- Both type 1 (T1DM) and type 2 diabetes mellitus (T2DM) can affect the psychologic and neuropsychologic status of children and adults. In most instances, these effects are modest in magnitude and are most likely to be associated with certain events that occur during the course of diabetes or its management.
- Children show remarkable psychologic resilience to the diagnosis of diabetes. About one-third report some psychologic distress shortly after diagnosis but this generally subsides within 6 months. This “adjustment disorder” is characterized by increased depressive symptomatology, more anxiety, social withdrawal and sleep disturbances. A similar adjustment reaction is often seen in parents, particularly mothers, of newly diagnosed children. Diagnoses of post-traumatic stress disorder are also more common in parents, occurring at rates comparable with that reported in children diagnosed with cancer. Increased rates of depression are also found in adults newly diagnosed with T1DM, whereas diagnosis of T2DM does not appear to increase the risk of depression unless accompanied by multiple medical co-morbidities.
- During the first 5–10 years of their diabetes, most children and adolescents show adequate psychologic functioning; however, after 10 years of diabetes, their rates of anxiety, depression or eating disorders are markedly increased, with as many as one-third of adolescents with diabetes meeting criteria for one or more psychiatric disorders. Adults with diabetes also show elevated rates of depression which are twice as high as those reported in the general population. At greatest risk are people who have been hospitalized, are older with multiple medical problems, have a history of past psychopathology or are female. Metabolic control is only weakly associated with the occurrence of a mood disorder.
- Macrovascular disease, chronic foot ulceration and proliferative retinopathy increase the risk of psychopathology, an understandable reaction to serious complications; however, lifetime psychiatric disorders such as depression may also increase the risk of later development of complications such as retinopathy. Recurrent diabetic ketoacidosis, particularly in females, is also predicted by poor psychologic functioning, and by high rates of family dysfunction.
- Phobic disorders are more common in adults with diabetes than in the general population. Fear of blood and injury may lead to less blood glucose self-monitoring and poorer control. Fear of hypoglycemia is common and may also lead to premature treatment as blood glucose levels begin to fall, resulting in persisting hyperglycemia.
- Quality of life for those with diabetes does not differ from patients with other chronic conditions, such as arthritis. Poor health-related quality of life is associated with biomedical complications, being female, physical inactivity, low income and recurrently hypoglycemia. Intensive diabetes management is not associated with a deterioration in quality of life.
- Interventions to reduce psychologic distress include individual psychotherapy or counseling, and group therapy; however, the efficacy of either–in terms of affecting mood or metabolic control–has not been studied extensively in clinical trials, but data from smaller studies suggests effects may be weak, at best. A promising technique for depressive symptoms is cognitive–behavior therapy, which teaches patients problem-solving strategies for stressful situations and “thinking away” distorted beliefs. Pharmacotherapy, including selective serotonin reuptake inhibitors and tricyclic antidepressants, has been found to reduce symptom severity but specific drugs may differentially influence glycated hemoglobin values.
- Cognitive dysfunction in diabetes is generally mild, but children diagnosed in the first 5 to 7 years of life have an elevated risk of manifesting clinically significant impairments in all cognitive domains. Those with a later onset of diabetes show modest effects, most evident on measures of intelligence, academic achievement and psychomotor efficiency. Deficits appear early in the course of the disease, and are evident within 2–3 years after diagnosis. Hypoglycemia was long considered to be the cause of this neurocognitive dysfunction but more recent research suggests that adverse effects on cognition may only occur when blood glucose levels are very low for an extended period of time. Although the etiology of neurocognitive changes in children remains poorly understood, a growing body of evidence implicates chronic hyperglycemia and, in particular, the development of microvascular and macrovascular complications.
- In adults with T1DM, neurocognitive deficits are quite modest and are most apparent on measures of intelligence, psychomotor speed and executive function. Older adults with T2DM also manifest marked reductions in memory function and, in both groups, the strongest predictor of cognitive dysfunction is chronic hyperglycemia and the presence of biomedical complications, particularly retinopathy and peripheral neuropathy.
- Structural damage to the brain is also common in both children and adults with either T1DM or T2DM. Not only is there a reduction in cortical gray matter density, but white matter structures may be disrupted. Cerebral atrophy is often present, neural slowing is evident on electroencephalography and regional cerebral blood flow is altered.
- Diabetes management and health outcomes are influenced by reciprocal relationships between metabolic control and psychologic variables. The latter include enduring psychologic traits such as locus of control, coping style, temperament and transitory psychologic states (stress, anxiety, depression). Diabetes is also strongly related to family functioning, especially in children and adolescents: low family conflict, good communication, cohesion and marital satisfaction relate to better diabetes control.
- Adherence or self-care behaviors (taking medicine, complying with diet, glucose monitoring, exercise regimens) are only weakly related to diabetic control, but this may reflect the inaccuracy of self-reported behavior. For those who are most seriously non-compliant, psychologic interventions include psychotherapy, family therapy and patient empowerment programs to improve goal-setting, problem-solving, coping, managing stress and self-motivation.
- A psychologist or other mental health care professional should be part of the diabetes care team.
Introduction
Diabetes and psychology have long been linked. More than 50 years ago, the psychosomatic model of medical illness postulated that psychosocial factors could trigger or maintain various disorders, including diabetes. This notion was subsequently abandoned by most scientists, yet the person living with diabetes and managing this behaviorally complex condition may nevertheless identify numerous points where diabetes and psychology interact. This chapter examines three major points of intersection: psychologic reactions to the development of diabetes and the appearance of complications, the neuropsychologic or cognitive consequences of diabetes, and psychologic factors that influence, or are influenced by, the everyday management of diabetes.
Psychologic impact of diabetes
Patients with diabetes ought to manifest significant psychologic distress, or so goes conventional clinical wisdom. After all, they have a disorder that shortens their lifespan, leads to debilitating biomedical complications such as blindness or neuropathy, and requires them to take complete daily responsibility for managing their health with drugs or insulin injections and careful monitoring of diet, exercise and blood glucose levels, for the remainder of their lives. Chronic illnesses like diabetes also mark individuals, particularly children, as different from their healthy peers, and burden families with demanding health care responsibilities that they may be unwilling or unable to meet. Given all of these factors, one should not be surprised to find that many people with diabetes, and their families, have elevated rates of emotional disturbances and behavioral problems.
Psychologic distress shortly after diagnosis
Observations in children and their parents
Children and adults show remarkable psychologic resilience in response to a diagnosis of diabetes. In what may be the most comprehensive prospective psychologic study of children with diabetes and their families, Kovacs et al. [1] assessed 95 children, 8–13 years of age, shortly after discharge from their initial hospitalization, and followed them for 6–10 years. Within 3 months of diagnosis, 36% of the children experienced sufficient psychologic distress to meet criteria for a diagnosable psychiatric disorder [2]. Most had “adjustment disorder,” defined as a transient reaction that exceeds the normal and expectable response to a stressor, develops within 3 months of onset of the stressor and lasts no more than 6 months. The occurrence of such a disorder signals that the child is beginning to come to terms with the diagnosis of diabetes, and can be considered to be a component of the “mourning process” that often accompanies the development of any chronic illness [3]. As one would expect with an adjustment disorder, recovery was rapid, with 93% showing complete remission of these psychiatric symptoms within 9 months. Other investigators, using different outcome measures, have also demonstrated rapid psychologic adaptation to the diagnosis of diabetes [4–6].
Parents of children newly diagnosed with diabetes also manifest psychologic responses that are analogous to an adjustment disorder. For mothers, the strain associated with caring for a school-aged child with diabetes elicited mild levels of depressive symptomatology, anxiety and generalized distress, but this dissipated within 6 months [7]. Less symptomatology was evident in fathers, both shortly after diagnosis and approximately 1 year later; however, if fathers do report experiencing high rates of emotional dysfunction in the first 2 years following their child’s diagnosis, their child is more likely to manifest poorer metabolic control and greater blood glucose variability during the first 5 years of diagnosis [8]. Greatly elevated rates of post-traumatic stress disorder (PTSD) have also been reported in a prospective study of parents evaluated at 6 weeks and 6 and 12 months after their child’s diagnosis. Depending on time point, 16–22% of mothers met DSM-IV criteria for a PTSD diagnosis, as did 8–14% of fathers [9]. Theses rates were significantly higher than those reported in the general population but are comparable with those seen in mothers of children diagnosed with cancer. The best predictor of PTSD severity at 12 months was PTSD severity at 6 months. Poorer disease outcomes, particularly episodes of hypoglycemia, were associated with an increased level of PTSD severity.
Elevated rates of worry and concern are also present in parents, but these differ by gender: 46% of mothers reported worry all or almost all of the time, whereas only 13% of the fathers reported similarly high levels of concern [10]. Mourning was the primary coping process engaged in by mothers, whose responses most commonly included feeling sad, worried and/or tired, and who manifested bouts of crying and irritability. As duration of illness increased, levels of psychologic distress, particularly depression, showed slight increments, with the greatest increases occurring in those mothers who were most distressed at the time of their child’ s diagnosis and in those mothers of higher socioeconomic status. The latter finding may reflect the possibility that these women were more knowledgeable about diabetes, and hence may have been more discouraged about its management and long-term outcomes. Despite the stresses and strains that the diagnosis of diabetes exerts on both the child and the family, there is little evidence of major family disruption in the first 2–3 years following diagnosis. Measures of parental perception of overall quality of family life as well as estimates of the quality of parents’ marriage show essentially no change during that period [11].
Diagnosis in adulthood
The onset of diabetes during adulthood ought to produce similar adjustment disorders within several months of diagnosis in both the patient as well as the spouse or other family member. A German sample of newly diagnosed, adult patients (aged 17–40 years of age) with type 1 diabetes mellitus (T1DM) had a rate of major depressive episodes that was twice that of a reference group drawn from a representative national population (5.8% vs 2.7%), although more careful analysis shows that these differences were statistically significant only for women with diabetes (9.3% vs 3.2% in reference group) [12]. There were no differences in rates of major depression between men with diabetes and the reference group (3.6% vs 2.2%), nor for other psychiatric disorders. Because this is a cross-sectional study, it is impossible to determine whether this is truly a “classic” depression or whether it is a transient adjustment disorder, as is typically seen in children and adolescents with diabetes. In contrast, several studies of adults with type 2 diabetes mellitus (T2DM) have found little psychologic morbidity in the first year following diagnosis. One smaller clinical study reported that of the 71 subjects studied, more than 50% expressed no emotional reaction to the diagnosis and felt that they could cope with diabetes [13]. Negative emotional reactions, along with feelings of being incapable of coping with this disorder, were expressed by only 26% of the sample, and this may reflect the fact that these individuals had significantly less social support than the others. A larger, more recent study assessing symptoms of depression, similarly found little evidence of a psychologic reaction in 824 patients newly diagnosed with T2DM [14]. Although there were marked gender differences, with women with diabetes reporting higher rates of significant depressive symptomatology than men with diabetes (16.1% and 8.2%, respectively), those values were comparable to UK normative data for women and men (13% and 8%, respectively). Greater depressive symptomatology was associated with more medical co-morbidities: newly diagnosed patients with diabetes taking both lipid-lowering and antihypertensive medications reported more symptoms of depression than those on only one, or on no concurrent medication.
Psychologic reactions emerging in the course of diabetes
Depressive symptomatology in children and adolescents
After resolution of their adjustment disorder, are children able to get on with their lives, or is there an increased likelihood of subsequent psychologic distress in the individual with diabetes? As duration of diabetes continues, it now appears that most children and adolescents with diabetes function well psychologically, although small increases may be evident in depressive symptomatology and internalizing behaviors, such as somatic complaints, social withdrawal, sleep disturbance and symptoms of depression and anxiety). In both school-aged [6,15,16] and pre-school children [17], this was evident after 2–3 years of diabetes, yet the magnitude of these changes was not so large as to be indicative of clinically significant psychopathology. Somewhat higher rates of externalizing, or aggressive, behaviors have also been reported, with this phenomenon especially pronounced in boys with diabetes [15], and strongly associated with consistently elevated blood glucose levels [18]. High levels of family conflict and the occurrence of multiple stressful life events appear to be particularly potent predictors of more aggressive behavior in children and adolescents with diabetes [19].
After 6 years of follow-up, Kovacs et al. [16] found trivially small increases in symptoms of depression for all subjects, and increased symptoms of anxiety for girls but not boys. Children who reported more difficulties in managing their diabetes also showed more symptoms of psychologic distress. Level of psychologic distress shortly after diabetes onset was the best predictor of symptomatology 6 years later, whereas the degree of metabolic control, as indexed by glycated hemoglobin (HbA1c) values, was not a viable predictor in this or in most other studies [20,21]. Neither age nor age at onset predicted increased psychologic distress in Kovacs et al. s cohort, although others have found an association between developmental stage and emotional or behavioral problems in so far as adolescents manifested more problems than pre-adolescents [21].
Even after 10 years of diabetes, older adolescents and young adults with childhood onset of diabetes may report little psychologic distress, although they tend to have lower levels of self-esteem and express concerns about their sociability and their physical appearance [22]. What is remarkable, however, is that when patients are formally evaluated with structured psychiatric interviews to assess for clinically significant psychopathology over this extended time period, marked elevations are found in rates of psychiatric disorder. Not only were females more often affected than males [23,24], but their risk of subsequently experiencing a recurrence of depression was nine times greater than males [25]. Kovacs et al. [1] reported that 40% of their sample had experienced at least one episode of a psychiatric disorder during the first 10 years of living with diabetes, and 26% had two different psychiatric disorders. Major depression was the most common diagnosis (26% of sample), followed by some form of anxiety disorder (20%). By comparison, similarly aged, subjects without diabetes drawn from the community had rates of depression that ranged from 9–16%, and rates of anxiety that ranged from 11–25% [26].
Remarkably similar rates of clinically significant DSM-IV disorders have been reported more recently by Northam et al. [23], who followed a cohort of newly diagnosed Australian children over a 10-year period. By year 10, 37% of those with diabetes received at least one diagnosis; of those, 60% met criteria for two or more disorders and 55% met criteria for three or more disorders [23]. In that study, mood, anxiety and eating disorders were each present in 17% of the sample; nearly 20% of the sample manifested a behavior disorder. Of note is their observation that those adolescents who met criteria for a DSM-IV psychiatric disorder were also more likely to have manifested significant externalizing problems shortly after diagnosis. Although not evaluated by Northam et al., other investigators have identified maternal psychopathology as a potent predictor of subsequent psychiatric disorder [1] and increased depressive symptomatology in children and adults with diabetes [27].
Significantly elevated rates of suicidal ideation have also been reported for adolescents with diabetes, with lifetime prevalence rates noted to be 26%, compared to rates for adolescents without diabetes ranging from 9–12% [28]. Although the rate of actual suicide attempts is low amongst youth with diabetes (4%), suicidal ideation was associated with greatly increased rates of non-compliance with medical treatment.
Clinically significant mood disorders in adults
The process of psychologic adaptation to the diagnosis of diabetes in adulthood remains incompletely understood, largely because few longitudinal studies have been conducted with adults [29]. In what remains the largest follow-up study of adults with T1DM, the Diabetes Control and Complications Trial (DCCT) Research Group [30] found no change in self-reported psychologic symptomatology over a follow-up period of 6–9 years, and found no relationship between type of treatment (conventional or intensive insulin therapy) and levels of psychologic distress [31]. Rates of clinically significant distress were higher in both treatment groups (25%) compared with rates of depression measured by self-report in the general population (14.4%) [32]. A smaller study that followed adults for 2 years from the diagnosis of T1DM also reported either no changes in psychologic state over time, or a significant improvement in functioning (e.g. reduced depressive symptomatology) [33].
Cross-sectional studies of adults with either T1DM or T2DM have demonstrated repeatedly that rates of psychologic distress, particularly depression and anxiety, tend to be higher than the general population, but are usually comparable to those reported in individuals with other chronic diseases [34–38]. Using self-report measures of psychologic symptoms, Peyrot & Rubin [39] found greatly elevated rates of both depressive (41%) and anxiety symptomatology (49%), with 38% of their entire sample showing elevations in both domains; however, repeated reassessment of these patients over a 6-month period indicated that these effects are quite unstable. Across all three assessments, only 13% of the sample was consistently disturbed. The strongest predictors of persisting distress included being female, having less than a high school education, being middle-aged and having more than two diabetes-associated biomedical complications [40]. Prevalence rates for any depressive disturbance were nearly twice as high in this study [39] than DCCT reports (41% vs 25%, respectively), but this is likely to be because of sampling differences. The DCCT study group was comprised of highly motivated patients with minimal diabetes-associated complications who were vigorously followed by a treatment team that included a psychologist or psychiatrist [41], whereas those patients studied by Peyrot & Rubin included a mix of patients with T1DM and T2DM who were recruited during a 1-week diabetes management outpatient program.
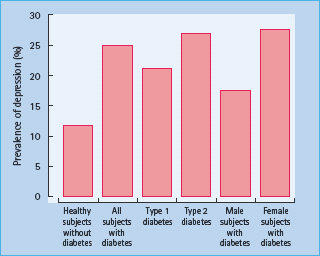
Numerous other studies have also demonstrated high rates of anxiety disorder [42] and depressive symptomatology in adults with either T1DM or T2DM [35,43]. An early analysis of 42 studies indicated that the risk of depression was doubled in people with diabetes, as compared to people without diabetes, and this occurred regardless of type of diabetes (Figure 49.1) [32]. Similar results have been reported in a more recent meta-analysis of 10 studies that included 9028 patients with T2DM and 42 272 adults without diabetes; again, rates of self-reported depression were significantly elevated in patients with diabetes (17.6% vs 9.8%) [44].
Figure 49.1 The increased prevalence of depression in people with diabetes compared with subjects without diabetes. The data are from a meta-analysis of 42 studies; figures for subjects with diabetes are the aggregate of both controlled and non-controlled studies. Adapted from Anderson et al. [32].
Variations in prevalence rates are common across individual studies, and appear to be related to the method used to ascertain depression. Those studies using self-report symptom scales yielded prevalence rates that were nearly three times higher than rates obtained using formal structured interviews with clinically established diagnostic criteria (31% vs 11%) [32]. Discrepancies amongst studies have also been found to be caused by differences in subject characteristics, particularly age and medical history [45,46]. The highest rates of current psychiatric distress tend to be found in hospitalized patients [47] or in older adults with multiple medical co-morbidities [48,49]. Frequently [50,51],but not invariably [45,52], adults with diabetes and more emotional problems also have poorer metabolic control. A review of more than 30 studies indicates that although depression is associated with higher HbA1c values, the magnitude of this effect is extremely small, with the exact value being a function of how depression is ascertained. In studies using symptom self-reports, less than 3% of the variance in HbA1c was explained by depression; when standardized diagnostic interviews are used, approximately 8% of the variance in HbA1c was accounted for by depression [53].
Studies of children with diabetes have indicated that the best predictor of future psychopathology is past psychopathology [16], and the same principle applies to adults. Lustman et al. [29] reassessed a group of adults with diabetes 5 years after they had first met criteria for current depression, and found that 67% were depressed at follow-up, whereas only 15% of the initially non-depressed adults met criteria for psychiatric disorder at follow-up. Repeated episodes of depression were common in the initially depressed group, with subjects having an average of 4.2 episodes during the 5-year follow-up period. Recurrence of depression was apparently unrelated to duration of disease, type of diabetes or development of diabetes-associated complications, but it was associated with a family history of psychiatric disorder. A subsequent study found that the severity of recurrent depressive episodes was related to the presence of neuropathy, but no other biomedical complication, at study entry and it has been suggested that the discomfort associated with this complication may serve as a stressor capable of provoking an episode of depression in vulnerable individuals [54]. The generally weak relationship between diabetes-related variables and reoccurrence of a mood disorder suggests that depression is not merely a psychologic reaction to the development of diabetes or its complications, but may be influenced significantly by underlying genetic or constitutional factors [25,55,56].
Depression as a risk factor for subsequent development of type 2 diabetes
Most readers would assume from this literature that the emergence of depression is a direct consequence of coping with its demanding burden of care [48] and/or its associated co-morbid medical conditions [57]. but there is growing evidence, at least from studies of older adults with T2DM, that the reverse may be true, and that being depressed greatly increases the risk of subsequently developing diabetes. In a meta-analysis that included 13 comparative prospective studies of depression and T2DM, a robust relationship was noted between depression and the subsequent incidence of T2DM (60% increase in risk), whereas the relationship between having T2DM and subsequently developing depression was modest at best (15% increase in risk) [58]. Although the exact pathologic mechanisms have not yet been established, it is certainly plausible that depression could greatly increase the likelihood of developing diabetes, in so far as depressive symptomatology is associated with a variety of behavioral (e.g. poorer compliance with dietary and weight loss recommendations; less physical activity) [59] and physiologic (e.g. increased activation of the hypothalamic-pituitary-adrenal axis, and increased inflammation) [60] risk factors for T2DM.
The diagnosis and treatment of depression and other psychiatric disorders in patients with diabetes is described in detail in Chapter 55.
Psychologic reactions to biomedical complications
Microvascular and macrovascular complications as triggers for psychologic distress
Diabetic complications may not only disrupt the individual’s usual lifestyle and interfere with self-care activities, but may also serve as a reminder that despite their best efforts, patients have failed to manage their disease adequately. In the same way that the child recently diagnosed with diabetes manifests an anxious or depressed mood as part of an adjustment disorder, older patients might be expected to show psychologic distress soon after a complication appears. This conjecture has not been tested empirically: it is not known how adults with diabetes react psychologically shortly after a complication appears, although as a group, adults with complications usually [61], but not invariably [62], have greater levels of psychologic distress.
Three types of diabetic complications are known to increase the risk of psychopathology: macrovascular disorders, chronic foot ulceration and sight-threatening proliferative retinopathy. Adults with diabetes and macrovascular disease often have elevated rates of depression [52] and poorer quality of life [55]. although this is not always the case [46,63]. Similarly, patients with chronic unilateral foot ulceration secondary to diabetic neuropathy have higher rates of depression and report greater dissatisfaction with their lives than age-matched adults with diabetes but no history of foot ulceration [64]. Results from a prospective cohort study noted that 24% of adults with diabetes presenting with their first diabetic foot ulcer had clinically significant major depression, and this was associated with a threefold risk of death during an 18-month follow-up period [65]. Other studies have also demonstrated marked increases in depressive symptomatology and peripheral neuropathy, and have attributed this psychologic distress to the physical distress associated with reduced feeling in the feet and unsteadiness, as well as its unpredictability [66,67].
Increased psychiatric symptomatology is also seen in patients with proliferative diabetic retinopathy, compared with those without retinopathy [68]. Both level of visual acuity and duration of visual problems affect mental health. Wulsin et al. [69] followed patients with diabetic retinopathy for 8 months, beginning shortly after a vitreous hemorrhage, and found that greater impairment of visual acuity was associated with increased psychologic distress and poorer coping efforts. Unlike a classic adjustment disorder, these relationships grew stronger, rather than weaker, over time. Fluctuations in visual impairment may also increase psychologic distress [70], although that is not inevitable [71], and it is important to keep in mind that the degree of psychologic distress secondary to visual loss is not unique to patients with diabetes; at least one study of older adults has reported no significant difference in psychologic adjustment between those with and without diabetes, either at the onset of visual loss or when re-evaluated 12 months later [72].
Depression e xacerbates the d evelopment and course of complications
Distress and depression are usually assumed to be a direct response to the occurrence of a complication, but there is growing evidence for the alternative possibility; that depression, at least under certain circumstances, may increase the likelihood that an individual will subsequently develop complications. The most compelling support for this view remains Kovacs’ sophisticated statistical analysis of 10-year follow-up data from their childhood-onset cohort study which demonstrated that severity of retinopathy could be predicted from three antecedent variables [73]. These variables had an additive effect in so far as the likelihood of retinopathy increased with increasing duration of diabetes, with length of time spent in poor control and with overall proportion of time depressed. Depression was not a reaction to retinopathy in this cohort but predated the diagnosis of retinopathy by several years.
An analogous pattern of results has been reported more recently in a study of 483 African-Americans with T2DM who were followed over a 6-year period. Patients with high depression scores at both baseline and 6.year follow-up had significantly higher baseline HbA1c values and were more likely to show progression of diabetic retinopathy (odds ratio 2.44) and progression to proliferative diabetic retinopathy (odds ratio 3.19), compared with patients with low depression scores at both visits [74]. Baseline HbA1c values accounted for 21% of the progression to diabetic retinopathy, while being depressed at both visits accounted for an additional 6% in the regression model.
These intriguing findings suggest that depression may be a risk factor, not only for the development of subsequent psychopathology, but also for the development of subsequent diabetes complications, at least in certain individuals. The physiologic basis for this relationship remains unknown but, as discussed below, depression could interfere with patients’ ability to manage their diabetes [75]. and so contribute to poor metabolic control. It follows that early treatment of depression may not only improve the individual’s mental health, but may improve metabolic control and delay the appearance of diabetic complications [76]. Support for that possibility comes from one small placebocontrolled medication trial clinical study that used a selective serotonin reuptake inhibitor (SSRI) in a group of older adults with T2DM [77], but several other studies, taking a “collaborative care” approach have had no impact on metabolic control or other diabetes-related variables despite marked success in reducing depression in people with diabetes [78,79].
Interactions between acute metabolic complications and psychologic distress
Acute complications of diabetes, particularly recurrent diabetic ketoacidosis (DKA), may also be predicted in children from indicators of poor psychologic functioning in the first year following diagnosis [80]. This is particularly true in girls, for whom more behavior problems and lower levels of social competence were associated with higher rates of DKA. Family dysfunction in the first year following diagnosis was also predictive, with higher levels of family conflict and lower levels of family cohesion associated with more recurrent DKA. Again, this relationship is limited to girls and is independent of variations in HbA1c values. Boys showed relatively low rates of recurrent DKA (6% vs 20%) but very high rates of recurrent hypoglycemia (22.6% vs 3%); the latter were unrelated to psychosocial variables shortly after diagnosis. Even after several years of diabetes, family functioning processes can have a major impact on risk for DKA. Adolescents who reported their parents expressed more negativity to their diabetes regimen had a greater risk of experiencing DKA [81]. It is likely that the link between early behavior problems and DKA in girls is mediated by psychosocial factors, such as poor adherence [82], although definitive evidence for that possibility is currently lacking.
Diabetes treatment-induced fears and phobias
Phobic disorders are twice as common in adults with diabetes than the general population [46]. Earlier work failed to explore the possible reasons for that difference, but an increasing body of research has identified injection or blood and injury phobia, and fear of hypoglycemia, as two sequelae of insulin treatment for diabetes [83]. For example, Berlin et al. [84] studied more than 100 adults with T1DM and not only found that 94% of their sample reported at least one phobic symptom, but that patients with poorer glycemic control had more symptoms of fear of blood or injury than did those with better control. Those individuals also measured their blood glucose less frequently and endorsed more symptoms of anxiety and depression. Statistical modeling techniques support the possibility that the association between poorer metabolic control and lower rates of daily blood glucose monitoring is mediated by patients’ fear of blood and injury. The prevalence of this phobia remains controversial, with estimates ranging from approximately 1.3% [85] to approximately 25% [86], depending on the questionnaire used to ascertain injection phobia and the criterion used operationally to define the severity of the disorder.
Fear of hypoglycemia is also common in children [87] and adults [88,89] with diabetes, as well as in spouses [90] and parents [91]. The development of hypoglycemic fear, and the corresponding effort to avoid any situation that may lead to a recurrence of a hypoglycemic event, is not at all surprising. Acute hypoglycemic episodes are uncomfortable and unpredictable. They are accompanied by autonomic arousal characterized by aversive symptoms such as trembling, sweating, light-headedness, pounding heart, nervousness [92], feelings of anger and “tensetiredness” [93,94] and worries that this episode could lead to a seizure, coma or death if not treated promptly.
Individuals who experienced recurrent hypoglycemia [88], or even a single episode of severe hypoglycemia when accompanied by seizure or coma [95]. have higher hypoglycemic fear scores, although this is likely to be a consequence of several factors, including pre-existing personality traits, particularly neuroticism [89] or trait anxiety [96], and current level of psychologic distress [88]. In addition to being associated with higher levels of generalized psychologic distress, fear of hypoglycemia may lead patients with diabetes, and the parents of pediatric patients, to avoid hypoglycemia by treating falling blood glucose levels prematurely and hence maintain ambient blood glucose at higher values than desirable [97]. Programs that teach insulin-treated patients to recognize and anticipate blood glucose fluctuations have also been successful in reducing fear of hypoglycemia [98]. One might expect that fear of microvascular and macrovascular complications would also influence the self-management of the diabetes by the patient, but there has been little formal research on this topic. The recent development of a psychometrically sound “fear of complications” scale is an important first step [99].
Quality of life
Psychologic distress has so far been the primary focus of this discussion, but the extent to which diabetes affects the individual’s perceived quality of life is also crucially important. Defining and measuring quality of life remains controversial, although it is generally agreed that this concept should include an understanding of how health-related variables affect physical, social and mental functioning as well as the individual’s overall feelings of well-being and satisfaction with life [100].
General health-related quality of life
Large-scale studies of groups of individuals with various chronic illnesses have typically found little evidence that quality of life is differentially disrupted in adults with diabetes. Patients with diabetes do not differ from those with arthritis, renal disease, dermatologic disorders or from the general population on measures of anxiety, depression, positive affect, emotional ties, loss of control or overall mental health [101]. When health-related quality of life was assessed in a large cohort of adults with diabetes with the Medical Outcome Study (MOS-36) questionnaire, patients with diabetes reported more problems in physical and social functioning than patients without chronic conditions, but tended to function better than patients with cardiovascular, pulmonary or gastrointestinal disorders [102].
For individuals with T1DM, poorer health-related quality of life is associated with being older, having biomedical complications, being female, being less physically active and having a lower income [103]. Even the presence of a single biomedical complication can have a measurable impact on quality of life, and as the number of complications increase, there is a corresponding decline in quality of life as assessed by virtually all MOS-36 scales [103,104]. Chronic hyperglycemia, as indexed by higher HbA1c levels, is also associated with poorer general health, even after the presence of complications is taken into account statistically [105]. Recurrent hypoglycemia, defined as one or more episodes a month, also predicts poorer health-related quality of life, particularly on measures of mental health and social function [103] and physical health [105,106]. Many of these same variables are associated with poorer health-related quality of life in older adults with T2DM. Among the best predictors are the presence of diabetes-related complications [107], certain demographic characteristics (female, poorly educated, lower income) and lower levels of physical activity [105,108]. The relationship between metabolic control and health-related quality of life remains controversial, with some [105], but not all [109] studies finding statistically reliable correlations.
Diabetes-specific quality of life
The use of diabetes-specific quality of life measures leads to similar conclusions [104]. The Diabetes Quality of Life (DQOL) measure, first developed to assess changes in quality of life during the course of the DCCT, explicitly examines factors such as satisfaction, impact of diabetes, social/vocational worry and diabetes-related worry [110]. When evaluated with either a generic pediatric quality of life measure [111] or with disease-specific questions, adolescents reported a relatively good quality of life which was only moderately affected by their diabetes, yet there was a great deal of inter-individual variation in response [112]. Differences in metabolic control or other biomedical or psycho-social factors may be responsible for this variability, although there is little consistency across studies. For example, improved metabolic control has sometimes [113], but not invariably [112], been found to be associated with better diabetes quality of life in adolescents.
Healthy adults with T1DM also report being satisfied overall with life, and indicate that diabetes has had little impact on their lives [114]. These relationships occur regardless of type of treatment (conventional or intensive insulin therapy) [31], or type of diabetes-associated quality of life measures employed [115]. Despite the demands made on individuals treated with intensive therapy, there is no evidence that treatment over a long period of time (6–9 years) adversely affects quality of life [31]; in fact, over a short-term (4 month) period, it is associated with improvements in diabetes quality of life [116]. The relatively benign experience of intensively treated patients participating in the DCCT may be a consequence of the greater level of psychologic and medical support that is provided to the participants in such clinical trials [41] or, alternately, may reflect the relative insensitivity of a measure like the DQOL to small changes in quality of life over time [100]. More recent studies evaluating changes in quality of life associated with the use of continuous subcutaneous insulin infusion have been mixed, with some indicating no benefit, while others suggesting modest benefit [117], particularly amongst children and their parents [118].
Age at onset of diabetes may also affect certain aspects of life quality. One survey of marital satisfaction found that adults diagnosed with T1DM before 9 years of age were more satisfied with their marriage, and were more likely to have children than those diagnosed later [119]. Those authors suggest that individuals diagnosed earlier in development may be more adept at integrating the disease as part of their lifestyle and thus find less disruption from diabetes later in life. More recent work also suggests that the linkages between marital satisfaction, higher levels of diabetes-related satisfaction and better metabolic control may reflect better psychosocial adaptation to a variety of illness-related and marital role stresses and strains [120].
Diabetes-specific quality of life measures have been used less frequently with older adults with T2DM, but those studies have typically obtained results that do not vary appreciably from those noted in patients with T1DM [104]. When quality of life is operationally defined by measures of mood, cognitive mistakes or work symptoms, most adults with T2DM report little disruption in most areas of life, although an appreciable minority (27%) report loss of enjoyment from previously valued activities, particularly social eating and drinking [121]. Worse quality of life seems to be especially evident in patients with T2DM treated with insulin [122], although several studies, including the UK Prospective Diabetes Study, have found no effect of intensive therapy regimens on quality of life [123].
Interventions to reduce psychologic distress
Traditional psychotherapy
Individual or group psychotherapy or supportive counseling should be as effective in children or adults with diabetes as in individuals without diabetes but with similarly high levels of psychologic distress [124]. Many recommendations have been made for such interventions [16,125]. and while there are now a large number of studies that have made use of these, data from several recent meta-analyses have generally found them to have, at best, modest effects in reducing psychologic distress and in improving metabolic control [126–128].
Traditional individualized and group therapy has been used to provide emotional support for both children and adults with diabetes, and may be particularly beneficial for patients who are confronting the development of complications such as blindness [129,130]. Studies of individual psychotherapeutic interventions are rare, but data from a single, very small randomized trial are quite promising. When a time-l imited problem-oriented individualized treatment was compared with standard insulin treatment counseling in adults with T1DM, patients receiving the psychotherapeutic treatment showed greater reductions in both problem severity and in HbA1c values than those receiving conventional diabetes care [131].
Group therapy programs, which are far more common, have similarly shown either very small positive effects or no reduction in distress. Such groups are typically led by mental health professionals, have 4–12 participants, meet weekly or fortnightly for 90 minutes; they may run for as long as a year [132], although interventions as brief as 7 weeks have been conducted [129]. A major focus for such groups, at least early on, is to provide participants with more medical information about diabetes [130,132]. Over time, participants become more comfortable in discussing personal concerns, diabetes-related and otherwise. The broad range of topics discussed may include coming to terms with unfinished grieving over diabetes-associated problems, dealing with guilt that they may have caused their own complications and coping with fears about loss of independence [133]. As with individual psychotherapy, the efficacy of formal group therapy, in terms of improved mood or metabolic control, has not been studied extensively in large, carefully designed studies [134] and the quality of the current research is considered “weak” from a meth-odologic perspective [128]. Anecdotal reports suggest that many participants leave the group happier, or better adjusted, and various “curative factors” have been identified by patients as being a major benefit of group therapy, including interpersonal learning, the experience of catharsis, development of insight into problems and an understanding that the individual is not alone in experiencing disease-related psychologic distress [135,136], but analyses of outcomes from formal clinical trials with children and adults with diabetes have been less sanguine [126,128,137].
Cognitive-behavioral therapy
Of the different psychotherapeutic modalities available, cognitive–behavioral therapy (CBT) appears to be particularly promising in reducing the severity of depressive symptomatology. CBT teaches patients to use problem-solving strategies to reduce stressful situations and trains them to use cognitive techniques to “think away” their distorted beliefs, and replace them with more accurate and adaptive thoughts. Comparing 10 weeks of individualized CBT with non-therapeutic diabetes education (control condition), Lustman et al. [138] found that 85% of patients with T2DM receiving CBT achieved remission of depressive symptoms, in contrast to 27% in the control condition; at 6-month follow-up, similar rates of remission were seen (70% vs 33%). Individuals who are most likely to benefit from CBT are those with fewer complications and/or better compliance with blood glucose monitoring [139]. Variations of this approach have been used in studies of adults with T1DM, and while there were marked reductions in diabetes-related distress and in symptoms of depression, they were no differences between those subjects treated with group CBT and those in an active comparator condition who were treated with blood glucose awareness training. In neither group were there meaningful changes in glycemic control at 6 or 12 months after therapy initiation, but both were equally effective in lowering depressive symptoms to a modest degree [140]. The fact that blood glucose awareness training, which has a minimal psychologic component, is effective in reducing psychologic distress is quite surprising, and emphasizes the potential effects that simple training in diabetes management may have on an individual’s level of psychologic distress.
Pharmacotherapy
Diabetes-related depression and anxiety disorders have also been treated successfully with pharmacotherapy [141,142]. Placebo-controlled studies have demonstrated that SSRIs such as fluoxetine [143] and sertraline [77], and tricyclic antidepressants such as nortriptyline [144] effectively reduce severity of depression and its subsequent recurrence in patients with diabetes, whereas the benzodiazepine alprazolam [145] effectively reduces anxiety. These different psychoactive drug classes differentially affect metabolic control. Nortriptyline produces a sustained increase in HbA1c values; in contrast, both fluoxetine and alprazolam reduce HbA1c values significantly. The physiologic basis for these differential effects remains unknown, but most experts believe that pharmcotherapy-induced hyperglycemia can be handled readily with appropriate adjustments to the diabetes management regimen [146].
Pharmacotherapy alone, or paired with a form of psychotherapy such as CBT, may not only improve mental health, but it may also reduce the risk of death, as recently demonstrated in the PROSPECT trial [147]. In that medical practice-based study, older depressed adults with and without diabetes were randomized to usual care or to an intervention in which trained depression-care managers monitored psychopathology, adherence to treatment regimens, patients’ responses and side effects, and provided subjects either with a first-line antidepressant medication (an SSRI) or interpersonal psychotherapy. All subjects were followed for a median of 52 months, and only mortality outcomes were reported. There was a significant reduction in all cause mortality, but only for those depressed patients with diabetes who received this simple depression management strategy, demonstrating that even minimal management of depression can have salutary effects in the health of older adults with diabetes. Given the nature of that study’s design, however, it is impossible to identify the processes underlying these effects.
Neuropsychologic impact of diabetes
Diabetes has long been thought to affect cognition, as well as emotion, but it was not until the development of clinical neuropsychology (the study of brain-behavior relationships) that researchers were able to demonstrate unequivocally that mental efficiency can be disrupted by diabetes, its complications and its management, and that this neuropsychologic dysfunction reflects changes occurring in the CNS. The magnitude of these effects is relatively modest in most individuals, and few patients with diabetes manifest cognitive changes that would be characterized as being “clinically significant” – unless they developed diabetes early in life. Until very recently, when cognitive dysfunction was found in patients with diabetes it was invariably attributed to the adverse effects of severe and/or recurrent hypoglycemia on the CNS. New research suggests that chronic hyperglycemia, and the metabolic and vascular complications that are associated with it, underlie the development of most structural and functional changes to the CNS, particularly in adults. Although hypoglycemia can never be considered to be entirely benign, it may have a relatively small role in the etiology of neurocognitive changes in patients with diabetes [148,149].
CNS sequelae of diabetes in children and adolescents
Cognitive manifestations
The nature and extent of cognitive dysfunction in children and adolescents differs depending on age of diagnosis. Those diagnosed in the first 5–7 years of life appear to have an elevated risk of manifesting a moderately severe cognitive impairment which is evident across a broad range of cognitive domains, including measures of attention, mental flexibility, psychomotor efficiency, learning, memory, problem-solving and overall intelligence [150–156]. In contrast, those diagnosed after that early “critical period” show very mild cognitive dysfunction which is limited primarily to measures of overall intelligence and to performance on speeded tasks, particularly those having a visuoperceptual component [156]. Learning, memory and problem-solving skills are largely intact in this “l ater onset” patient population, or are only very minimally [157] and inconsistently affected [158,159]. Regardless of age at diagnosis, children with diabetes also tend to achieve lower scores than their peers without diabetes on measures of academic achievement [157,160], and have somewhat poorer grades in school [161], with these latter effects especially pronounced in children with a very early onset of diabetes [162].
The magnitude of the cognitive dysfunction seen in children with diabetes tends to be quite modest, as demonstrated by a formal meta-analysis of 19 pediatric studies encompassing 1393 children with diabetes and 731 healthy comparison subjects. When effect sizes were calculated (between-group difference divided by pooled standard deviation: “Cohen’s d” [163]) for studies comparing later-onset children with control subjects, d values were approximately 0.20 or less-indicative of “small” to negligible effects. In contrast, effect sizes were more than twice as large when comparing early-onset subjects with diabetes with their peers without diabetes [156]. Using clinical rather than statistical criteria, one similarly finds marked differences between children with an early, as compared with a later, onset of diabetes. One large study found that 24% of children with an early onset of diabetes meet criteria for clinically significant impairment, as compared with only 6% of children with a later onset of diabetes, and 6% of a comparison group without diabetes [153].
This age at onset phenomenon has also been reported in adults diagnosed with diabetes early in life. Young adults who developed diabetes before 7 years of age performed more poorly on measures of information processing speed, and earned lower performance IQ scores than their peers with diabetes diagnosed at or after age 7 [164]. Abnormalities in brain structure are also evident. Magnetic resonance imaging (MRI) scans showed higher rates of mild to moderate ventricular atrophy (61% vs 20%), as well as somewhat higher rates of small punctate white matter lesions within the hippocampus (14% vs 2%). Smaller brain volumes were also correlated with poorer cognitive test performance, supporting the view that cognition dysfunction is necessarily linked to changes in CNS morphology.
Neurocognitive abnormalities appear relatively early in the course of diabetes, having been reported within 2–3 years of diagnosis. In the largest longest prospective pediatric study to date, a representative sample of 90 newly diagnosed youngsters with diabetes and 84 healthy children drawn from the community have been followed over a 12.year period. No between. group differences were evident at study entry [165] but, 2 years later, those children diagnosed before age 4 manifested developmental delays in so far as their scores on both the Wechsler Vocabulary and Block Design subtests improved less over time, relative to either children with a later diabetes onset or to community control subjects [166]. After 6 years of follow-up, children with diabetes-regardless of age at diagnosis-performed worse than their peers without diabetes on measures of intelligence, attention, processing speed, long-term memory and executive skills. Children with an early age at onset were particularly affected, and performed significantly worse on measures of attention and executive function than those with a somewhat later onset of diabetes [151]. After 12 years of follow-up, these children with diabetes-now young adults-continued to earn lower verbal and full-scale IQ scores, demonstrating that these effects are not a “developmental delay,” but reflect a true, albeit modest, loss in cognitive efficiency, relative to those without diabetes [167]. Several other reports have also noted gradual decline in IQ scores as diabetes duration increases [161,167,168].
Effects of hypoglycemic episodes on brain function
Hypoglycemia has long been considered to be the cause of these neuropsychologic deficits, particularly in children with an early onset of diabetes [153,155]. Not only are rates of severe hypoglycemia significantly higher in children younger than 5 years of age, compared with children older than 5 (48% vs 13%), but hypoglycemia is also more likely to reccur in this younger group [169]. Behavioral factors could also contribute to the high rates of hypoglycemia early in life. Falling blood glucose levels may go unrecognized and untreated because very young children cannot adequately communicate that they are developing hypoglycemic symptoms. Although that view seems quite plausible, recent large well-designed cross-sectional [164,170] and longitudinal [171,172] studies completely failed to find any relationship between recurrent episodes of hypoglycemia and cognitive impairment, whereas others have reported only very weak and inconsistent findings [159] or have data sets in which severe hypoglycemia and age at onset tend to be confounded [173]. Similarly, both animal neuropathology [174] and human neuroimaging studies [167,175,176] are consistent with that view. For example, no changes in neuronal morphology were found in very young (1 month old) rats despite recurrent bouts of experimentally induced severe hypoglycemia, whereas 2 months of insulin-controlled diabetes caused a reduction in dendritic branching and fewer dendritic spines on neurons, and this was associated with poorer performance on measures of spatial memory [174]. These findings suggest that hypoglycemia is unlikely to be sufficient to induce significant brain dysfunction in most children, at least in those diagnosed with diabetes after the age of 7 years; however, for children with an early onset of diabetes, hypoglycemia may have a contributory role in the development of brain dysfunction [154,159].
Other CNS changes associated with diabetes in childhood
In addition to cognitive dysfunction, children with diabetes manifest multiple other changes in the CNS. Slowed neural activity is a very robust phenomenon. Compared with their peers without diabetes, adolescents with diabetes in good metabolic control showed significant increases in delta and theta (slow wave) activity, significant declines in alpha peak frequency in frontal brain regions, and declines in alpha, beta and gamma fast wave activity in posterior temporal regions [177]. When electroencephalogram (EEG) recordings were evaluated clinically, one large study noted that 26% of their subjects with diabetes had abnormal EEG records, compared to only 7% of healthy controls [178]. Both earlier age of diabetes onset and episodes of severe hypoglycemia were strong predictors of abnormality in that study, as well as in several earlier studies [179]. When auditory or visual evoked potentials were recorded, children and adolescents with a 2 years or more history of diabetes showed significant neural slowing, as evidenced by increased latencies, whereas those with less than 2 years of diabetes had normal latencies [180].
Cerebral blood flow has been measured only infrequently in children with diabetes, but the one study that used single photon emission computed tomography (SPECT) found lower levels of cerebral blood flow in children with diabetes than in healthy comparison subjects [181]. The greatest reductions in brain perfusion were found in the basal ganglia and frontal regions, followed by parietal and temporal areas. This pattern is similar to that reported in adults with T1DM [182]. and provides limited support for the view that changes in brain perfusion may occur relatively early in the course of diabetes. The extent to which these cerebrovascular changes contribute to cognitive dysfunction remains to be determined.
Brain structure anomalies, documented with MRI, have only recently been reported in children with diabetes. The one study that focused exclusively on children with an early onset of diabetes noted greatly elevated rates of a very unusual brain anomalymesial temporal sclerosis. Within this cohort, 16% manifested this anomaly compared to less than 1% of the general pediatric population [175]. These anomalies apparently developed within a relatively brief period of time (mean duration of diabetes in this sample was approximately 7 years), and were unrelated to a past history of hypoglycemia. By contrast, children who experienced one or more episodes of severe hypoglycemia (seizure or coma) had smaller gray matter volumes than those with no such history (724 vs 764 cm.), regardless of whether the hypoglycemic event occurred early in life or at a somewhat later age.
A second neuroimaging study used a semi-quantitative voxel-based morphometry technique to ascertain gray and white matter volumes in 108 children with diabetes and 51 age-matched children without diabetes who were 7–17 years of age [176]. Total brain volume was comparable in the two groups, but those children with diabetes who experienced one or more episodes of severe hypoglycemia had a slight reduction in gray matter in the left (but not right) temporal occipital region. This pattern of highly circumscribed effects, localized primarily to the left hemisphere, is consistent with what has been reported in adults with a long history of childhood-onset diabetes [183]. as well as in several case reports [184]. Lifetime HbA1c values, used to estimate of chronic hyperglycemia, were associated with less cortical volume in the right posterior brain regions (particularly the right cuneus and precuneus), also replicating findings in adults with diabetes [183]. Chronic hyperglycemia was also associated with less white matter, and these effects were most pronounced in parietal brain regions.
At this point, the underlying pathophysiologic basis for the development of CNS dysfunction in children remains unresolved, because at least some studies have found relationships between neurocognitive outcomes and both hypoglycemia and chronic hyperglycemia; however, there is increasing interest in studying the many metabolic – and potentially neurotoxic – events that occur around the time of diabetes diagnosis such as changes in brain glucose during the peri-onset period, dramatic metabolic perturbations, including the occurrence of DKA, that now seem to have the potential to alter the structure of the developing brain in the child with diabetes [154,185].
Brain structure and function in adults with type 1 diabetes
Neurocognitive manifestations
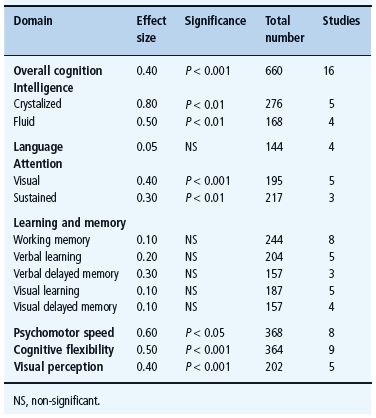
A highly circumscribed pattern of mild cognitive dysfunction characteristic of adults with T1DM has been identified from a systematic meta-analysis of data from 31 studies published in English between 1980 and 2004 that compared the performance of subjects with and without diabetes on multiple cognitive domains [186]. As illustrated in Table 49.1, subjects with diabetes, who were 18–50 years of age and in relatively good health, performed significantly more poorly on measures of intelligence, attention, psychomotor speed, cognitive flexibility and visual perception, whereas no between-group differences were found on measures of language, learning and memory. Even when differences were detected, they were modest at best, with effect sizes (d) ranging from 0.3 to 0.8 standard deviation units. It is important to note that not all cognitive domains were affected. Learning and memory skills, which are generally considered to be sensitive to early brain damage [187], were well preserved in this diverse patient population, despite an average of 20 or more years of T1DM. Moreover, with only one exception (“crystallized intelligence”), virtually all of the cognitive tasks on which patients with diabetes perform more poorly were those that also required rapid responding. That is, mental slowing appears to be the fundamental deficit associated with T1DM in adulthood [188]. A similar pattern of results has been found in adults with T1DM who are over the age of 60 [189]. Remarkably, the magnitude of the cognitive differences found in these older adults was similar (d = 0.30.5) to that reported in their younger counterparts, despite their longer duration of diabetes.
Table 49.1 Cognitive characteristics of adults with T1DM, based on a meta-analysis of published papers. Standardized effect sizes (Cohen’s d) for each cognitive domain reflect differences between subjects with and without diabetes. Adapted from Brands et al.[186].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree