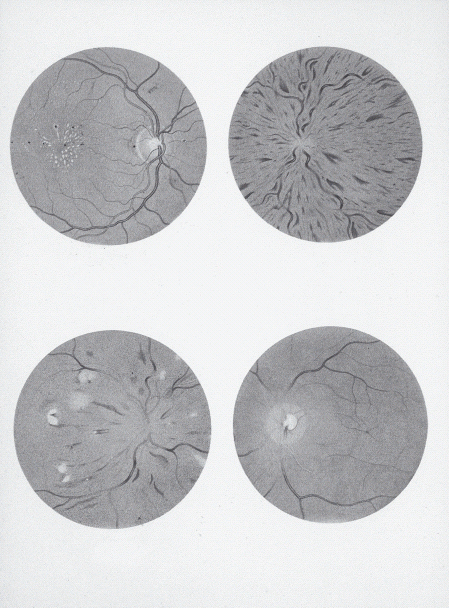
Figure 1.8 Pictures from Jaeger’s Atlas of the Optic Fundus, 1869 [14]. Top left: Bright’s disease. Top right: Jaeger’s retinitis hemorrhagica is now recognized as central retinal vein occlusion. Bottom left: A 22-year-old man with suspected diabetes. Bottom right: Central retinal artery occlusion. Courtesy of W.B. Saunders.
Clinical diabetes in the 19th century
Doctors in the 19th century were therapeutically impotent; their main role was as taxonomists who described symptom complexes and the natural history of disease. As a result, most of the major complications of diabetes were well described before 1900.
Eduard von Jaeger (1818–1884) is credited with the first description of diabetic retinopathy, in his beautiful Atlas of Diseases of the Ocular Fundus, published in 1869 [14]. In fact, the features illustrated (Figure 1.8), from a 22-year-old patient, look more like hypertensive retinopathy. In 1879, Stephen Mackenzie (1844–1909) and Sir Edward Nettleship (1845–1913) found microaneurysms in flat preparations of the retina and, in 1888, Nettleship described new vessels and the beaded appearance of retinal veins [15]. The full picture of diabetic retinopathy was described in 1890 by Julius Hirschberg (1843–1925) who was the first to claim that it was specific to diabetes [16].
Neuropathic symptoms in patients with diabetes had been mentioned by Rollo at the end of the 18th century, and in 1864 Charles Marchal de Calvi (1815–1873) concluded that nerve damage was a specific complication of diabetes. In 1885, the Guy’s Hospital physician, Frederick Pavy (1829–1911), gave a description of neuropathic symptoms which would grace any modern textbook [17]:
“The usual account given by these patients of their condition is that they cannot feel properly in their legs, that their feet are numb, that their legs seem too heavy – as one patient expressed it, “as if he had 20 lb weights on his legs and a feeling as if his boots were great deal too large for his feet.” Darting or “lightning” pains are often complained of. Or there may be hyperaesthesia, so that a mere pinching of the skin gives rise to great pain; or it may be the patient is unable to bear the contact of the seam of the dress against the skin on account of the suffering it causes. Not infrequently there is deep-seated pain located, as the patient describes it, in the marrow of the bones which are tender on being grasped, and I have noticed that these pains are generally worse at night.”
Pavy also recorded unusual presentations, including a 67-year-old who complained of “lightning pains on the right side of the waist” and cases in which the third nerve was affected with “dropped lid and external squint” [18].
Kidney disease was known to be relatively common in diabetes. In 1859, Wilhelm Griesinger (1817–1868) reported 64 autopsies in adults, half of whom had renal changes which he attributed to hypertension and atherosclerosis [19]; however, the histologic features of diabetic kidney disease and the importance of renal complications were not reported until the 1930s.
In the latter part of the 19th century it was becoming apparent that there were at least two clinically distinct forms of diabetes. In 1880, the French physician Etienne Lancereaux (1829–1910) identified lean and obese patients as having diabète maigre and diabète gras [20], and this observation laid the foundations for subsequent etiologic classifications of the disease.
The 20th century
Murray’s cure of myxoedema in 1891 led to a belief that pancreatic extract would soon result in a cure for diabetes, but, in the face of repeated failures over the next 30 years, even believers in an antidiabetic internal secretion were depressed about the likelihood of isolating it, and diverted their attention to diet as a treatment for the disease.
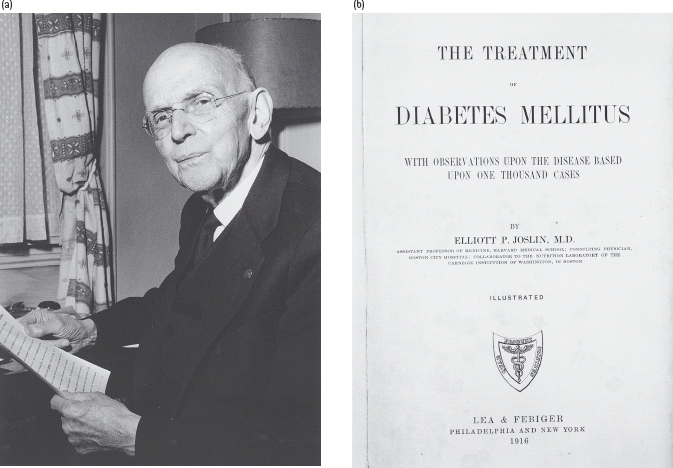
Best known was the starvation regimen of Frederick Madison Allen (1876–1964), which Joslin (Figure 1.9) described in 1915 as the greatest advance since Rollo’s time [21], This approach was an extreme application of one that had been proposed as early as 1875 by Apollinaire Bouchardat (1806–1886), who advocated intensive exercise and “manger le moins possible” Starvation treatment did work in a limited sense, in that some patients could survive for many months or even years, instead of a few weeks or months with untreated type 1 diabetes. The quality of life, however, was very poor, and some patients died of malnutrition rather than diabetes. In 1921, Carl von Noorden (1858–1944) -proponent of the “oatmeal cure” – turned away in disapproval when he saw Joslin’s prize patient, 17-year-old Ruth A, who at just over 1.52 m in height weighed only 24.5 kg (a body mass index of 10.6 kg/m2).
Figure 1.9 Elliott P. Joslin (1869–1962), arguably the most famous diabetes specialist of the 20th century and the frontispiece to his 1916 textbook [22]. Courtesy of the Wellcome Library, London.
Discovery of insulin
Many attempts were made between 1889 and 1921 to isolate the elusive internal secretion of the pancreas. These largely failed because the extracts were inactive or had unacceptable side effects; some preparations may have had limited biologic activity, but this was not recognized, either because hypoglycemia was misinterpreted as a toxic reaction or because blood glucose was not measured. Those who came closest were the Berlin physician, Georg Zuelzer (1840–1949) in 1907 [23], Ernest Scott (1877–1966) in Chicago in 1911 [24] and Nicolas Paulesco (1869–1931) in Romania in 1920–1921 [25] (Figure 1.10).
Figure 1.10 (a) Georg Zuelzer (1840–1949) and (b) the title page from his paper (1907) reporting that a pancreatic extract reduced glycosuria in pancreatectomized dogs [23] (top). (c) Nicolas Paulesco (1869–1931).

The story of how insulin was discovered in Toronto in 1921 is well known, at least superficially (Figure 1.11). A young orthopedic surgeon, Frederick Banting, inspired after reading an article by the pathologist Moses Barron (1884–1975), wondered whether the antidiabetic pancreatic principle was digested by trypsin during extraction, and decided to prevent this loss by ligating the pancreatic duct, thus causing the exocrine tissue to degenerate. He approached the Professor of Physiology in Toronto, J.J.R. Macleod, an authority on carbohydrate metabolism, who poured scorn on the idea and suggested that the only likely outcome would be “a negative result of great physiological importance.”
Figure 1.11 The discoverers of insulin. Clockwise from top left: Frederick G. Banting (1891–1941); James B. Collip (1892–1965); J.J.R. Macleod (1876–1935); and Charles H. Best (1899–1978). Courtesy of the Fisher Rare Book Library, University of Toronto.

Eventually, Macleod relented and installed Banting in a rundown laboratory, later leaving for Scotland and a fishing holiday. A student, Charles Best, was chosen by the toss of a coin to help Banting. Within 6 months of this unpromising start, Banting and Best (referred to in Toronto academic circles as B2) had discovered the most important new therapy since the antisyphilitic agent Salvarsan. These events are described in detail in the excellent book by Michael Bliss [26].
Their approach began with the injection of extracts of atrophied pancreas (prepared according to Macleod’s suggestions) into dogs rendered diabetic by pancreatectomy). Subsequently, they discovered that active extracts could be obtained from beef pancreas which Best obtained from the abbatoir. The extraction procedure (using ice-cold acid-ethanol) was greatly refined by James B. (Bert) Collip, a biochemist who was visiting Toronto on sabbatical leave.
The first clinical trial of insulin (using an extract made by Best) took place on January 11, 1922, on 14-year-old Leonard Thompson, who had been on the Allen starvation regimen since 1919 and weighed only 30 kg (Figure 1.12). After the first injection, his blood glucose level fell slightly, but his symptoms were unchanged and he developed a sterile abscess. On January 23, he was given another extract prepared by Collip, and this normalized his blood glucose by the next morning; further injections over the next 10 days led to marked clinical improvement and complete elimination of glycosuria and ketonuria. Initial clinical results in seven cases were published in the March 1922 issue of the Canadian Medical Association Journal [27], which concluded dramatically that:
Figure 1.12 Leonard Thompson, the first patient to receive insulin, in January 1922. Courtesy of the Fisher Rare Book Library, University of Toronto.

The term “insulin” was coined by Macleod, who was unaware of de Meyer’s earlier suggestion of insuline, News of its miraculous effects spread astonishingly rapidly [28]. In 1922, there were only 19 references in the world literature to “insulin” or equivalent terms such as “pancreatic extract”; by the end of 1923, there were 320 new reports, and a further 317 were published during the first 6 months of 1924.
By October 1923, insulin was available widely throughout North America and Europe. International recognition followed rapidly for its discoverers, and the 1923 Nobel Prize for Physiology or Medicine was awarded jointly to Banting and Macleod. Banting was angered by the decision, and announced publicly that he would share his prize with Best, whereupon Macleod decided to do the same with Collip.
The postinsulin era
It was confidently anticipated that insulin would do for diabetes in the young what thyroid extract had done for myxoedema, but it soon became obvious that insulin was a very different type of treatment. Thyroid was given once a day by mouth and at a fixed dosage. Insulin had to be injected in measured amounts which varied from day to day, and carried the ever-present danger of hypoglycemia. One often reads that insulin “revolutionized” the treatment of diabetes; it did so in the sense that it saved the lives of many who would otherwise have died, but its unforeseen effect was to transform an acute, rapidly fatal illness into a chronic disease with serious long-term complications. For example, only 2% of deaths among Joslin’s young patients with diabetes before 1937 were caused by kidney disease, while over 50% dying between 1944 and 1950 had advanced renal failure. Strategies to avoid and prevent the chronic complications of diabetes remain important scientific and clinical priorities today.
The rest of this chapter highlights some developments that can be regarded as landmarks in the understanding and management of the disease: to some extent, this is a personal choice, and it is obvious from the other chapters in this book that the “history” of diabetes is being rewritten all the time.
Causes and natural history of diabetes
The recognition that diabetes was not a single disease was important in initiating research that has helped to unravel the causes of hyperglycemia.
The broad etiologic subdivision into type 1 (juvenile-onset, or insulin-dependent) and type 2 diabetes (maturity-onset, or non-insulin-dependent) stemmed ultimately from Lancereaux’s diabète maigre and diabète gras distinction, as well as observations soon after the discovery of insulin that some patients did not react “normally” to insulin. In the 1930s, Wilhelm Falta (1875–1950) in Vienna [29] and Harold Himsworth (1905–93) in London [30] proposed that some individuals with diabetes were more sensitive to the glucose-lowering effects of insulin, whereas others were insulin-insensitive, or insulin-resistant. The former were usually thin and required insulin to prevent ketoacidosis, while the latter were older, obese and ketosis-resistant.
The “insulin clamp” technique developed in the 1970s by Ralph DeFronzo et al, [31] in the USA was the first to measure rigorously the hypoglycemic action of insulin, and has led to countless studies of insulin resistance and its relationship to type 2 diabetes and vascular disease. Various groups, including DeFronzo’s, have helped to clarify the role of β-cell failure in type 2 diabetes, and how it relates to insulin resistance. Maturity-onset diabetes of the young (MODY) was recognized in 1974 by Robert Tattersall (b. 1943) as a distinct, dominantly inherited subset of type 2 diabetes [32], since 1993, five different molecular defects have been identified in this condition.
The causes of the profound β-cell loss that led to the severe insulin deficiency of type 1 diabetes remained a mystery for a long time. “Insulitis”, predominantly lymphocytic infiltration of the islets, was noted as early as 1901 by Eugene L. Opie (1873–1971) and colleagues [33], but because it was apparently very rare, found in only six of 189 cases studied by Anton Weichselbaum (1845–1920) in 1910, its importance was not appreciated. The possible role of insulitis in β-cell destruction was not suggested until 1965, by the Belgian Willy Gepts (1922–1991) [34], The theory that type 1 diabetes results from autoimmune destruction of the β-cells was first made in 1979 by Deborah Doniach (1912–2004) and GianFranco Bottazzo (b. 1946) [35]. Unlike other autoimmune endocrine diseases where the autoantibody persists, islet cell antibodies (ICA) turned out to be transient and disappeared within a year of the onset of diabetes. An unexpected finding from the Barts–Windsor prospective study of the epidemiology of diabetes in childhood started by Andrew Cudworth (1939–1982) was that ICA could be detected in siblings of young people with diabetes up to 10 years before they developed apparently acute-onset diabetes. This long lead-in period raised the possibilty of an intervention to prevent continuing β-cell destruction. Cyclosporine in people with newly diagnosed type 1 diabetes prolongs the honeymoon period but without permanent benefit once the drug is stopped [36]. Nicotinamide and small doses of insulin (together with many other interventions) prevent diabetes in the non-obese diabetic (NOD) mouse but were without effect in relatives of people with type 1 diabetes with high titers of ICA [37,38].
From 1967, when Paul Lacy (1924–2005) showed that it was possible to “cure” diabetes in inbred rats with an islet cell transplant, it always seemed that the problem of islet cell transplantation in humans was about to be solved. Hope was rekindled in 2000 by a team in Edmonton, Canada. After 5 years 80% of their transplanted patients were producing some endogenous insulin but only 10% could manage without any injected insulin [39].
Chronic diabetic complications
It had been assumed that arteriosclerosis caused chronic diabetic complications, but this notion was challenged by two papers published in the mid-1930s, which pointed to specific associations of diabetes with retinal and renal disease (Table 1.2). In 1934, Henry Wagener (1890–1961) and Russell Wilder (1885–1959) from the Mayo Clinic reported patients who had retinal hemorrhages but no other clinical evidence of vascular disease [40], and concluded that “The very existence of retinitis in cases in which patients have no other signs of vascular disease must mean that diabetes alone does something to injure the finer arterioles or venules of the retina, probably the latter.”
Table 1.2 Milestones in the scientific understanding of diabetes and its complications.
| Matthew Dobson (England, 1776) | Diabetic serum contains sugar |
| Michel Chevreul (France, 1815) | The sugar in diabetic urine is glucose |
| Claude Bernard (France, 1850s) | Glucose stored in liver glycogen and secreted during fasting |
| Wilhelm Petters (Germany, 1857) | Diabetic urine contains acetone |
| Paul Langerhans (Germany, 1869) | Pancreatic islets described |
| Adolf Kussmaul (Germany, 1874) | Describes ketoacidosis Oskar Minkowski and Josef von |
| Mering (Germany, 1889) | Pancreatectomy causes diabetes in the dog |
| Gustave Edouard Laguesse (France, 1893) | Glucose-lowering pancreatic secretion produced by islets |
| M.A. Lane (USA, 1907) | Distinguished A and B islet cells |
| Jean de Meyer (Belgium, 1909) | Hypothetical islet secretion named “insuline” |
| Frederick Banting, Charles Best, J.J.R. Macleod, James Collip (Canada, 1922) | Isolation of insulin |
| Richard Murlin (USA, 1923) | Discovered and named glucagon |
| Bernado Houssay (Argentina, 1924) | Hypophysectomy enhances insulin sensitivity |
| Frederick Sanger (England, 1955) | Determined primary sequence of insulin |
| W.W. Bromer (USA, 1956) | Determined primary sequence of glucagon |
| Rosalyn Yalow and Solomon Berson (USA, 1959) | Discovered radioimmunoassay for insulin |
| Donald Steiner (USA, 1967) | Discovered proinsulin |
| Dorothy Hodgkin (England, 1969) | Determined three-dimensional structure of insulin |
| Pierre Freychet (USA, 1971) | Characterized insulin receptors |
| Pedro Cuatrecasas (USA, 1972) | Isolated insulin receptor protein |
| Axel Ullrich (USA, 1977) | Reported sequence of rat insulin |
| Ralph DeFronzo and Reuben Andres (USA, 1979) | Invented insulin clamp technique |
| Graham Bell (USA, 1980) | Reported sequence of human insulin gene |
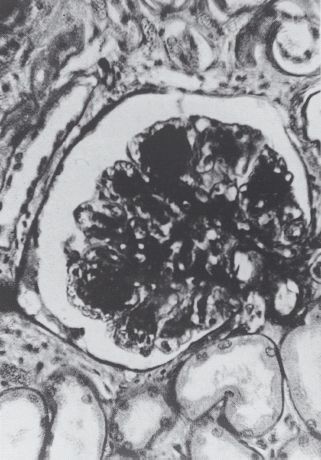
In 1936, Paul Kimmelstiel (1900–1970) and Clifford Wilson (1906–1997) described the striking histologic finding of “intercapillary glomerulosclerosis” – large hyaline nodules in the glomeruli – in the kidneys of eight subjects at autopsy (Figure 1.13) [41], Seven of the eight patients had a known history of diabetes, and Kimmelstiel and Wilson noted the common features of hypertension, heavy albuminuria with “oedema of the nephrotic type,” and renal failure. In fact, this paper led to considerable confusion during the next 15 years: according to one writer, the “Kimmelstiel-Wilson syndrome” came to mean all things to all men [42], Nonetheless, it was significant because it drew attention to a specific diabetic renal disease.
Figure 1.13 Nodular glomerulosclerosis. Figure from the paper by Kimmelstiel and Wilson, 1936 [41]. Courtesy of the British Medical Association Library.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree