Summary of key facts
- •
SARS-CoV-2 was initially detected in December 2019 and is an enveloped, single-stranded RNA virus causing an extremely contagious severe respiratory illness with a high rate of mortality.
- •
Whole genome sequencing confirmed COVID-19 to be 79.6% similar to SARS and MERS, which belong to β-CoV genera, one of four identified coronavirus genera.
- •
SARS-CoV-2 is a respiratory virus and spreads mainly through person-to-person contact and inhalation of microdroplets and aerosols containing viral particles.
- •
To establish infection, the COVID-19 virus must target and enter cells to undertake viral replication. It does so through the virus S1 subunit of the spike protein, which binds to the human angiotensin-converting enzyme-2 (ACE2) receptor on host cells.
- •
Tηε World Health Organization declared SARS-CoV-2 (COVID-19) a global pandemic on March 11, 2020.
- •
Health care systems were rapidly overwhelmed with critically ill patients with COVID-19, causing significant disruptions in delivery of active cancer therapy and cancer screening.
- •
mRNA expression of ACE2 is upregulated in renal cell cancer, gastrointestinal cancer, and lung adenocarcinoma. ACE2 upregulation also correlates with increased PD-L1 expression.
- •
The promotor of ACE2 expression is hypomethylated during COVID-19 infection.
- •
Initial COVID-19 innate immune response occurs by germ-line encoded pattern recognition receptors (PRRs) and their recognition of viral PAMPs and infected host cell DAMPs (cDAMPs and iDAMPs). PRRs involve the Toll-like receptors, especially TLR-7, in innate system immunity.
- •
A weakened innate immune system response may be observed in individuals of advanced age, with obesity, and with associated chronic diseases including cancer.
- •
Patients with cancer have been highly vulnerable to COVID-19 infection, especially patients with hematologic malignancies, lung cancer, and patients receiving B-cell targeted therapy.
- •
Excessive and prolonged innate immune system response with secretion of cytokines in response to COVID-19 infection may cause organ damage (especially lung) in patients already responding to cancer and cancer immune therapies. High levels of cytokines correlate with greater COVID-19 morbidity and mortality.
- •
Increasing evidence supports infection-induced genetic alterations generating a form of innate response immunity/memory to infecting agents.
- •
Innate immune response goals are to (1) eliminate COVID-19 virus replication, (2) establish a proinflammatory response to kill infected cells, and (3) rapidly prime the antigen-specific adaptive immune response.
- •
Adaptive system goals are (1) generation of antigen-specific B-cell neutralizing antibodies, (2) expansion of antigen-specific cytotoxic CD8 + T cells to eliminate virus infected cells, and 3) expansion of activated CD4 + subpopulations and robust lymphocyte-based immune memory.
- •
The response of T lymphocytes is critical to surviving the COVID-19 acute infection and generating a robust vaccination response.
- •
Frequent reverse transcription polymerase chain reaction (RT-PCR) testing and timing of the use of vaccination are key to safely managing the patient with cancer during the COVID-19 pandemic.
- •
Evidence is accumulating that immune checkpoint inhibitor therapy does not predict a worse course or outcome if such patients become COVID-19 positive. Combination immunotherapy appears safe with careful patient selection and monitoring for the vaccinated patient with cancer.
- •
Avoiding therapy-induced lymphopenia (anti-CD20 therapy and chemotherapy are examples of lymphopenia inducing anti-cancer therapies) should be the goal by carefully managing therapy to minimize infection risk.
Introduction
The world of cancer care continues to be significantly affected by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2; coronavirus disease 2019 [COVID-19]) global pandemic. COVID-19 has demonstrated unprecedented transmissibility as a highly infectious RNA respiratory virus causing serious morbidity and mortality. On March 11, 2020, the World Health Organization declared COVID-19 a global pandemic. Health care systems everywhere were quickly overwhelmed as they were called upon to provide hospitalization for a sudden tremendous surge in COVID-19-infected patients. Hospitalized patients with COVID-19 often required intensive care (ICU) beds and ventilator support for severe respiratory distress. As expected, it was quickly observed that individuals with malignant disease were at a higher risk for COVID-19 infection and for having greater disease severity and mortality. The tremendous effect of the pandemic on health care systems and health care workers immediately caused disruptions to the delivery of normal cancer care, both anticancer therapy and cancer screening. Patients with cancer frequently delayed or abandoned active treatment out of fear of becoming infected if they were to actually go to their point of care. ,
Stressed hospitals were forced to delay surgical procedures. Cancer clinical trials were severally limited or even placed on hold as staff had to be shifted to COVID-19 patient care and research support staff was transferred to remote work. At major academic centers, clinical investigators were called upon to urgently shift their focus to COVID-19 research. At Johns Hopkins, a special COVID-19 Institutional Review Board (IRB) was established and throughout the remainder of 2020 and 2021 met every day of the week to expedite proposed COVID-19 research protocol reviews. By the end of 2020, there was a glimmer of hope, as safety and efficacy data generated by large-scale clinical trials of several candidate vaccines received U.S. Food and Drug Administration (FDA) review and emergency use authorization (EUA) in the United States as well as review and similar approval in other countries.
Over the months since the introduction of the vaccines and priority access to vaccination for patients with cancer, cancer care, cancer research, and cancer screening have gradually begun to return to prepandemic status. Even with the current much lower incidence of infections in the population, it will take some time to completely understand the true disruption that COVID-19 has had on the incidence and mortality caused by cancer. Though the vaccines have had a major effect, it is important to recognize that the pandemic continues. On March 10, 2022, the Johns Hopkins Coronavirus Resource Center website reported that the number of global COVID-19 documented cases for the previous 28 days was 48,132,252, with 242,345 deaths. In the United States the 28-day total cases were 2,107,247 and there were 49,440 deaths. The total number of individuals documented as dying from COVID-19 in the United States since the beginning of the outbreak had reached 965,069. Many believe this to be a significant underestimation.
During the 2 years since January 2020, much has been learned about this specific coronavirus, and much knowledge has been gained regarding the public health management of a serious viral pandemic. Though there exists considerable knowledge regarding the innate and adaptive immune responses and the heterogeneity of those responses to tumors of different organ sites and histologies, there is still much to learn regarding the effect of COVID-19 infection on the existing anticancer immune response and on the timing of cancer therapies, especially immunotherapy. Such knowledge regarding COVID-19’s effect on cancer immune therapies is aided to some degree by previous experience in many patients with cancer harboring other chronic infections such as human immunodeficiency virus (HIV), human papillomavirus (HPV), hepatitis B, and hepatitis C. This chapter will attempt to summarize what we currently know regarding COVID-19 and to do so in the context of malignancy and anticancer immune therapy.
The emergence and global effect of SARS-CoV-2
In early December 2019, there was increasing awareness regarding an outbreak of a serious and novel life-threating acute respiratory viral illness spreading in and around the city of Wuhan in the Hubei Province of China. The exact origin of this virus has been an ongoing debate, with considerable difficulty encountered by epidemiologists in exercising a full-force investigation. The early cases of the severe respiratory illness were said to be related to an open-air market located in Wuhan, China. By January 26, 2020, there were 2794 laboratory-confirmed cases resulting in 80 deaths and evidence documenting the beginnings of global spread to at least 33 individuals in 10 countries.
A complete genome sequence of isolates from hospitalized patients documented the causative agent to be an RNA coronavirus virus. The complete sequence was initially obtained by scientists at the Wuhan Institute of Virology, Center for Biosafety Mega-Science, Chinese Academy of Sciences, Wuhan, China. The initial sequence and subsequent analysis was based on specimens obtained from seven severely ill hospitalized patients in Wuhan and showed that the novel disease-causing virus was 96% identical to a bat coronavirus and shared 79.6% sequence identity with viruses known to cause SARS. , Coronaviruses have previously been determined to cause SARS and Middle East respiratory syndrome (MERS).
Spread of the COVID-19 respiratory virus is mainly through person-to-person contact and the inhalation of microdroplets and aerosols containing viral particles. This occurs when an infected person coughs, sneezes, or simply talks during direct person-to-person contact. Less common modes of spread include a fecal–oral route, leading to identification of the virus in site-specific sewage and in wastewater at municipal treatment plants. There has been evidence in a small percentage of cases of vertical maternal transmission to the newborn especially occurring during late stages of pregnancy.
SARS-CoV-2 causes nasopharyngeal and lower respiratory tract infections. Most infections appear to be mild, and though it is difficult to be certain, somewhere between 20% and 40% of those infected are asymptomatic or only mildly symptomatic. However, for a great number of patients the disease is much more severe, leading to hospitalization and acute respiratory distress syndrome (ARDS). Patients requiring hospitalization can present with a severe interstitial pneumonia, sometimes further complicated by systemic inflammation, thromboembolic events, evidence of cardiac complications, and massive cytokine release. , , Currently recognized risks for severe disease requiring hospitalization include lack of complete vaccination and medical comorbidities such as immunodeficiency, obesity, cardiopulmonary diseases, and cancer. During the beginning of the pandemic, the risk of COVID-19 infection was estimated to be seven times greater for the patient with cancer and even greater for the non-White population of patients with cancer. ,
COVID-19 rapidly became a pandemic, putting extreme pressure on health care workers and the infrastructure of health care systems. Despite well-proven public health measures required to manage a global pandemic caused by a rapidly spreading respiratory virus, the attempted implementation of these measures in the United States became a major political challenge. This unfortunate turn of events and the lack of our medical system’s preparedness for such an occurrence will certainly be the subject of debate for many years to come. At the time of this writing, we are improving from the latest wave of COVID-19, Omicron BA.1, but by no means are we able to declare that the pandemic is behind us as Omicron BA.2 begins to spread in the United States ( https://coronavirus.jhu.edu/map.html , accessed March 10, 2022). We must continue to be vigilant, increase the rate of maximum vaccination, increase the ease and availability of testing, and, above all, significantly enhance our genetic surveillance searching for new viral variants. One important lesson to be learned from our experience attempting to manage this global pandemic is the tremendous need for public education regarding the very basics of infectious disease and the methodologies of sound public health to minimize the effect of future pandemics.
The vast global and extremely rapid spread of COVID-19 provides significant opportunities for the occurrence of viral mutations as the virus replicates within the host’s cells, producing new variants of the virus. The world has already experienced the challenges of several of these new variants of the original COVID-19, namely, Delta and Omicron BA.1 and BA.2. , The U.S. government through the Centers for Disease Control and Prevention (CDC) has significantly increased its approach to population sampling and virus sequencing in an effort to stay ahead of the development of new COVID-19 variants circulating in the population. The CDC has established a classification process for new COVID-19 variants that places newly identified viral variants into one of three groups: variants of interest, variants of concern, and variants of high consequence (for in-depth review, see Xu et al. ).
SARS-CoV-2 structure and biology
SARS-CoV-2 (COVID-19) is an enveloped, single-stranded RNA virus and has proved to cause an extremely infectious and severe respiratory illness with clinical features of fever, cough, dyspnea, malaise, severe rapidly progressing interstitial pneumonia, and acute respiratory distress syndrome (ARDS). SARS-CoV, MERS-CoV, and COVID-19 all belong to the β-CoV genera, one of the four described Coronaviridae genera. , Currently the β-CoV subgroup is recognized as having the highest human mortality rates among the four coronavirus genera. , ,
Structurally, COVID-19 is composed of four major proteins: the spike protein (S); the nucleocapsid protein (N); the membrane protein (M), which has a short N-terminal ectodomain with a cytoplasmic tail; and the hydrophobic envelope protein (E). , The nucleocapsid protein (N) is complexed with the genomic RNA to form a helical capsid. The spike or S protein is a type 1 glycoprotein that forms peplomers on the virus surface. The RNA virus has several open reading frames (ORFs) encoding accessory proteins ( Fig. 13.1 ).
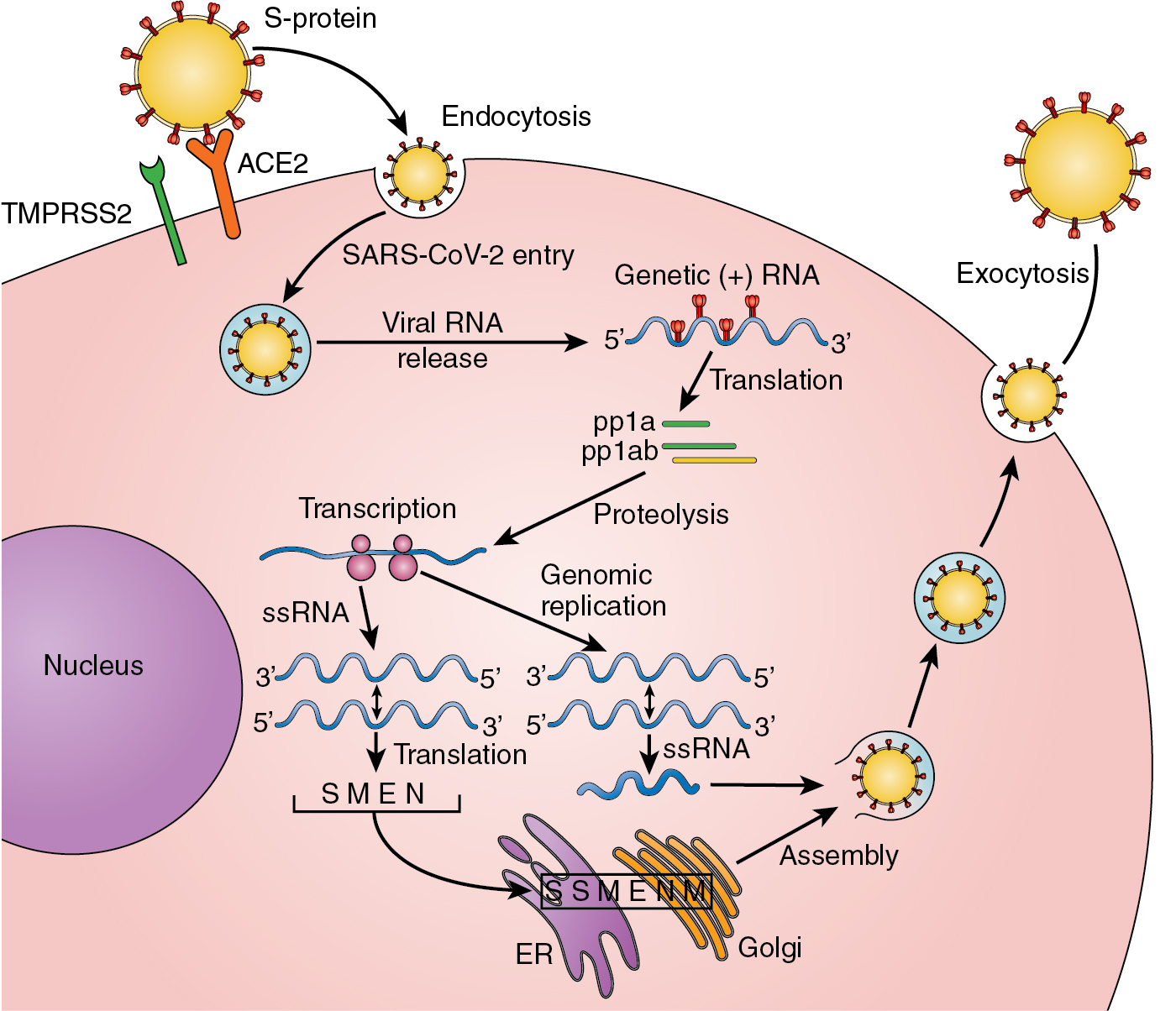
The respiratory tract is the primary site for viral particle entry in humans. The main transmission of the virus occurs in the form of COVID-19 encapsulated virus particle aerosols and microdroplets released by infected hosts. For the virus to survive and replicate, it must enter into host cells. , This occurs via the S1 subunit of the spike protein on the virus surface that recognizes and binds to the human angiotensin-converting enzyme-2 (hACE-2) receptor expressed on respiratory tract cells, vascular endothelium, cardiovascular tissue, renal tissues, and intestinal epithelium. , , The normal function of ACE2 is to convert angiotensin II to angiotensin-(1-7). A serine protease, TMPRSS2, is also involved and acts to prime the S protein for ACE2 binding and host cell entry.
The host cell entry process is assisted by other proteins including neurophilin-1, heparin sulfate proteoglycans, and C-type lectins. In the infected cell cytoplasm, the viral RNA is translated into two polyproteins pp1a and pp1ab and 16 nonstructural proteins that function to form the viral replication–transcription complex generating an antisense negative-strand template of the viral RNA. Double-membrane vesicles formed from membranes of the endoplasmic reticulum (ER) and the Golgi compartmentalize and isolate within the cytoplasm the process of viral replication. The assembly of new viral particles within the ER–Golgi compartment generates virion-containing vesicles that fuse with the host cell plasma membrane for exocytosis and release of the virus into the extracellular space. , From these several steps in the pathologic process of viral infection and replication, there are numerous opportunities for the virus to be recognized as nonself, to initiate a host inflammatory response, and to first activate the innate immune response, followed quickly by the more critical adaptive immune system response ( Fig. 13.2 ) (for in-depth review, see Diamond and Kanneganti ).
The angiotensin-converting enzyme 2, SARS-CoV-2 infection, and cancer
Angiotensin-converting enzyme 2 (ACE2) is the cell surface receptor required for SARS-CoV-2 entrance into the host cells. , , As expected, studies quantitating the expression of ACE2 in various tissues correlate both the risk of becoming infected and the severity of the viral infection. , , Ren and colleagues reported studies early in the pandemic (March 2020) focused on determining the level of ACE2 expression in normal patient tissues and in tissues of patients with cancer. Their studies using primarily the Gene Expression Profiling Interactive Analysis (GEPIA) database and ONCOMINE to compare mRNA expression of ACE2 in tissues found that the kidneys, duodenum, intestine, gallbladder, and testis had the highest level of ACE2 expression. The colon, rectum, and seminal vesicles displayed a moderate level of expression, with the lungs having the lowest expression.
ACE2 is recognized to have a significant role in cancer prognosis, demonstrating a protective anticancer effect. In these studies, ACE2 expression was upregulated in renal cancer, gastrointestinal tumors, and lung cancer. Other studies of malignant tumors and cancer cell lines suggest that essentially all cancer tissues can express ACE2. Studies indicated that ACE2 is overexpressed in colon adenocarcinoma, renal papillary cell carcinoma, pancreatic adenocarcinoma, rectal adenocarcinoma, gastric adenocarcinoma, and lung adenocarcinoma. , , ,
In patients with cancer infected with COVID-19, there is evidence that the promotor for ACE2 expression is hypomethylated but not to the degree that it is in non-COVID-19-infected patients with cancer. , Studies documenting the importance of the level of ACE2 expression in patients with cancer demonstrate that upregulation of ACE2 in multiple cancer types is associated with suppression of multiple oncogenic pathways, including cell cycle proteins, vascular endothelial growth factor (VEGF), transforming growth factor beta (TGF-β), and the Wnt and Notch signaling pathways. Increased levels of ACE2 in cancer are also directly correlated with enhancement of an antitumor immune response. Taken together, ACE2 upregulation results in enhanced disease prognosis. More specific to the use of immunotherapies in cancer, ACE2 expression has been shown to directly correlate with expression of programmed death ligand 1 (PD-L1) in patients with cancer. Tumors express PD-L1 to varying degrees, which binds to T cells, causing immunosuppression of the antitumor immune response and tumor progression.
SARS-CoV-2 viral infection and innate immunity
The infection and host inflammatory response is initiated by the binding of the COVID-19 S1 glycoprotein to the ACE2 receptor on the target cell membrane followed by entry of a viral RNA genome into the target cell cytoplasm. Studies show that the infected cells, shortly after viral entry, stimulate local inflammatory cells to secrete interleukin (IL)-8, which functions as a chemoattractant for monocytes and macrophages, dendritic cells, neutrophils, and T lymphocytes. These form the first stage of inflammation initiating the innate immune response to COVID-19 infection.
Host cell–based sensors may detect the COVID-19 virion during this initial ACE2 binding process. This recognition occurs through pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPS) that occur within the inflammatory cells responding to the invading virus. PAMPs are evolutionarily highly conserved pathogen-specific structures on the invading COVID-19 virus. DAMPs (sometimes termed alarmins ) are self molecules found in tissue macrophages and monocyte-derived macrophages under conditions of cell stress and cell damage as occurs with viral infection–induced inflammation. DAMPs are a heterogeneous group of host inflammatory cell–derived molecules and have been classed as continuous DAMPs (cDAMPs) and inducible DAMPs (iDAMPs).
The host has a limited but quite effective number of germ line-encoded pattern recognition receptors (PRRs). In the case of COVID-19 infection, PPRs have been shown to involve the family of Toll-like receptors (TLRs), especially TLR-7. These PPR TLRs recognize PAMPs and DAMPs that are present in the infected cell’s endosomes, resulting in secretion of a number of inflammatory cytokines. Other PRRs, such as retinoid acid–inducible gene I (RIG-I)-like receptors (RLRs), nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), C-type lectin receptors, and absent in melanoma 2 (AIM2)-like receptors also contribute to the initial virus-induced inflammatory response.
There are a number of innate immune system cells that provide the source of PRRs capable of the early recognition of the COVID-19 array of PAMPs and DAMPs, including tissue resident macrophages, monocytes, antigen-presenting dendritic cells, neutrophils, cytotoxic natural killer (NK) cells, and gamma delta T lymphocytes (γδT cells). Many of these cell types are rapidly recruited by the initial PRR’s, recognition of COVID-19 PAMPs and infected host cell DAMPs. The result is the initial release of interferon gamma (IFN-γ) and IL-8 followed by cytokines IL-1β, IL-6, TNF, IL-12, IL-18, and others along with a number of chemoattractants.
The Toll-like receptor TLR-7 is expressed on monocytes–macrophages and dendritic cells. TLR-7 activates several key signaling pathways and transcription factors such as Janus kinase/signal transducer and activator of transcription (JAK/STAT), nuclear factor κB (NF-κB), activator protein 1 (AP-1), interferon response factor 3 (IRF3), and IRF7. In the next stage, the virus and the initial inflammatory response acts to trigger an adaptive immune response involving T and B lymphocytes. It should be noted that much of the knowledge regarding the immune system’s response to SARS-CoV-2 comes from studies of prior coronaviruses SARS and MERS. , Neutrophils are rapidly recruited to sites of viral infection where extracellular traps (NETS) trap and eliminate virus and virus infected cells.
For SARS-CoV-2 to be the cause of such a destructive global pandemic, it must have ways in which it can successfully evade immune attack. One therefore can conclude that COVID-19 is very capable of evading the initial innate recognition and innate system immune response. Failure to control the early stage of COVID-19 infection and a high risk of hospitalization, serious morbidity such as ARDS, and mortality has been associated with lack of early IFN I and IFN III responses leading to an ineffective or delayed innate immune response. Elevated C-X-C motif chemokine ligand 10 (CXCL 10), IL-6, and IL-8 in patients with COVID-19 appear to be indicators of a weak or delayed innate immune response. This provides the COVID-19 virus with a damaging head start and, most important, a significant delay in the innate immune system’s priming of the adaptive anti-COVID-19 antigen-specific immune response.
A weakened innate immune system response may be observed in individuals of advanced age, those with obesity, and those with other chronic diseases such as cancer, all known to affect the integrity of the host immune response. A concern in patients with COVID-19 with a compromised immune system must be the occurrence of a prolonged and overactive innate immune response that tries to overcome the lack of a good adaptive immune response. The excessive and prolonged secretion of cytokines and chemokines cause an increased inflammatory response with organ damage, especially damage to the lung. There is increasing evidence in patient studies that the cytokine profile of COVID-19 appears unique to the virus compared with other infections and inflammatory states. The presence of high levels of cytokines does correlate with a greater COVID-19 morbidity and mortality. ,
In recent years (2010-2022) and perhaps relevant to individual susceptibility to SARS-CoV-2 infection, there has been experimental evidence supporting the presence of “innate immune memory” or “trained immunity.” This is a significant change in our understanding of the innate immune system. The concept of innate immune memory was first suggested in 2007 by experiments that observed histone modifications to be present in macrophages responding to bacterial-derived lipopolysaccharide (LPS). These findings were supported by others demonstrating that there were other stimuli of the innate immune response that also produced specific persistent changes of histone acetylation and methylation resulting in a persistent alteration of the innate immune response to those stimuli. This form of innate immune system memory involves long-term changes in gene transcription. ,
Recognizing that trained immunity in the innate immune system is a relatively new discovery raises a number of important questions regarding exactly when this memory response is positive for the host and when it might be detrimental. In addition, how does innate memory correlate with an early and/or enhanced adaptive immune response? Is there a role for trained immunity in the innate system with the initiation of cancer and cancer surveillance? These critical questions will be the subject of exciting future research.
One of the most worrisome effects of acute COVID-19 infection in the patient with cancer is an overly aggressive innate immune response to the virus inducing a massive release of a variety of cytokines, resulting in cytokine storm or, as it is more appropriately termed, cytokine release syndrome (CRS), causing added damage to the lung and other critical organ tissues. Patients with cancer undergoing new immune-stimulating anticancer therapies such as immune checkpoint inhibitors (ICIs), chimeric antigen receptor (CAR) T-cell therapies, and bispecific T-cell engagers (BiTEs) have the potential, if infected with COVID-19, of experiencing an enhanced CRS, causing an even more aggressive COVID-19 attack and severe physiologic complications secondary to an immune attack on normal tissues.
Clearly, in the majority of COVID-19 cases the initial host innate immune response is sufficient to effectively clear the COVID-19 virus, as it does so often with the many viruses we regularly encounter. There are several goals of this initial host recognition of SARS-CoV-2 infection by the cells that comprise the innate immune response. The first goal is to eliminate replication of the virus. The second is to induce production of a number of cytokines and chemoattractants to establish a proinflammatory response to eliminate infected host cells and, third, to rapidly prime the process of adaptive antigen-specific immune activation, culminating in the production of neutralizing antibodies and cytotoxic T lymphocytes (for an in-depth review, see Mogensen ).
Though this is just the early phase of research to carefully define the many aspects of the innate immune system’s response to COVID-19, there are some important messages from these initial studies and from past efforts to clarify the protective role that the innate immune system plays regarding viral infections. For example, it has become clear that the innate immune system has a great deal of actual specificity through PRRs providing the host with the ability to quickly recognize nonself. Further, there exists a much greater connectivity between the innate response and the activation and imprinting of the adaptive immune response than was previously believed. In addition, there is now increasing evidence indicating the occurrence of genetic reprogramming of the cells involved in innate immunity to generate innate response memory. This latter observation holds significant promise regarding our resistance to cancer initiation as well.
The adaptive immune response to SARS-CoV-2 infection
The adaptive immune system comprises three major cell types, B lymphocytes, CD4 + T lymphocytes, and CD8 + T lymphocytes. B lymphocytes are the producers of the anti-COVID-19 neutralizing antibodies. CD4 + T lymphocytes are responsible for evolving a number of different T-cell subpopulations including T helper cells (Th1, Th2), T regulator cells, and other T cells with a variety of functional roles, including assisting the B-cell humoral response. The acute immune response to COVID-19 rapidly stimulates naïve CD8 + T lymphocytes to undergo antigen-specific vigorous proliferation to become antigen-specific cytotoxic cells (CTLs). , These three major cell populations of the immune system provide highly restricted antigen-specific recognition of the COVID-19 virus.
In response to the COVID-19 infection, the lymphocyte populations function in an integrated and complex fashion to generate SARS-CoV-2-specific neutralizing antibodies, to generate cytotoxic T lymphocytes to kill virus-infected cells, and, importantly, to create long-lasting host immunity through a successful response to the infection and, of course, through vaccination. The level of response and the balance of each of the involved lymphocyte subpopulations in the adaptive immune response is host dependent and influenced by comorbid diseases such as cancer. A healthy adaptive immune system capable of COVID-19 antigen-specific recognition and the generation of robust immune memory is key to a successful vaccination program and protection of patients with cancer against the risk of serious COVID-19 disease , (for an in-depth review, see Sette and Crotty ).
With the priming assistance of the front-line response by the innate immune system, viral COVID-19 antigen(s) are presented in the context of major histocompatibility complex (MHC) class I and MHC class II molecules to stimulate antigen-specific humoral and cellular immunity. COVID-19 antigen presentation and cellular response occurs approximately on days 7 to 14 from the onset of COVID-19 disease. Viral antigens are presented by antigen-presenting cells (APCs) to antigen-specific B cells, which produce specific antiviral neutralizing antibodies, and to antigen-specific CD4 + and CD8 + T cells. Antigen-specific CD8 + cytotoxic T cells recognize and kill virus-infected cells. , The antigen triggering of the CD4 + T lymphocytes results in an aggressive antiviral inflammatory response and the differentiation of additional T-cell subtypes with unique supporting functions.
The B-cell antibody response occurs with the assistance of antigen recognition by T cells and includes the production of immunoglobulin (Ig) M, IgG, and IgA neutralizing antibodies that have been confirmed to recognize antigenic determinants of the spike glycoprotein and of the nucleocapsid. , Studies demonstrate that seroconversion occurs within 5 to 15 days of initial infection with 90% conversion by day 10 of infection. Observations in patients with COVID-19 indicate that the antibody response to the virus develops rapidly and primarily from naïve B cells and not from B cells with any memory to coronaviruses in general. It has been suggested that the B-cell responses in infected patients appear to be lower than expected, especially in the severely ill. Further, the presence of a demonstrable Fc antibody-dependent cytotoxic component of the immune response to the virus is fairly negligible. Severity of COVID-19 disease has been strongly correlated with the level of neutralizing antibody.
Alejo and colleagues, of the Department of Surgery, Johns Hopkins University School of Medicine, reported a study of healthy unvaccinated adults recruited between September 11, 2021, and October 8, 2021. They divided the cohort into the following three groups: (1) 295 subjects laboratory confirmed to have experienced a COVID-19 infection, (2) 275 subjects unconfirmed but who believed they had been infected, and (3) 246 subjects who never tested positive and believed they were never infected. Antibodies to SARS-CoV-2 spike protein receptor-binding domain (RBD) were detected in 99% of individuals in group 1, in 55% of group 2, and in 11% of group 3. Anti-RBD antibodies were observed after a confirmed positive COVID-19 test for up to 20 months. Though these findings are encouraging, it remains to be demonstrated how serologic testing results may correlate with actual immunity. ,
CD8 + T cells are the T-lymphocyte cell population with the major responsibility for clearance of the COVID-19 disease. They do so through their ability to directly destroy infected cells. SARS-CoV-2-specific CD8 + T cells have been detected very early in the disease and have been shown to recognize a range of COVID-19 antigens, primarily spike, nucleocapsid determinants, M-determinants, and ORF3a. , , A demonstrated strong CD8 + T-cell response correlates with a more favorable outcome to infection, which is also common for other severe viral infections such as SARS and MERS. Post-COVID-19 infection and after complete vaccination, protection from recurrent or breakthrough infection is strongly supported by the presence of memory SARS-CoV-2 CD8 + T cells.
CD4 + T cells can be considered to be the more functionally critical lymphoid cells to achieving a successful overall adaptive immune response to COVID-19. Antigen-specific CD4 + T cells, especially those specific to the spike antigenic determinants, are critically involved in supporting the generation of antigen-specific B-cell neutralizing antibodies. Antigen-specific CD4 + T cells are detectable early in the infection, and the robustness of the CD4 + T-cell response is more strongly associated with less severity of infection than the level of B-cell and CD8 + T-cell responses. In COVID-19, CD4 + T cells differentiate into Th1 cells and T-follicular helper cells (Tfh) that directly support B-cell responses. Th1 cells produce antiviral IFN-γ and a variety of antiviral cytokines. CD4 + T lymphocytes are also responsible for developing a critical memory element of the disease and from vaccination. Observations in patients infected with SARS-CoV-2 also showed that the response of the T lymphocytes is critically important to ultimately surviving the disease. Importantly, the response in T cells is critical to the development of strong protection against the disease via vaccination (for an in-depth review, see Shrotri et al. ).
COVID-19 infection in patients with cancer
As with any viral infectious disease, a robust, healthy immune system is essential to fighting the infection and to mounting a strong protective antiviral vaccine response. There are striking similarities between the host’s immune response to acute virus challenge and the response of the immune system to cancer initiation and progression. As we have seen, both challenges involve similar host cell populations and generate very similar inflammatory responses and humoral neutralizing antibody and antigen-specific T-cell immune responses.
Cancer, however, is a heterogeneous group of diseases with the resultant variability in the malignancy’s interaction with the host immune system. The antitumor immune response can be further altered by active therapeutic interventions including surgery and the effect of tissue injury, radiation with tissue damage, chemotherapy, and, more recently, immunotherapy. As a consequence, one can assume that in the patient with cancer there is immune imbalance. This immune imbalance in the patient with cancer creates a challenge to treating physicians in terms of managing the patient’s response to the added burden of an active COVID-19 infection or simply to the ever-present risk of COVID-19 infection.
Evidence accumulated during the pandemic has confirmed that T-cell immunity, in contrast to humoral immunity, is the critical component of survival from SARS-CoV-2 infection and also for long-term vaccine protection against COVID-19. Studies have proposed the importance of the balance in the roles of Th1 versus Th2 cells in fighting the invading virus and in developing long-term immunity. The balance or differences between the roles of Th1 and Th2 and their functional integrity exist in the portfolio of cytokines unique to each subtype and the potential for cytolytic activities generated by each CD4 + T-cell subtype. The balance between Th1 and Th2 response may, of course, be altered in cancer and therefore relevant to the development of a robust anti-COVID-19 immune response. A robustness in the integrity of Th1/Tc1 also appears particularly important to the development of vaccination-induced strong immunity. Perhaps in the future, being able to test for the Th1/Tc1 ratio and Th2 balance in functional T-cell integrity will be useful in optimizing the design of future antiviral vaccines.
To further expand on the relevance of the Th1/Th2 CD4 + subtypes, it is worth examining a recent extensive prospective cross-sectional analysis of several groups of healthy subjects and patients with cancer. In this study, investigators attempted to determine how the virus-specific T cell correlates of protection against COVID-19 infection are affected by the presence of cancer. They found that an imbalance between Th1/Th2 population recall responses conferred a greater susceptibility to COVID-19 in both cancer and noncancer populations. A more robust Th1/Tc1 level of immunity and their respective cytokines (IL-2/IL-5 ratio > 1) appeared to be significantly more protective against infection by COVID-19. The results of these studies suggest that any defects/weaknesses in the performance of the Th1/Tc1 response affecting the recognition of the COVID-19 S1-RBD are associated with an increased susceptibility to infection by COVID-19. Importantly, the Th1/Tc1 defect was more prominent in patients with hematologic malignancies.
As anticipated, COVID-19 infection can be especially challenging for the patient with cancer undergoing treatment, especially anticancer immunotherapies or therapies that are immunosuppressive. As it progresses, cancer generates a network of stromal cells and inflammatory immune cells. Early in the onset of the pandemic, it appeared that of COVID-19-infected patients, approximately 2% were being actively treated for cancer. , Multiple studies have reported that patients with cancer have a greater incidence of complications and mortality if they develop COVID-19 infection that is severe enough to require hospitalization. The hospitalized COVID-19-infected patient with cancer has a reported 30-day mortality rate of ~30% compared with 21% for noncancer patients. , , As a result, there is consensus regarding the high priority for vaccination of patients with cancer, including those actively receiving therapy. Exceptions to this recommendation includes patients undergoing stem cell transplantation and adoptive cell-based therapy. Vaccination of these patients should be delayed appropriately until the immune system has had adequate time for recovery.
The cancer inflammatory process consists of antitumor immune responses and protumor inflammation blocking antitumor immunity as well as exerting tumor promoting signals. The acute immune response to COVID-19 stimulates naïve CD8 + T cells to undergo vigorous proliferation and differentiation to become effective killer cells like the natural killer (NK) cells of the first line of antiviral immune defense. The innate immune system response is directed against infected cells harboring the virus and expressing specific viral antigens. These viral antigens are presented in the context of MHC class I and class II molecules to the cells of the adaptive immune system. The resultant T-cell response is also associated with the release of a number of different inflammatory cytokines and chemokines.
Upon COVID-19 activation, CD8 + T cells express increased numbers of inhibitory receptors (immune checkpoints) such as PD-1, Cytotoxic T-lymphocyte Antigen-4 (CTLA-4), and others that function to control for an excessive response to antigenic stimulation and, in doing so, avoid any damage to self tissues. Once the CD8 + T cells have accomplished clearance of the COVID-19-infected cells, the majority of these antigen-specific T cells undergo activation-induced cell death and are cleared, with a small percentage remaining as CD8 + antigen-specific memory cells. ,
For the patient with cancer infected with COVID-19, there exists the concern that their CD8 + T cells, in responding to a progressing tumor, are already undergoing a chronic intense exposure to a variety of specific tumor antigens and are being pushed to their limits of potential immune response. This raises the possibility that if infected by the COVID-19 virus, the added CD4 + and CD8 + antigen stimulation could result in a phenomenon known as T-cell exhaustion . T-cell exhaustion results in a decreased production of effector cytokines such as IL-2, decreased T-cell proliferation, impaired cytotoxicity, and decreased production of antigen-specific memory cells. ,
Patients with cancer undergoing chemotherapy and/or immunotherapy are recognized as having a weakened or altered immune response of both innate and adaptive immune systems. Cancer, more commonly a disease among those of older age (>65 years), is commonly associated with other diseases such as obesity, type 2 diabetes, and cardiopulmonary disease. These comorbidities may further compromise the integrity of the immune system. As a result, patients with cancer are at a greater risk for contracting infectious diseases, especially when faced with the severity of the current SARS-CoV-2 pandemic. , , In a report by Lee and associates comparing cohorts of patients with cancer with and without COVID-19 infection in the United Kingdom, patients with hematologic malignancies experienced a more serious COVID-19 infection (odds ratio [OR] = 1.57; 95% confidence interval [CI], 1.15–2.15; P < .0043). The in-hospital mortality for those patients recently receiving chemotherapy who became infected with COVID-19 was also increased (OR = 2.09; 95% CI, 1.09–4.08; P = .028). ,
SARS-CoV-2 viral load has been reported to be an independent predictor for in-hospital mortality in patients with cancer contracting COVID-19. Patients with documented high, midrange, or low viral load had mortality rates of 45.2%, 28%, and 12.1%, respectively ( P = .008). Jee and colleagues reported that both the severity of the hematologic malignancy in patients and the severity of the lung cancer stage in patients with lung cancer were predictive of an increase in the severity of a COVID-19 infection. An interesting observation by Kong and colleagues suggests that patients with lung adenocarcinoma were actually more at risk of contracting COVID-19 infection than patients with squamous cell lung cancer. Clearly, because the major feature of COVID-19 infection is pulmonary, patients with lung cancer are at higher risk of experiencing a more serious course of COVID-19 than patients with other solid tumor malignancies.
COVID-19 vaccination and the patient with cancer
The protection of patients with cancer from the devastating morbidities and added mortality associated with infection by SARS-CoV-2 depends on the physical protective measures of masking and upon achieving long-term B- and T-cell immune memory protection by adequate vaccination. , As the year 2020 came to a close, the FDA had reviewed the results of clinical trials and granted emergency use authorization to two lipid nanoparticle-formulated, nucleoside-modified mRNA vaccines that encoded the S1 spike glycol of SARS-CoV-2 protein. The FDA also granted emergency use authorization to a replication-deficient adenovirus type 26 vaccine (Ad26.COV2.S).
Initial clinical trials to confirm the efficacy and safety of COVID-19 vaccines, as expected, did not include patients with cancer with active disease. Studies indicate that patients with cancer do not mount the same antibody response to mRNA platform vaccines as obtained in normal healthy subjects. In Shroff et al.’s reported studies, the data indicated that most patients with cancer developed both CD4 + and CD8 + T-cell responses. Reports indicate a fairly rapid decline in the neutralizing capacity of vaccine induced antibodies against COVID-19 spike and nucleocapsid antigens, especially in the face of the development of variants of the original SARS-CoV-2 strain. It appears that COVID-19 vaccination of patients with cancer even when they are undergoing active anticancer therapy will result in some degree of detectable neutralizing antibody and T-cell response, especially the latter.
A meta-analysis that included 621 patients with cancer and 256 control noncancer patients provides a useful look at the efficacy and safety of mRNA COVID-19 vaccines in patients with cancer. The meta-analysis identified and screened 39 citations of potential relevance that were subjected to inclusion/exclusion screening. The screening resulted in six manuscripts deemed suitable for meta-analysis inclusion. The meta-analysis confirmed that the majority of patients with cancer can be expected to have a good immunologic response to mRNA anti-COVID-19 vaccinations. The seropositive rates (anti-S IgG) in 281 patients with solid tumors after two doses of an mRNA vaccine were >90% in the group of patients with cancer and 100% in the control group. The cohort of 340 reported patients in the analysis with hematologic malignances consistently had a slightly lower (~85%) seroconversion. Recent chemotherapy (<15 days), patient frailty, patients with thoracic malignancies, and patients who received highly immunosuppressive cancer treatments showed significantly lower seroconversion rates. Obviously, the numbers available for analysis in these latter groups were small and heterogeneous, but the trends appeared as expected. , Patients with hematologic malignancies are certainly candidates for closer follow-up and specific testing to determine optimal timing of booster vaccination. More longitudinal clinical studies are needed to understand the duration of vaccine protection as well as the development of high-risk variants of the virus.
All accumulated data indicate that SARS-CoV-2 vaccination is safe when given to patients with cancer even for patients known to be immunocompromised. Patients with hematologic malignancy and those patients with cancer receiving immunosuppressive systemic therapy should receive a third mRNA vaccine dose of the mRNA vaccine at least 28 days after receiving their second dose. This third dose has been shown to significantly improve the neutralizing antibody response, especially against the Omicron variants, in patients with cancer. The benefit is unfortunately less in patients with hematologic malignancy. In a prospective study, neutralizing antibodies were detected in only 19% of patients with hematologic malignancy after the second dose. The number of patients with detectable neutralizing antibodies was increased to 56% with the third dose. ,
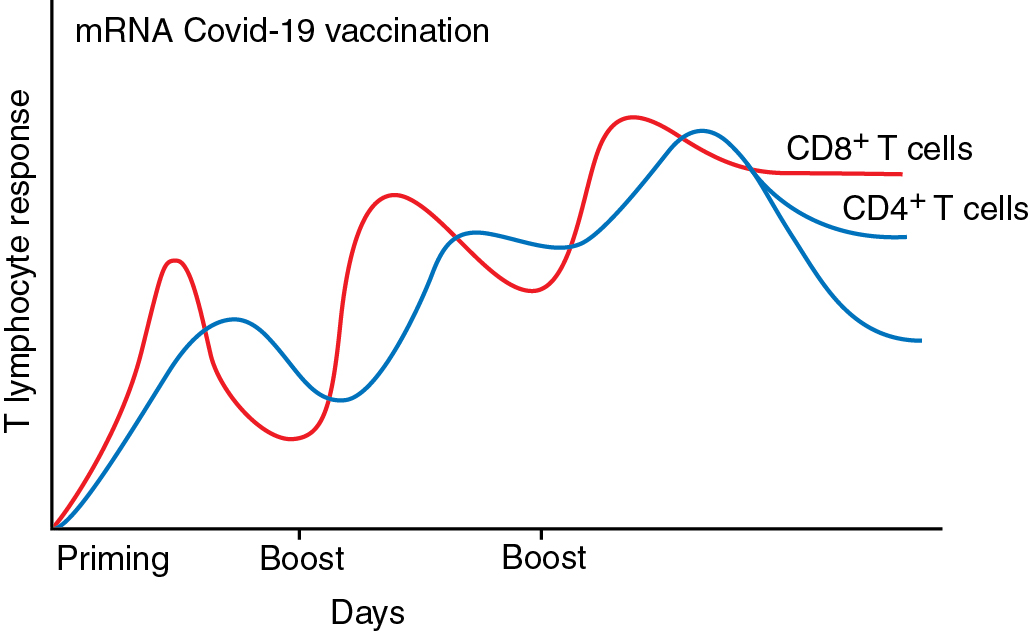
It is important to understand that the three-dose primary regimen is not equivalent to the common use of a “booster” dose for noncancer patients and nonimmunosuppressed patients. Studies involving patients with cancer support the use of a three-dose primary regimen followed by the administration of a booster immunization (a fourth dose) 6 months after the three-dose primary regimen. More studies will be needed to develop a more formal regimen for the administration of booster doses of the vaccines. The correlation between assays for B-cell immunity (enzyme-linked immunosorbent assay, ELISA) by determining levels of neutralizing antibody and by testing for T-cell function (enzyme-linked immune absorbent spot, ELISPOT) to determine the level of protection against COVID-19 remains to be demonstrated by cohort studies ( Fig. 13.3 ).