- Gastrointestinal manifestations are frequent in diabetes.
- Diabetes may not be the cause of gastrointestinal symptoms.
- Glycemia influences development of neuropathy and acutely changes gastric emptying.
- Extrinsic and enteric neuropathies are key mechanisms in diabetic gastroenteropathy.
- Motor dysfunctions may affect any region of the gastrointestinal tract in diabetes and cause fast or slow transit.
- Incontinence, a frequently unvoiced symptom, may reflect sphincter or sensation deficits.
- Assessments of hydration, nutrition and metabolic status are essential.
- Measurements of regional transit and anorectal motor and sensory functions are key to management of diabetic gastroenteropathy.
- For management of gastroparesis and dyspepsia, correct hydration, nutrition and metabolic status; exclude any iatrogenic factor, such as GLP-1 analog, and “gastroparesis” and treat the pathophysiologic process.
Introduction
Although most attention has traditionally focused on the stomach, diabetes can affect the entire gastrointestinal tract. The term diabetic enteropathy refers to all the gastrointestinal complications of diabetes. Gastrointestinal involvement may be asymptomatic or manifest as symptoms (i.e. dysphagia, heartburn, nausea and vomiting, abdominal pain, constipation, diarrhea and fecal incontinence). These manifestations may affect quality of life, impair nutrition, and affect glycemic control.
Epidemiology
Studies in selected patient groups, often from tertiary referral centers, suggest that gastrointestinal symptoms are common in diabetes mellitus [1,2]; however, these studies are prone to selection and other biases, which are avoided by studies conducted among people with diabetes in the community, where the prevalence of gastrointestinal symptoms is either not different, or only slightly higher than people without diabetes. Thus, in the Rochester Diabetic Neuropathy Study, only 1% of patients had symptoms of gastroparesis and only 0.6% had nocturnal diarrhea [3]. In another study from Olmsted County, Minnesota, the prevalence of gastrointestinal symptoms (i.e. nausea and/or vomiting, dyspepsia, heartburn, irritable bowel syndrome, constipation and fecal incontinence) was not significantly different between individuals with either type 1 (T1DM) or type 2 diabetes mellitus (T2DM) and age-matched controls [4]; however, people with T2DM and men with T1DM used laxatives more frequently than in controls. Moreover, that study and a Finnish population-based study reported that people with T1DM had a lower prevalence of heartburn [5].
In contrast to these studies, a study from Australia found that the prevalence of several upper and lower gastrointestinal symptoms was higher in 423 patients with predominantly (95%) T2DM than in controls [6]. Taken together, these data suggest that gastrointestinal manifestations are not uncommon among patients with diabetes presenting for care. In the general population, however, the prevalence of gastrointestinal manifestations is not substantially higher among people with diabetes and matched controls, perhaps partly because the prevalence of gastrointestinal symptoms, mostly attributable to functional gastrointestinal disorders (e.g. irritable bowel syndrome), among people without diabetes in the community is relatively high, and approaches 20%. In Olmsted County, Minnesota, the age-adjusted incidence per 100,000 person-years of definite gastroparesis for the years 1996–2006 was 2.4 (95% confidence interval [CI] 1.2–3.8) for men and 9.8 (95% CI 7.5–12.1) for women. The age-adjusted prevalence of definite gastroparesis per 100000 persons on January 1, 2007, was 9.6 (95% CI 1.8–17.4) for men and 37.8 (95% CI 23.3–52.4) for women [7].
Diabetic gastroparesis may cause severe symptoms and result in nutritional compromise, impaired glucose control and a poor quality of life, independently of other factors such as age, tobacco use, alcohol use or type of diabetes [8]. Studies of the natural history of gastroparesis have been limited by relatively small numbers of patients, potential referral bias or short follow-up periods. The data suggest that gastric emptying and its symptoms are generally stable during 12 years of follow-up or more [9]. In a study of 86 patients with diabetes who were followed for at least 9 years, gastroparesis was not associated with mortality after adjustment for other disorders [10]. Among patients with gastroparesis, overall survival was significantly lower than the age- and sex-specific expected survival computed from the Minnesota white population [7]; the most common causes of death were cardiovascular disease (24.6%), respiratory failure (23.2%), malignancy (15.9%), chronic renal failure in 11 (15.9%), cerebrovascular accident (10.1%) and other causes(10.1%).
Pathophysiology
Gastrointestinal dysmotility in diabetes is caused by extrinsic (i.e. sympathetic and parasympathetic) neural dysfunction, hyperglycemia and hormonal disturbances. More recently, a role for intrinsic (i.e. enteric) neuronal dysfunctions, resulting from loss of excitatory and inhibitory neurons and interstitial cells of Cajal, has also been implicated [11]. Neural dysfunction has been attributed to several mechanisms (e.g. oxidative stress) described below and detailed elsewhere [12].
Normal gastrointestinal motor functions
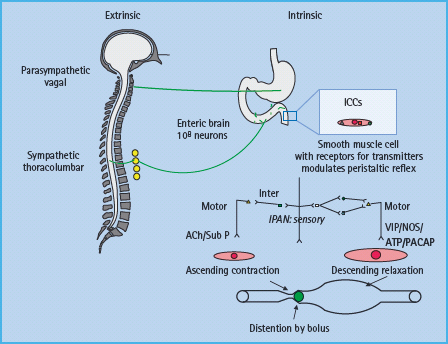
Gastrointestinal motor function is primarily controlled by the intrinsic or enteric nervous system (i.e. the “little brain” in the digestive tract) and modulated by the extrinsic (i.e. parasympa-thetic and sympathetic) nervous system (Figure 46.1) [13]. While intrinsic and extrinsic controls are independent, the prevertebral ganglia integrate afferent impulses between the gut and the central nervous system and provide additional reflex control of the abdominal viscera. The parasympathetic arm is excitatory to non-sphincteric muscle and inhibits sphincters. The sympathetic component has opposite effects. The enteric nervous system consists of 100 million neurons that are organized in distinct gangli-onated plexi including the submucous plexus, which is primarily involved in absorption and secretion, and the myenteric plexus, which regulates motility. The interstitial cells of Cajal serve as pacemakers and also convey messages from nerve to smooth muscle. As with the somatic and autonomic nerves elsewhere, the gut’s autonomic and enteric nervous system can be affected in diabetes. Derangements of the extrinsic nerves at any level may alter gastrointestinal motility and secretion [14].
Figure 46.1 Control of gastrointestinal motility. Note the extrinsic or autonomic nervous system modulates the function of the enteric nervous system, which controls smooth muscle cells through excitatory (i.e. acetylcholine [Ach], substance P [Sub P]) or inhibitory (nitric oxide [NO], vasoactive intestinal peptide [VIP], pituitary adenylate cyclase activating peptide [PACAP]) neurotransmitters. ICC, interstitial cells of Cajal; IPAN, intrinsic primary afferent neuron. Adapted from Camilleri M, Phillips SF. Disorders of small intestinal motility. Gastroenterol Clin North Am 1989; 18:405–424.
Gastrointestinal digestion and absorption require gastrointestinal motility, gastric and pancreatic secretion, and gastrointestinal hormonal release, which in turn, modulate motor, secretory and absorptive functions in the upper gut [15]. Traditionally, these processes are considered in three phases (cephalic, gastric and intestinal), which are integrated and overlap. Normally, liquids, particularly non-caloric liquids, empty rapidly from the stomach in a linear fashion. In contrast, gastric emptying of solids follows an exponential pattern. During the first 45 minute postprandial period (i.e. the lag phase), the gastric antrum grinds solids into particles smaller than 2 mm in size, so they can be emptied through the pylorus. During the lag phase, the stomach relaxes or accommodates, providing room for digestion to occur (Figures 46.2 & 46.3). Thereafter, solids are emptied in a linear fashion, with approximately 50% emptying in 2 hours and 100% emptying in 4 hours.
Figure 46.2 Assessment of gastric motor functions. Gastric accommodation can be assessed by measuring the post – prandial change in gastric volume using single photo emission computed tomography (SPECT). (top panel) The stomach wall is labeled with intravenous 99m Tc pertechnetate. Food is subsequently transferred to the antrum. Manometry (bottom panel) demonstrates that the distal antrum and pylorus contract synchronously to grind food into smaller particles. Only particles 2 mm or smaller can be emptied through the pylorus.
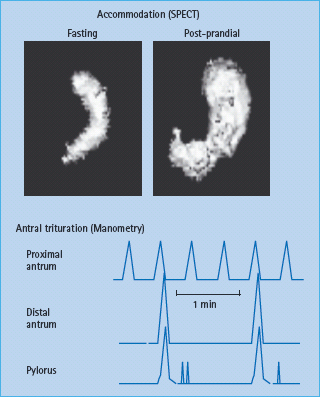
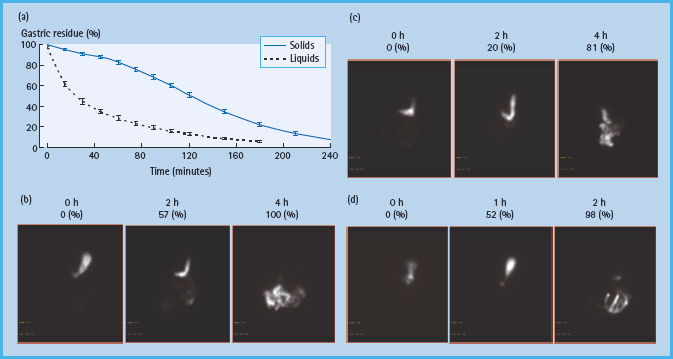
Figure 46.3 Assessment of gastric emptying by scintigraphy. Normally liquids are emptied in a linear manner while solid emptying has an exponential profi le, characterized by an initial lag phase, followed by a more rapid, linear emptying (a). The lag phase corresponds to the time required for antral trituration and gastric accommodation, during which approximately 10% of solids are emptied. (b, c, and d) Representative examples of normal, delayed and accelerated gastric emptying respectively of an egg meal, labeled with 99mTc, in patients with diabetes. At time 0 (i.e. first image in each panel), the entire meal was in the stomach. Thereafter, the normal ranges for gastric emptying are 11–39% at 1 hour, 40–76% at 2 hours and 84–98% at 4 hours.
Pancreatobiliary secretions and mechanical processes ensure small intestinal digestion, which precedes absorption. The small intestine transports solids and liquids at approximately the same rate; the head of the column of liquid chyme may reach the cecum as early as 30 minutes after ingestion. As a result of the lag phase for the transport of solids from the stomach, liquids typically arrive in the colon before solids. It takes about 150 minutes for half the solid and liquid chyme of similar caloric density (assuming solids are presented in a triturated form to the small bowel) to traverse the small bowel. Complex carbohydrates or fat in the distal small intestine exert feedback control of proximal small intestinal motility, a process known as the small intestinal brake. Chyme is transferred from the ileum to colon in intermittent boluses. On average, it takes 36 hours, with an upper limit of 65 hours, to transfer contents from the cecum to the rectum. Compared to the stomach and small intestine, colonic transit is relatively prolonged, permitting digestion of fiber and absorption of water and electrolytes to be completed.
Pathophysiology of diabetic enteropathy: insights from animal studies
In animal models, extrinsic neural dysfunction has been primarily implicated to a loss of myelinated and unmyelinated fibers without much neuronal loss [16,17]. The loss of nerve fibers is often multifocal, suggestive of ischemic injury. Within the enteric nervous system, reduced neuronal staining, and to a lesser extent neuronal loss, particularly inhibitory neurons expressing nitric oxide synthase (NOS) have been described in several animal models of diabetes [12]. In theory, this reduction in nitrergic inhibitory functions may contribute to impaired gastric accommodation and accelerated intestinal transit in diabetes. Reduced sympathetic inhibition may also contribute to accelerated intestinal transit. Because nitric oxide (NO) is a mediator of pyloric relaxation, loss of NOS may impair pyloric relaxation and thereby retard gastric emptying. Loss of intestinal cells of Cajal (ICC), documented in several animal models and case reports of diabetes, may also contribute to gut dysmotility [11,18].
Several mechanisms, including apoptosis, oxidative stress, advanced glycation end products and neuroimmune mechanisms may be responsible for neuronal loss and gut dysmotility [12]. The loss of ICC has been attributed to a reduction in heme-oxygenase (HO-1) and other protective mechanisms against hyperglycemia [11]. The effects of diabetes on neuronal morphology and functions are reversible. Insulin or pancreas transplantation improved glycemic control and the axonopathy affecting autonomic nerves in rats with diabetic autonomic neuropathy [19]. Insulin also restored expression of NOS and gastric emptying in animal models of diabetes while insulin and insulin-like growth factors prevented the loss of ICC in cultures [20,21]. Because ICC do not express receptors for either hormone, these effects are perhaps mediated by smooth muscle secretion of stem cell factor, which is the most important growth factor for ICC rather than directly by insulin and insulin-like growth factors [11]. Overexpression of glial cell line-derived neurotrophic factor, a trophic factor for enteric neurons, in transgenic mice reversed hyperglycemia-induced apoptosis of enteric neurons, improved gastric emptying and intestinal transit [22].
Pathophysiology of diabetic enteropathy in humans
Gastric dysfunctions
Neuropathy
Diabetes is associated with accelerated or delayed gastric emptying, increased and reduced gastric sensation, and impaired gastric accommodation (Figure 46.4). A vagal neuropathy can cause antral hypomotility and/or pylorospasm, which may delay gastric emptying [23]. The pathophysiology of rapid gastric emptying in diabetes is less well understood. Conceivably, impaired gastric accommodation resulting from a vagal neuropathy [24] may increase gastric pressure and thereby accelerate gastric emptying of liquids. However, the relationship between rapid gastric emptying and impaired gastric accommodation has not been substantiated. The relationship between vagal neuropathy and impaired post-prandial accommodation is unclear because accommodation may be preserved even in people with diabetes and vagal neuropathy [25], perhaps reflecting non-vagal adaptive mechanisms involving enteric neurons [26]. Some patients with diabetes and gastroparesis also have small intestinal dysmotility, more frequently characterized by reduced than by increased motility [27]. Small bowel dysmotility may also contribute to gastric stasis.
Figure 46.4 Pathophysiology of diabetes enteropathy in humans. Adapted from Camilleri M. Gastrointestinal problems in diabetes. Endocrinol Metab Clin North Am 1996; 25:361–378.
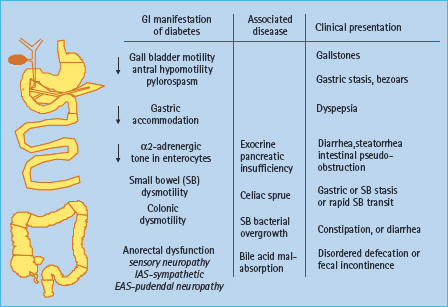
Figure 46.5 Management of diarrhea in diabetes mellitus. Adapted from Camilleri M. Gastrointestinal problems in diabetes. Endocrinol Metab Clin North Am 1996; 25:361–378.
Hyperglycemia
Acute hyperglycemia delays gastric emptying in healthy subjects and in T1DM [28–31]. These effects may be explained by hyper-glycemia-lnduced suppression of antral motility and migrating motor activity, the so-called intestinal “housekeeper” [32–34]. The effects of acute hyperglycemia on gastric emptying are modest. Indeed, even in T1DM, severe acute hyperglycemia (i.e. 16–20 mmol/L vs 4–8mmol/L) prolonged the gastric emptying half-lime by only 17 minutes from 124 to 141 minutes. Acute modulation of blood glucose within the physiologic post-prandial range (4–8 mmol/L) can also delay gastric emptying, to a lesser degree [31], Cross-sectional studies suggest that higher glycated hemoglobin concentrations are associated with a higher prevalence of gastrointestinal symptoms and slower gastric emptying among people with diabetes in the community [6,35]. While strict glycemic control improves neural, renal and retinal functions in diabetes, the impact on gastric emptying is unclear [36]. Indeed, in the only study that assessed this question, improved glycemic control did not improve gastric emptying 1 week later in 10 patients with T2DM [37]. In addition to hyperglycemia, electrolyte imbalances caused by diabetic ketoacidosis (e.g. hypokalemia) and uremia may also aggravate impaired motor function in patients with diabetes. Iatrogenic gastroparesis may result from treatment with amylin or GLP-1 analogs.
Diabetic diarrhea
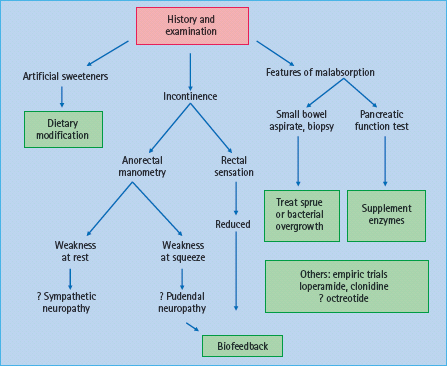
It is useful to categorize the pathophysiology of diabetic diarrhea into conditions that are associated with malabsorption and those that are not (Figure 46.5). Involvement of sympathetic fibers, which normally inhibit motility and facilitate absorption via a2-adrenergic receptors, can result in accelerated small intestinal transit and cause diarrhea [38]. Artificial sweeteners such as sorb-itol may also contribute to diarrhea. Patients with rapid ileal transit may have bile acid malabsorption [39,40] and deconju-gated bile acids induce colonic secretion.
Features suggestive of malabsorption such as anemia, macro-cytosis or steatorrhea should prompt consideration of bacterial overgrowth, small bowel mucosal disease or pancreatic insufficiency. Small intestinal dysmotility predisposes to bacterial overgrowth, which can cause bile salt deconjugation, fat malabsorption and diarrhea. Although T1DM is associated with celiac disease, most patients with celiac disease and T1DM in the community are asymptomatic [41]. Chronic pancreatic insufficiency may result from pancreatic atrophy, disruption of cholinergic entero-pancreatic reflexes, or elevated serum hormonal levels of glucagon, somatostatin and pancreatic polypeptide, which reduce pancreatic enzyme secretion [42]. Nevertheless, the association between chronic pancreatic insufficiency and diabetes is uncommon. Moreover, because there is sufficient pancreatic reserve, only 10% of pancreatic function is sufficient for normal digestion.
Fecal incontinence
Loose stools and anorectal dysfunctions contribute to fecal incontinence in diabetic diarrhea. Compared to continent people with diabetes and healthy controls, patients with diabetes and fecal incontinence have a higher threshold for rectal perception of balloon distention, a marker of reduced sensation [43,44]. A sympathetic neuropathy may impair internal anal sphincter function and anal resting pressures while a pudendal neuropathy may result in reduced anal squeeze pressure.
Constipation
The mechanisms of constipation in diabetes have not been carefully studied and are poorly understood. Clinical observations suggest that, similar to idiopathic chronic constipation, both colonic dysmotility and anorectal dysfunctions, such as impaired anal sphincteric relaxation during defecation, may contribute to constipation in diabetes [45]. Patients with colonic dysmotility have an impaired colonic contractile response to a meal and delayed colonic transit [46]. Patients with reduced rectal sensation may not perceive the desire to defecate. Compared to euglycemia, acute hyperglyemia inhibits the colonic contractile response to gastric distention and proximal colonic contraction elicited by colonic distention in healthy subjects [47]. By contrast, acute hyperglycemia did not significantly affect fasting or postprandial colonic tone, motility, compliance and sensation, or rectal compliance and sensation in healthy people [48].
In addition to these factors, it is also important to consider the role of psychologic factors in the perception of gastrointestinal symptoms. Indeed, psychosomatic symptoms are significantly associated with the reporting of gastrointestinal tract symptoms [4]. Several medications have gastrointestinal side effects; for example, metformin can cause diarrhea while among other medications, verapamil and anticholinergic agents, can cause constipation.
Clinical manifestations
Dysphagia and heartburn
Esophageal dysmotility, typically characterized by impaired peristalsis with simultaneous contractions, is common, may cause dysphagia, and may be related to cardiovascular autonomic neuropathy in diabetic mellitus [49]. The amplitude of peristaltic contractions and basal lower esophageal sphincter pressures are generally normal. Symptoms of gastroesophageal reflux are also common, particularly in patients with impaired gastric emptying who have vomiting. Rarely, recurrent vomiting may lead to Mallory–Weiss tears and bleeding.
Dysphagia and heartburn should prompt upper gastrointestinal endoscopy to exclude reflux and other incidental mucosal diseases, such as candidiasis and neoplasms. While manometry may reveal esophageal peristaltic disturbances in patients with significant dysphagia that is not explained by a structural lesion, it is unlikely to alter management, except for rare patients in whom another disorder, such as achalasia, is responsible for dysphagia. Because of the high prevalence of coronary atherosclerosis in diabetes, testing for coronary artery disease should be considered when necessary in patients with chest pain.
Dyspepsia and gastroparesis
Although gastroparesis refers to a syndrome characterized by symptoms of nausea, vomiting, early satiation after meals and impaired nutrition and objective evidence of markedly delayed gastric emptying, gastric retention may be asymptomatic [50], perhaps because of the afferent dysfunction associated with vagal denervation [51], Nausea and vomiting often occur in episodes lasting days to months or in cycles. Nausea and vomiting may be associated with impaired glycemic control and often cause hypoglycemia, perhaps because delivery of food into the small bowel for absorption is not sufficient to match the effects of exogenous insulin.
Consistent with the concept of a paralyzed stomach, the term gastroparesis should be restricted to patients with markedly delayed gastric emptying. When the delay in gastric emptying is not severe, the term diabetic dyspepsia is perhaps more appropriate. Dypepsia is characterized by one or more, generally post-prandial, upper gastrointestinal symptoms, including bloating, post-prandial fullness and upper abdominal pain. Typically, vomiting is not severe but significant weight loss secondary to reduced caloric intake is not unusual. In addition to delayed gastric emptying, impaired gastric accommodation and abnormal, either increased or decreased, gastric sensation may also contribute to symptoms in diabetes [52,53]. Nonetheless, the distinction of dyspepsia from gastroparesis is challenging because there is no official distinction between moderately and severely delayed gastric emptying. Perhaps, gastric emptying of less than 65% at 4 hours reflects a significant delay as it is often associated with nutritional consequences, the need for nutritional supplementation, jejunal feeding or gastric decompression [54].
Patients with diabetic gastroparesis frequently have long-standing T1DM with other microvascular complications, including retinopathy, nephropathy, peripheral neuropathy and other forms of autonomic dysfunction. These can present as abnormal pupillary responses, anhidrosis, gustatory sweating, orthostatic hypotension, impotence, retrograde ejaculation and dysfunction of the urinary bladder) (Table 46.1). In contrast, it has been suggested that rapid gastric emptying of liquids is a relatively early manifestation of T2DM [55–59]. Clinicians have relied on these manifestations, and on certain symptoms, such as vomiting of undigested food eaten several hours previously and weight loss, and signs (e.g. a gastric succussion splash or features of an autonomic neuropathy) to predict delayed gastric emptying in patients with diabetes who present with upper and gastrointestinal symptoms. Several studies have shown, however, that symptoms are of limited utility for predicting delayed gastric emptying in diabetes [60–66], Similarly, the type and duration of diabetes, glycated hemoglobin levels and extraintestinal complications were, in general, not useful for discriminating normal from delayed or rapid gastric emptying; however, significant weight loss and a neuropathy were risk factors for delayed and rapid gastric emptying, respectively [67].
Table 46.1 Symptoms and signs of autonomic dysfunction. Reproduced from Camilleri M. Disorders of gastrointestinal motility in neurologic disease. Mayo Clin Proc 1990; 65:825–846, with permission.
| Sympathetic | Parasympathetic |
| Failure of pupils to dilate in the dark | Fixed dilated pupils |
| Fainting, orthostatic dizziness | Lack of pupillary accommodation |
| Constant heart rate with orthostatic hypotension | Sweating during mastication of certain foods |
| Absent piloerection | Decreased gut motility |
| Absent sweating | Dry eyes and mouth |
| Impaired ejaculation | Dry vagina |
| Paralysis of dartos muscle | Impaired erection |
| Difficulty emptying urinary bladder; recurrent urinary tract infections |
In patients with upper gastrointestinal symptoms, an upper gastrointestinal endoscopy is necessary to exclude peptic ulcer disease and neoplasms, either of which can cause gastric outlet obstruction. Upper endoscopy may reveal gastric bezoars, which suggest antral hypomotility. Metabolic derangements, such as diabetic ketoacidosis or uremia, and medications, particularly opiates, calcium-channel blockers and anticholinergic agents, may contribute to dysmotility. Rarely, patients with gastroparesis present with retrosternal or epigastric pain and cardiac, biliary or pancreatic disease may be considered.
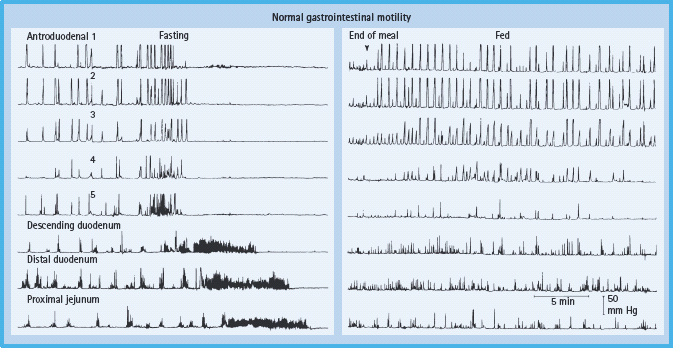
Barium X-rays of the small intestine or enterography with computed tomography should be considered only when the clinical features raise the possibility of small intestinal obstruction. Gastric emptying of solids should be quantified by scintigraphy and antroduodenal manometry should be considered in selected circumstances. Measurement of pressure profiles in the stomach and small bowel can confirm the motor disturbance and may facilitate the selection of patients for enteral feeding (Figure 46.6). Patients with selective antral hypomotility may tolerate feeding delivered directly into the small bowel while those with a more generalized motility disorder may not.
Figure 46.6 Normal manometric profile (fasting and post-prandial). The migrating motor complex characteristic of fasting state is demonstrated by the presence of quiescence (phase I), intermittent activity (phase II) and an activity front (phase III). Post-prandial profile shows high amplitude, irregular but persistent phasic pressure activity at all levels. Reproduced from Malagelada J-R, Camilleri M, Stanghellini V. Manometric Diagnosis of Gastrointestinal Motility Disorders. New York: Thieme Publishers, 1986, by permission of Mayo Foundation for Medical Education and Research. All rights reserved.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







