Summary of key facts
- •
Immuno-oncology (IO) is one of the fastest-growing therapeutic areas within oncology.
- •
IO agents work indirectly via the host’s adaptive and innate immune systems to recognize and eradicate tumor cells.
- •
Current FDA-approved classes of IO agents include immune checkpoint inhibitors (ICIs), chimeric antigen receptor (CAR) T-cell therapy, bispecific T-cell engager (BiTE) antibody therapy, cancer vaccine therapy, and oncolytic virus therapy.
- •
Cancer immunotherapy has made progress in several cancer types including melanoma, non-small cell lung cancer (NSCLC), renal cell carcinoma (RCC), and urothelial carcinoma; however, several cancers remain refractory to immunotherapy.
- •
CAR T-cell therapies have a promising role for the treatment of many liquid malignancies.
- •
Immune-related adverse events (irAEs) associated with ICIs may present immediately or delayed and can affect any tissue or organ. Early recognition, diagnosis, and prompt treatment are needed. Severe toxicities will generally require permanent discontinuation of the offending agent.
- •
Future directions of IO include exploration in the neoadjuvant setting, combination strategies, and optimizing patient selection through improved biomarkers.
Introduction
Conventional chemotherapy has been the cornerstone of systemic treatment for oncologic malignancies. However, advances in medicine in the late 1990s and early 21st century have led to breakthroughs in the field of immuno-oncology (IO) therapy, an oncologic subspecialty widely regarded as one of the fastest growing areas within oncology. Whereas conventional chemotherapy mounts an indiscriminate, toxic, and direct attack on both malignant and normal cells, immunotherapy agents work indirectly via the host’s adaptive and innate immune system to recognize and eradicate tumor cells. Such an approach makes cancer immunotherapy extraordinarily promising given its potential for specificity, breadth of response, memory, and durability.
Historical background
The historical origins of the clinical application of immunotherapy in the treatment of cancer dates as far back as the late 19th century. In 1863, Rudolf Virchow noted infiltration of immune cells in human tumors. Approximately 30 years later, William Coley, an American surgical oncologist, observed cancer remission in some patients who had sustained bacterial infections. Coley hypothesized that the link between the potential positive effect of infection on malignant tumors may be due to the body’s provoked immune response. This led to an experiment where a bacterial broth containing streptococcus, now known as “Coley’s toxins,” was injected into patients’ unresectable soft tissue tumors with the hope of inducing immune responses that would attack tumor cells. Though Coley was met with skepticism during his lifetime, his scientific contributions have since been appropriately acknowledged. Advances in science and in medicine from the 1970s onward have spurred the development of engineered antibodies, cytokine treatments, and vaccines. It was not until the 1990s to 2000s, however, that a major breakthrough would transform the field of IO. A crucial discovery was that T-cell immune responses are controlled through immune checkpoints that function like on/off switches via the cytotoxic T lymphocyte–associated protein 4 (CTLA-4) pathway or the programmed cell death protein 1 (PD-1)/programmed cell death protein 1 ligand (PD-L1) pathway. The characterization of immune checkpoints and their mechanisms of action would ultimately revolutionize the field and lead to the development of promising immunotherapies for previously refractory cancers.
General classes of cancer immunotherapy agents
There are five classes of U.S. Food and Drug Administration (FDA)-approved cancer immunotherapy agents that target various stages of the antitumor response and can be used for treatment: immune checkpoint inhibitors (ICIs), chimeric antigen receptor T-cell therapy (CAR-T), bispecific T-cell engager (BiTE) antibody therapy, cancer vaccine therapy, and oncolytic virus therapy.
ICIs use monoclonal antibodies to target immune checkpoints, which ultimately leads to enhanced T-cell effector function. Targets of currently approved ICIs include the CTLA-4 pathway and the PD-1/PD-L1 pathway. CTLA-4 is an immune checkpoint molecule that competes with CD28 to bind to CD80 and CD86, thereby downregulating T-cell activation. PD-1 is also a negative regulator of T-cell activity and is induced by T-cells at the time of an inflammatory response. PD-1 is expressed on a variety of immune cell types and limits immune response and T-cell activity in peripheral tissues. PD-L1 and PD-L2 are important mediators of the PD-1 pathway. ,
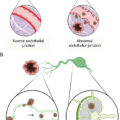
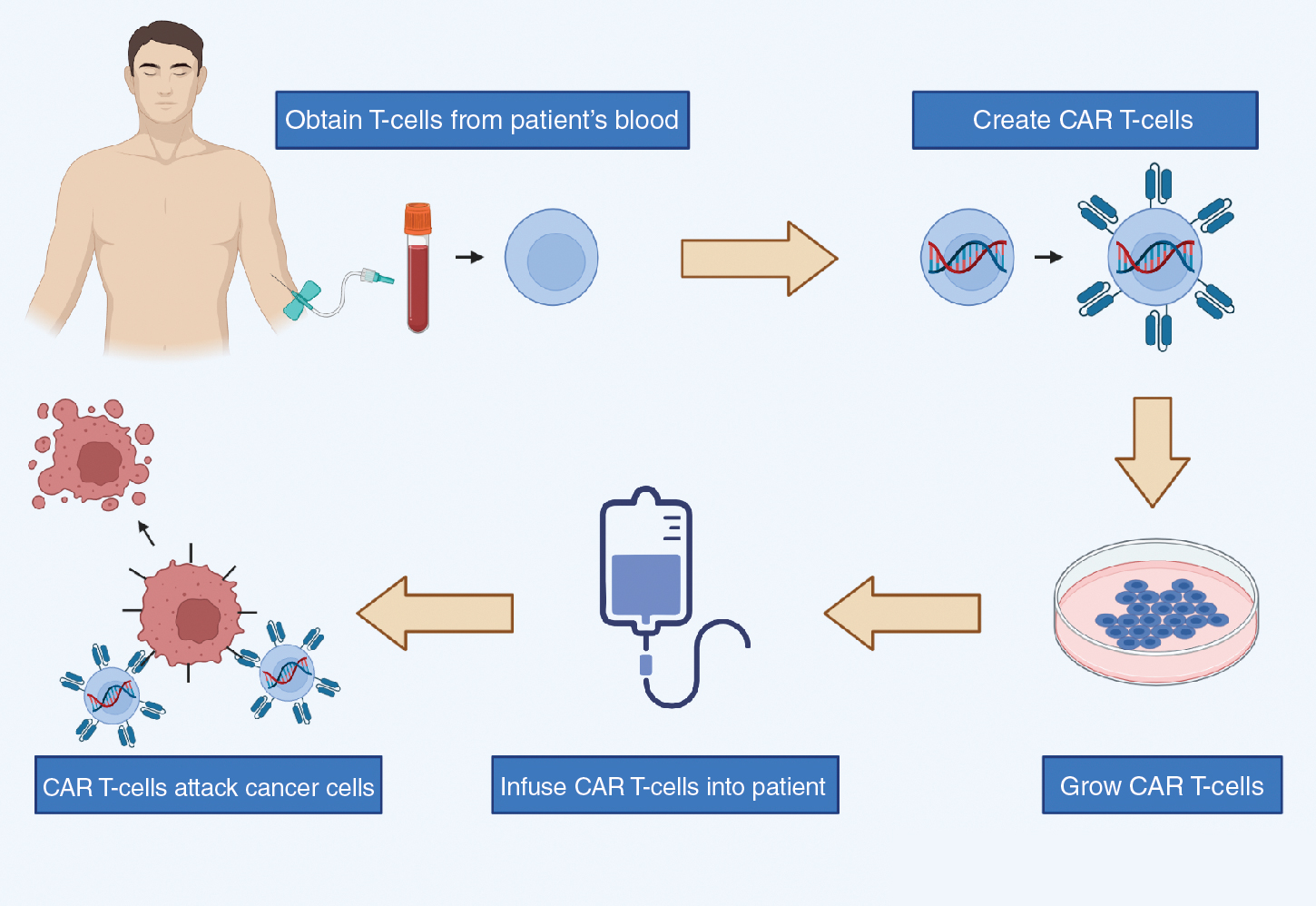
CAR T-cell therapy is a class of immunotherapy that combines the antigen-binding site of a monoclonal antibody with the signal-activated machinery of a T-cell, thereby circumventing the more restrictive process of antigen recognition by major histocompatibility complex (MHC). In CAR T-cell therapy, a patient’s own T-cells are genetically engineered to express a synthetic receptor to bind to a tumor antigen; these cells are then infused into the patient’s body to attack chemotherapy-resistant cancer ( Fig. 12.1 ).
BiTE antibodies are designed to engage and enhance T-cell activation by directing cytotoxic T-cells to CD19-expressing B cells. BiTE antibodies can recruit antigen-experienced T-cells without pre- or costimulation to directly kill tumor-associated antigen cells.
Cancer vaccines have been tested for the treatment of cancers, with the aim to elicit immune response against antigens expressed by tumor cells such as tumor-associated antigens or mutation-derived antigens (neoantigens). Finally, oncolytic virus therapy is another class of cancer immunotherapy that uses viruses to infect and kill tumor cells.
Skin cancer
Melanoma
Melanoma is the malignant transformation and proliferation of melanocytes, found predominantly in the skin but also present elsewhere in the gastrointestinal and genitourinary mucosa, the uvea, and the meninges/central nervous system. Though localized melanoma is highly curable with surgery, those with unresectable, metastatic disease have poor survival outcomes, with a 5-year overall survival (OS) of 29.8%. The development of ICIs has revolutionized the treatment of advanced/metastatic melanoma. In 2011, the FDA approved ipilimumab as a first-line therapy for unresectable stage III/IV melanoma after it demonstrated an improved OS of 11.2 months compared with 9.1 months in the placebo group. This marked the beginning of the use of ICIs to treat cancers and transformed the oncologic therapeutic landscape. Since then, numerous clinical trials have been conducted with various ICIs approved for specific melanoma indications, as detailed in Table 12.1 .
| Melanoma | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinical Trial | FDA Agent | Phase | N | Study Population | Treatment Arm | Control Arm | Primary Endpoint | Results | Grade 3+ AE |
| First-Line Monotherapy | |||||||||
| NCT00324155 | Ipilimumab (Yervoy) | 3 | 502 | Unresectable stage III/IV melanoma | Ipilimumab + Dacarbazine | Placebo + Dacarbazine | OS | OS: 11.2 vs 9.1 months | 56.3% vs 27.5% |
| KEYNOTE-006 (NCT01866319) | Pembrolizumab (Keytruda) | 3 | 834 | Unresectable stage III/IV melanoma with no more than one prior systemic therapy | Pembrolizumab every 2 weeks; Pembrolizumab every 3 weeks | Ipilimumab | PFS, OS | ORR: 33.7%; 32.9% vs 11.9%; 1-year OS: 74.1%; 68.4% vs 58.2%; 6-month PFS: 47.3%; 46.6% vs 26.5% | 13.3%; 10.1% vs 19.9% |
| CheckMate 066 (NCT01721772) | Nivolumab (Opdivo) | 3 | 418 | Unresectable stage III/IV melanoma without BRAF mutation | Nivolumab | Dacarbazine | OS | ORR: 40.0% vs 13.9%; 1-year OS: 72.9% vs 42.1%; median PFS: 5.1 vs 2.2 months | 11.7% vs 17.6% |
| Second-Line Monotherapy | |||||||||
| NCT00094653 | Ipilimumab (Yervoy) | 3 | 676 | Unresectable stage III/IV melanoma previously treated with dacarbazine, temozolomide, fotemustine, carboplatin, or IL-2 | Ipilimumab + GP100; Ipilimumab alone | GP100 | OS | Median OS: 10.0; 10.1 vs 6.4 months | 10%–15% vs 3% |
| CheckMate 037 (NCT01721746) | Nivolumab (Opdivo) | 3 | 631 | Unresectable stage IIIC/IV metastatic melanoma BRAFV600+ pts with progression on anti-CTLA-4 and BRAF inhibitor BRAF wild-type pts with progression after anti-CTLA-4 | Nivolumab | Chemotherapy | ORR, OS | ORR: 31.7% vs 10.6% | 5% vs 9% |
| KEYNOTE-002 (NCT01704287) | Pembrolizumab (Keytruda) | 2 | 540 | Unresectable stage III/IV melanoma with progression after ipilimumab, BRAF, and/or MEK inhibitor | Pembrolizumab 2 mg/kg; Pembrolizumab 10 mg/kg | Chemotherapy | PFS | 6-Month PFS: 34%; 38% vs 16% | 11%; 14% vs 26% |
| First-Line Combination Therapy | |||||||||
| CheckMate 069 (NCT01927419) | Nivolumab (Opdivo) + Ipilimumab (Yervoy) | 2 | 142 | Unresectable stage III/IV melanoma | Nivolumab + Ipilimumab | Placebo + Ipilimumab | ORR | 2-Year OS: 63.8% vs 53.6%; median OS: not reached | Tx arm: grade 3: 44%; grade 4: 11% Control arm: grade 3: 17%; grade 4: 2% |
| CheckMate 067 (NCT01844505) | Nivolumab (Opdivo) + Ipilimumab (Yervoy) | 3 | 945 | Unresectable stage III/IV melanoma | Nivolumab + Ipilimumab Nivolumab alone | Ipilimumab | PFS, OS | Median PFS: 11.5; 6.9 vs 2.9 months; PD-L1 positive: median PFS: 14.0 vs 5.3 months PD-L1 negative: median PFS 11.2 vs 5.3 months | 55.0%; 16.3% vs 27.3% |
| IMspire150 (NCT02908672) | Atezolizumab (Tecentriq) | 3 | 514 | Unresectable stage IIIC/IV BRAFV600 + melanoma | Atezolizumab + Vemurafenib + Cobimetinib | Placebo + Vemurafenib + Cobimetinib | PFS | Median PFS: 15.1 vs 10.6 months | 79% vs 73% |
| Adjuvant Therapy | |||||||||
| CheckMate 238 (NCT02388906) | Nivolumab (Opdivo) | 3 | 906 | Resected stage IIIB/IIIC/IV melanoma | Nivolumab | Ipilimumab | RFS | 1-Year RFS: 70.5% vs 60.8% | 14.4% vs 45.9% |
| EORTC 18071 (NCT00636168) | Ipilimumab (Yervoy) | 3 | 951 | Resected stage IIIA/IV melanoma metastatic to LN only | Ipilimumab | Placebo | RFS | 5-Year RFS: 40.8% vs 30.3%; 5-year OS: 65.4% vs 54.4%; 5-year distant metastasis–free survival: 48.3% vs 38.9% |
|
| KEYNOTE-054 (NCT02362594) | Pembrolizumab (Keytruda) | 3 | 1019 | Resected stage III melanoma | Pembrolizumab | Placebo | RFS | 1-Year RFS: 75.4% vs 61.0% PD-L1 positive: 1-year RFS 77.1% vs 62.6% | 14.7% vs 3.4% |
| Oncolytic Virus | |||||||||
| OPTiM (NCT00769704) | Talimogene laherparepvec [T-VEC] (IMLYGIC) | 3 | 436 | Unresectable stage IIIB/IV melanoma | T-VEC | GM-CSF | DRR | ORR: 26.4% vs 5.7%; median OS: 23.3 vs 18.9 months; DRR: 16.3% vs 2.1% | ≥2% |
The approvals of ipilimumab, pembrolizumab, and nivolumab as monotherapy agents paved way for subsequent trials assessing the role of combination therapies. Results from the CheckMate 069 trial led to the accelerated approval of the first immunotherapy combination immune checkpoint blockade of PD-1 (nivolumab) and CTLA-4 (ipilimumab) for patients with BRAFV600 wild-type unresectable/metastatic melanoma. Patients receiving combination CTLA-4 and PD-1 blockade demonstrated an improved 2-year OS of 63.8%, compared with a 2-year OS of 53.6% for patients receiving ipilimumab monotherapy. This combination was also approved for patients with unresectable BRAFV600 mutation-positive melanoma after the CheckMate 067 trial demonstrated improved median progression-free survival (PFS) of 11.5 months in the combination group compared with 2.9 months in the ipilimumab monotherapy group. For patients with disease progression despite prior systemic therapies, ICIs are also approved as second-line monotherapy, with several trials demonstrating improved response rates, median OS, and PFS compared with standard chemotherapy regimens.
Though ICIs have dominated the IO landscape for advanced/metastatic melanoma, the FDA has also approved talimogene laherparepvec (T-VEC), a genetically modified herpes simplex virus (HSV) type 1 oncolytic virus therapy, for unresectable stage III/IV melanoma, based on a phase 3 trial that demonstrated a significant overall response rate (ORR) of 26.4% in the T-VEC group compared with 5.7% in the subcutaneous granulocyte-macrophage colony-stimulating factor (GM-CSF) group. Although T-VEC is currently indicated for only melanoma treatment, it is the hope that oncolytic viruses, which are designed to selectively replicate within the tumor cell, eradicate the cells and, in doing so, induce tumor-directed immune responses, may eventually also demonstrate benefit for other cancer types.
Nonmelanoma skin cancers
ICIs have received FDA approval for the treatment of advanced/metastatic nonmelanoma skin cancers including Merkel cell carcinoma (MCC), cutaneous squamous cell carcinoma (cSCC), and basal cell carcinoma (BCC), based on several phase 2 clinical trials ( Table 12.2 ).
| Merkel Cell Carcinoma (MCC) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Clinical Trial | FDA Agent | Phase | N | Study Population | Treatment | Primary Endpoint | Results | Grade 3+ AE |
| First-Line Therapy | ||||||||
| KEYNOTE-017 (NCT02267603) | Pembrolizumab (Keytruda) | 2 | 50 | Metastatic/recurrent MCC refractory to chemo, unamenable to surgery and radiation | Pembrolizumab | ORR | ORR: 56%; 2-year PFS: 48.3%, median PFS: 16.8 months; 2-year OS: 68.7%, median OS: not reached | 28% |
| Second-Line Therapy | ||||||||
| JAVELIN Merkel 200 (NCT02155647) | Avelumab (Bavencio) | 2 | 88 | Stage IV MCC refractory to chemo | Avelumab | ORR | ORR: 31.8% | 5% |
| Cutaneous Squamous Cell Carcinoma (cSCC) | ||||||||
| Clinical Trial | FDA Agent | Phase | N | Study Population | Treatment | Primary Endpoint | Results | Grade 3+ AE |
| First-Line Therapy | ||||||||
| Study 1423 (NCT02383212) | Cemiplimab (LIBTAYO) | 1 | Phase 1: 26; Phase 2: 59 | Locally advanced/metastatic cSCC unamenable to surgery and radiation | Cemiplimab | ORR | ORR phase 1: 50%; ORR phase 2: 47% | 42% |
| Study 1540 (NCT02760498) | Cemiplimab (Libtayo) | 2 | 115 | Locally advanced/metastatic cSCC unamenable to surgery and radiation | Cemiplimab 250 mg q3w; Cemiplimab 3 mg/kg q2w | ORR | ORR: 41.1%; 49.2% | 39.3%; 50.8% |
| KEYNOTE-629 (NCT03283324) | Pembrolizumab (Keytruda) | 2 | 105 | Recurrent/metastatic cSCC unamenable to surgery and radiation | Pembrolizumab | ORR | ORR: 34.3%; disease control rate: 52.4%; median PFS: 6.9 months; median OS: not reached | 5.7% |
| Basal Cell Carcinoma (BCC) | ||||||||
| Clinical Trial | FDA Agent | Phase | N | Study Population | Treatment | Primary Endpoint | Results | Grade 3+ AE |
| Second-Line Therapy | ||||||||
| Study 1620 (NCT03132636) | Cemiplimab (Libtayo) | 2 | 84 | Locally advanced/metastatic BCC after first-line HHI therapy or if not candidates for HHI | Cemiplimab | ORR | ORR: 31% | 48% |
Two ICIs are currently FDA-approved for MCC. Avelumab was the first ICI approved in 2017 in the second-line setting for metastatic MCC, following results from the JAVELIN Merkel 200 trial that demonstrated an ORR of 31.8% at a median follow-up of 10.4 months. One year later, pembrolizumab was approved for patients with recurrent locally advanced/metastatic MCC who had not received prior systemic therapy, based on results from the KEYNOTE-017 trial that demonstrated an ORR of 56% at median follow-up of 14.9 months, a 2-year PFS of 48.3% with median PFS of 16.8 months, and a 2-year OS of 68.7%.
For cSCC, cemiplimab and pembrolizumab can be used as first-line agents for locally advanced/metastatic cSCC. Cemiplimab was FDA-approved in 2018 for locally advanced/metastatic cSCC, based on results from Study 1423 (phase 1) and Study 1540 (phase 2). In 2020, pembrolizumab was approved for recurrent/metastatic cSCC not amenable to surgery and radiation after the KEYNOTE-629 trial demonstrated an ORR of 34.3% at median follow-up of 11.4 months, a disease control rate of 52.4%, and a median PFS of 6.9 months. For BCC, cemiplimab was approved in 2021 as a second-line therapy for patients with locally advanced/metastatic disease.
Lung cancer
Non-small cell lung cancer
Lung cancer is the leading cause of all cancer mortality. Although lung cancer traditionally was characterized by late-stage disease at diagnosis with limited treatment options, immunotherapy breakthroughs over the early 21st century have provided encouraging results ( Table 12.3 ). Prior to ICIs, platinum-based chemotherapy was the cornerstone of treatment for early stage and advanced non-small cell lung cancer (NSCLC).
| Non-Small Cell Lung Cancer (NSCLC) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinical Trial | FDA Agent | Phase | N | Study Population | Treatment Arm | Control Arm | Primary Endpoint | Results | Grade 3+ AE |
| First-Line Monotherapy | |||||||||
| KEYNOTE-024 (NCT02142738) | Pembrolizumab (Keytruda) | 3 | 305 | Stage IV NSCLC with no EGFR/ALK mutations with PD-L1 TPS ≥ 50% | Pembrolizumab | Platinum-based chemo | PFS | ORR: 44.8% vs 27.8%; median PFS: 10.3 vs 6.0 months; 6-month OS: 80.2% vs 72.4% | 26.6% vs 53.3% |
| KEYNOTE-042 (NCT02220894) | Pembrolizumab (Keytruda) | 3 | 1274 | Stage III/IV NSCLC with no EGFR/ALK mutations unamenable to surgery or CRT with PD-L1 TPS ≥ 1% | Pembrolizumab | Platinum-based chemo | OS | Median OS: TPS ≥ 1% 16.7 vs 12.1 months; TPS ≥ 20% 17.7 vs 13.0 months; TPS ≥ 50% 20 vs 12.2 months | 8% vs 41% |
| IMPower 110 (NCT02409342) | Atezolizumab (Tecentriq) | 3 | 572 | Stage IV NSCLC with PD-L1 TPS ≥ 1% | Atezolizumab | Platinum-based chemo | OS | Median OS: 20.2 vs 13.1 months; median PFS: 8.1 vs 5.0 months; ORR 38% vs 29% | 30.1% vs 52.5% |
| EMPOWER-Lung 1 (NCT03088540) | Cemiplimab (Libtayo) | 3 | 710 | Stage IIIB-C/IV NSCLC with PD-L1 TPS ≥ 50% | Cemiplimab | Platinum-based chemo | PFS, OS | PD-L1 ≥ 50%: median OS not reached vs 14.2 months; median PFS: 8.2 vs 5.7 months | 28% vs 39% |
| First-Line Combination Therapy | |||||||||
| KEYNOTE-021 (NCT02039674) | Pembrolizumab (Keytruda) | 2 | 123 | Stage IIIB/IV NSCLC with no EGFR/ALK mutations | Pembrolizumab + Platinum-based chemo | Platinum-based chemo | ORR | ORR: 55% vs 29% | 39% vs 26% |
| KEYNOTE-189 (NCT02578680) | Pembrolizumab (Keytruda) | 3 | 616 | Stage IV nonsquamous NSCLC with no EGFR/ALK mutations | Pembrolizumab + Platinum-based chemo | Placebo + Platinum-based chemo | PFS, OS | 1-Year OS: 69.2% vs 49.4%; median PFS: 8.8 vs 4.9 months | 67.2% vs 65.8% |
| KEYNOTE-407 (NCT02775435) | Pembrolizumab (Keytruda) | 3 | 559 | Stage IV squamous NSCLC | Pembrolizumab + Platinum-based chemo | Placebo + Platinum-based chemo | PFS, OS, ORR | Median OS: 15.9 vs 11.3 months; median PFS: 6.4 vs 4.8 months; ORR: 58% vs 35% | 69.8% vs 68.2% |
| IMPower 150 (NCT02366143) | Atezolizumab (Tecentriq) | 3 | 692 | Stage IV nonsquamous NSCLC with wild-type or high effector T-cell gene (T eff ) signature | ABCP: Atezolizumab + Bevacizumab + Carboplatin + Paclitaxel | BCP: Bevacizumab + Carboplatin + Paclitaxel | PFS, OS |
| ABCP: grade 3–4: 55.7%; grade 5: 2.8% BCP: grade 3–4: 47.7%; grade 5: 2.3% |
| IMPower 130 (NCT02367781) | Atezolizumab (Tecentriq) | 3 | 724 | stage IV NSCLC | Atezolizumab + Platinum-based chemo | Platinum-based chemo | PFS, OS | Median OS: 18.6 vs 13.9; median PFS: 7.0 vs 5.5 months | Tx arm: grade 3: 50%; grade 4: 23%; grade 5: 2% Control arm: grade 3: 47%; grade 4: 13%; grade 5: <1% |
| CheckMate 227 (NCT02477826) | Nivolumab (Opdivo) | 3 | Part 1a: 793 | Recurrent/stage IV NSCLC with no EGFR/ALK mutations with PD-L1 ≥ 1% | Nivolumab + Ipilimumab | Platinum-based chemo | OS | Median OS: 17.1 vs 14.9 months; median PFS 5.1 vs 5.6 months; ORR 36% vs 30%; median DoR 23.2 vs 6.2 months | 32.8% vs 36.0% |
| CheckMate 9LA (NCT03215706) | Nivolumab (Opdivo) + Ipilimumab (Yervoy) | 3 | 1150 | Recurrent/stage IV NSCLC | Nivolumab + Ipilimumab + Platinum-based chemo | Platinum-based chemo | OS | Median OS: 15.6 vs 10.9 months | Tx arm: grade 3: 35%; grade 4: 12% Control arm: grade 3: 32%; grade 4: 6% |
| Second-Line Therapy | |||||||||
| CheckMate 017 (NCT01642004) | Nivolumab (Opdivo) | 3 | 272 | Previously treated, recurrent stage IIIB/IV squamous NSCLC | Nivolumab | Docetaxel | OS | Median OS: 9.2 vs 6.0 months; 1-year OS: 42% vs 24%; ORR: 20% vs 9%; median PFS: 3.5 vs 2.8 months | 7% vs 55% |
| CheckMate 057 (NCT01673867) | Nivolumab (Opdivo) | 3 | 582 | Previously treated, recurrent stage IIIB/IV nonsquamous NSCLC | Nivolumab | Docetaxel | OS | Median OS: 12.2 vs 9.4 months; 1-year OS: 51% vs 39%; 1.5-year OS: 39% vs 23%; ORR: 19% vs 12% | 10% vs 54% |
| KEYNOTE-010 (NCT01905657) | Pembrolizumab (Keytruda) | 2 + 3 | 1034 | Previously treated, stage IV NSCLC with PD-L1 TPS ≥ 1% |
| Docetaxel | PFS, OS | Median OS: 10.4; 12.7 vs 8.5 months; median PFS: 3.9; 4.0; vs 4.0 months PD-L1 positive: OS 14.9; 17.3 vs 8.2 months; PFS: 5.0; 5.2 vs 4.1 months | 13%; 16% vs 35% |
| POPLAR (NCT01903993) | Atezolizumab (Tecentriq) | 2 | 287 | Previously treated, stage III/IV NSCLC | Atezolizumab | Docetaxel | OS | OS: 12.6 vs 9.7 months | Tx arm: grade 3–4: 11%; grade 5: 1% Control arm: grade 3–4: 39%; grade 5: 2% |
| OAK (NCT02008227) | Atezolizumab (Tecentriq) | 3 | 1225 | Previously treated stage IIIB/IV NSCLC with PD-L1 TPS ≥ 1% | Atezolizumab | Docetaxel | OS | OS: 13.8 vs 9.6 months; OS PD-L1 ≥ 1%: 15.7 vs 10.3 months | 15% vs 43% |
| Maintenance Therapy | |||||||||
| PACIFIC (NCT02125461) | Durvalumab (IMFINZI) | 3 | 709 | Unresectable stage III NSCLC after platinum-based CRT | Durvalumab | Placebo | PFS, OS | Median PFS: 17.2 vs 5.6 months; 2-year OS: 66.3% vs 55.6% | 30.5% vs 26.1% |
| Adjuvant Therapy | |||||||||
| IMPower 010 (NCT02486718) | Atezolizumab (Tecentriq) | 3 | 990 | Resected stage II/IIIA NSCLC with PD-L1 TPS ≥ 1% | Atezolizumab | Platinum-based chemo | DFS |
| Tx arm: grade 3–4: 22%; grade 5: 2% Control arm: grade 3–4: 12%; grade 5: 1% |
| Neoadjuvant Therapy a | |||||||||
| CheckMate 816 (NCT02998528) | Nivolumab (OPDIVO) | 3 | 358 | Resectable stage IB-IIIA NSCLC | Nivolumab + Platinum-based chemo followed by surgery | Platinum-based chemo followed by surgery | pCR, EFS | pCR: 24.0% vs 2.2%; improved EFS in tx arm compared with control; MPR: 36.9% vs 8.9%; ORR: 53.6% vs 37.4%; radiographic down-staging rates: 30.7% vs 23.5% |
|
a NOTE: As of January 2022, no immunotherapy drugs have been approved by the FDA as neoadjuvant therapy for NSCLC. However, this is currently evolving with continual changes in clinical practice. Results from the CheckMate 816 trial have shown promising results for the possible role of immunotherapy as neoadjuvant for NSCLC.
ICIs were initially evaluated in the second-line setting for patients with advanced NSCLC. Several clinical trials demonstrated significant improved OS with either nivolumab (CheckMate 017, CheckMate 057 ), pembrolizumab (KEYNOTE-010 ), or atezolizumab (POPLAR, OAK ) as monotherapy compared with standard, second-line chemotherapy control groups, ultimately leading to FDA approvals.
The promising results from the use of ICIs in the second-line setting led to further evaluation of these drugs as potential first-line therapies. KEYNOTE-001 was a phase 1 study that assessed the efficacy and safety of pembrolizumab in advanced NSCLC and showed that previously untreated patients with PD-L1 expression (determined by Tumor Proportion Score [TPS]) ≥ 50%) had a response rate of 50%. KEYNOTE-024 subsequently compared pembrolizumab with platinum-based chemotherapy in patients with untreated advanced NSCLC with TPS expression ≥50%; results from this study found higher response rates (44.8% vs 27.8%), PFS (10.3 vs 6.0 months), and 6-month OS (80.2% vs 72.4%) compared with chemotherapy, leading to its FDA approval as a first-line therapy. To investigate whether pembrolizumab could also be used for patients with lower PD-L1 expression, the investigators of KEYNOTE-042 compared patients with untreated locally advanced/metastatic NSCLC with PD-L1 expression ≥1% to those on standard platinum-based chemotherapy and found a significant survival advantage of 16.7 months in the treatment arm compared with 12.1 months in the control arm. This paved the way for approval of pembrolizumab as a first-line agent for patients with untreated advanced/metastatic NSCLC with PD-L1 expression ≥1%. Atezolizumab (IMPower 110 ) and cemiplimab (EMPOWER-Lung1 ) were approved in 2020 and 2021, respectively, as first-line monotherapy agents for patients with advanced NSCLC with PD-L1 expression on tumors demonstrating improvement in OS compared with platinum-based chemotherapy.
Combinational therapy has also been an active area of investigation for treatment of this disease. Several clinical trials have led to the first-line approval of pembrolizumab in combination with chemotherapy (KEYNOTE-021, KEYNOTE-189, KEYNOTE-407 ). Similarly, atezolizumab with chemotherapy was approved for metastatic NSCLC based on the IMPower 130 trial. The IMPower 150 study, which randomized patients to receive atezolizumab + carboplatin + paclitaxel (ACP), bevacizumab + carboplatin + paclitaxel (BCP), or atezolizumab + BCP (ABCP), demonstrated improved PFS (8.3 vs 6.8 months) and median OS (19.2 vs 14.7 months) in the ABCP group compared with the BCP group, resulting in the approval of this combination regimen. Regarding the combination of multiple ICIs, nivolumab plus ipilimumab is currently approved for advanced NSCLC after results from the CheckMate 227 trial showed improved median OS (17.1 vs 14.9 months), PFS (5.1 vs 5.6 months), and duration of response (DoR; 23.2 vs 6.2 months) compared with chemotherapy for patients with PD-L1 TPS ≥1%. In 2020, nivolumab with ipilimumab and platinum-doublet chemotherapy was approved as first-line treatment for patients with recurrent/metastatic NSCLC after demonstrating improved median survival of 15.6 months compared with 10.9 months in the standard chemotherapy arm.
For locally advanced NSCLC, durvalumab has been approved as a maintenance therapy for patients with unresectable, stage III NSCLC after platinum-based chemoradiation therapy as a result of the PACIFIC trial. ,
One of the breakthroughs in the clinical application of immunotherapy in the 2020s is the use of ICIs in treating early-stage NSCLCs. As a result of the IMPower010 study, atezolizumab is indicated as a single-agent adjuvant therapy after resection and platinum-based chemotherapy for adult patients with stage II–IIIA NSCLC whose tumors have PD-L1 expression on ≥1% of tumor cells. Although the application of immunotherapy as neoadjuvant therapy for NSCLC has yet to be FDA-approved, several trials have demonstrated findings that may lead to changes in clinical practice. The CheckMate 816 trial is a phase 3 study that compares nivolumab plus platinum-based chemotherapy as neoadjuvant with platinum-based chemotherapy alone for patients with resectable stage IB-IIIA NSCLC. An interim analysis of this trial showed improved pathologic complete response (pCR; 24.0% vs 2.2%), major pathologic response (MPR; 36.9% vs 8.9%), ORR (53.6% vs 37.4%), and event-free survival (EFS) in the neoadjuvant nivolumab plus chemotherapy treatment arm compared with the control arm, with no effect on surgical outcomes. Together with the results from the IMPower010 trial, the findings from CheckMate 816 trial have changed the treatment paradigm of early-stage NSCLCs. Determining the role of neoadjuvant versus adjuvant ICI therapy for patients with stage IB–IIIA NSCLCs, however, remains to be addressed.
Small cell lung cancer
The positive results of PD-1/L1 inhibitors for the treatment of NSCLC ignited interest in evaluating these agents for the treatment of SCLC ( Table 12.4 ). Although SCLC is initially sensitive to chemoradiation, many patients may relapse within 6 months, with 5-year survival rates as low as 5% for patients with extensive-stage disease (ES-SCLC). In 2018, nivolumab was the first ICI to be granted accelerated approval by the FDA as a third-line option for stage IV SCLC. That same year, pembrolizumab was approved as third-line therapy for the same indication based on the KEYNOTE-028 and KEYNOTE-158 studies.
| Small Cell Lung Cancer (SCLC) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinical Trial | FDA Agent | Phase | N | Study Population | Treatment Arm | Control Arm | Primary Endpoint | Results | Grade 3+ AE |
| First-Line Therapy | |||||||||
| IMpower133 (NCT02763579) | Atezolizumab (Tecentriq) | 3 | 403 | ES-SCLC | Atezolizumab + Etoposide + Carboplatin | Placebo + Etoposide + Carboplatin | PFS, OS | Median OS: 12.3 vs 10.3 months; median PFS: 5.2 vs 4.3 months | Tx arm: grade 3–4: 56.6%; grade 5: 1.5% Control arm: grade 3–4: 56.1%; grade 5: 1.5% |
| CASPIAN (NCT03043872) | Durvalumab (Imfinzi) | 3 | 537 | ES-SCLC | Durvalumab + Etoposide + Platinum-based chemo | Platinum-based chemo | OS | Median OS: 13.0 vs 10.3 months; ORR 68% vs 58% | 62% vs 62% |
| Third-Line Therapy | |||||||||
| KEYNOTE-028 (NCT02054806), KEYNOTE-158 (NCT02628067) | Pembrolizumab (Keytruda) | 1 + 2 | 83 | Stage IV SCLC with progression after platinum-based chemotherapy and 1+ prior line of therapy | Pembrolizumab | N/A | ORR, DRR | ORR: 19%; 6-month DRR 94%; 1-year DRR 63%; 18-month DRR: 56% | 9.6%–9.9% |
Though standard, first-line treatment for ES-SCLC is platinum-based chemotherapy with etoposide, outcomes continued to remain poor with median OS of approximately 10 months. Two ICIs, atezolizumab (Impower133 ) and durvalumab (CASPIAN ), approved as first-line therapies for untreated ES-SCLC in combination with etoposide and a platinum-based chemotherapy regimen, have shown improvements in median OS to 12 to 13 months.
Malignant pleural mesothelioma
Malignant pleural mesothelioma (MPM) is an aggressive cancer often unresectable at the time of diagnosis. Though platinum agents with folate antimetabolites such as pemetrexed have been approved as standard first-line therapies, prognosis remains poor, with a median 5-year OS of <10%. Currently, nivolumab in combination with ipilimumab is the available immunotherapy for first-line treatment of unresectable MPM, based on the CheckMate 743 trial that demonstrated an OS of 18.1 months in the treatment arm compared with 14.1 months in the standard chemotherapy (platinum + pemetrexed chemotherapy) arm and a 2-year OS of 41% vs 27%, respectively ( Table 12.5 ).
| Mesothelioma | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinical Trial | FDA Agent | Phase | N | Study Population | Treatment Arm | Control Arm | Primary Endpoint | Results | Grade 3+ AE |
| First-Line Therapy | |||||||||
| CheckMate 743 (NCT02899299) | Nivolumab (Opdivo) + Ipilimumab (Yervoy) | 3 | 605 | Unresectable malignant pleural mesothelioma | Nivolumab + Ipilimumab | Platinum + Pemetrexed chemo | OS | OS: 18.1 vs 14.1 months; 2-year OS: 41% vs 27% | 30% vs 32% |
Triple-negative breast cancer
Triple-negative breast cancer (TNBC) is an aggressive subtype that is difficult to treat, owing to the lack of targeted agents. Though chemotherapy including taxanes or platinum-based agents remains the standard of care for systemic treatment, these tumors can become rapidly resistant to chemotherapy. In 2021, pembrolizumab in combination with chemotherapy was approved in the first-line setting for metastatic disease, as well as in the neoadjuvant and adjuvant setting ( Table 12.6 ). The approval of pembrolizumab as first-line therapy for metastatic TNBC stemmed from the KEYNOTE-355 study, which demonstrated significant PFS (9.6 vs 5.6 months) in the pembrolizumab plus chemotherapy arm compared with the placebo plus chemotherapy arm for patients with PD-L1 expression with combined positive score (CPS) ≥10. Pembrolizumab’s approval as neoadjuvant therapy in combination with chemotherapy for high-risk stage II/III TNBC followed by adjuvant treatment after surgery was based on findings from KEYNOTE-522 that showed a pCR rate of 63% in the pembrolizumab arm compared with 56% in the chemotherapy arm.
| Triple-Negative Breast Cancer (TNBC) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinical Trial | FDA Agent | Phase | N | Study Population | Treatment Arm | Control Arm | Primary Endpoint | Results | Grade 3+ AE |
| First-Line Therapy | |||||||||
| KEYNOTE-355 (NCT02819518) | Pembrolizumab (Keytruda) | 3 | 847 | Unresectable, recurrent/stage IV TNBC with PD-L1 (CPS ≥ 10) | Pembrolizumab + Chemo | Placebo + Chemo | PFS, OS | Median PFS: 9.6 vs 5.6 months | 68% vs 67% |
| Neoadjuvant/Adjuvant Therapy | |||||||||
| KEYNOTE-522 (NCT03036488) | Pembrolizumab (Keytruda) | 3 | 1174 | Untreated high-risk stage II/III TNBC | Pembrolizumab + Chemo | Placebo + Chemo | Pathologic complete response rate, event free survival | Pathologic complete response: 63% vs 56%; event-free survival: 16% vs 24% | 76.8% vs 72.2% |
Gastrointestinal malignancies
Gastric/gastroesophageal junction cancers
Gastric cancer, including gastroesophageal junction (GEJ) cancer, is the fourth leading cause of cancer-related deaths worldwide. Outcomes remain poor with standard-of-care fluoropyrimidine and platinum-based chemotherapy in unresectable disease. , Current ICIs approved as first-line agents for advanced or metastatic gastric/GEJ include nivolumab in conjunction with chemotherapy (CheckMate 649) and pembrolizumab in conjunction with chemotherapy and trastuzumab for human epidermal growth factor receptor 2 positive (HER2+) tumors (KEYNOTE-811). Nivolumab and pembrolizumab have also been approved as second-line indications for advanced or metastatic esophageal/GEJ cancers that have progressed after prior lines of therapy, based on findings from the KEYNOTE-180, KEYNOTE-181, and ATTRACTION-3 trials, as detailed in Tables 12.7 to 12.9 . In addition, nivolumab is approved in the adjuvant setting for patients with stage II/III disease after definitive treatment with chemoradiation and surgical resection with residual disease.
| Gastric Cancer | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinical Trial | FDA Agent | Phase | N | Study Population | Treatment Arm | Control Arm | Primary Endpoint | Results | Grade 3+ AE |
| First-Line Therapy | |||||||||
| CheckMate 649 (NCT02872116) | Nivolumab (Opdivo) | 3 | 1581 | Unresectable, locally advanced, stage IV gastric/GEJ/esophageal adenocarcinoma | Nivolumab + Chemo | Chemo | PFS, OS | Median OS: 13.8 vs 11.5 months CPS ≥ 5: PFS 7.7 vs 6.0 months, median OS 14.4 vs 11.1 months | 59% vs 44% |
| KEYNOTE-811 (NCT03615326) | Pembrolizumab (Keytruda) | 3 | 264 | Unresectable/stage IV HER2+ gastric/GEJ cancer | Pembrolizumab + Trastuzumab + Chemo | Placebo + Trastuzumab + Chemo | PFS | ORR: 74.4% vs 51.9% | 57.1% vs 57.4% |
| Esophageal/Gastroesophageal Junction (GEJ) Adenocarcinoma | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinical Trial | FDA Agent | Phase | N | Study Population | Treatment Arm | Control Arm | Primary Endpoint | Results | Grade 3+ AE |
| First-Line Therapy | |||||||||
| CheckMate 649 (NCT02872116) | Nivolumab (Opdivo) | 3 | 1581 | Locally advanced, unresectable/stage IV gastric/GEJ/esophageal adenocarcinoma | Nivolumab + Chemo | Chemo | PFS, OS | Median OS: 13.8 vs 11.5 months CPS ≥ 5: PFS 7.7 vs 6.0 months; OS 14.4 vs 11.1 months | 59% vs 44% |
| KEYNOTE-590 (NCT03189719) | Pembrolizumab (Keytruda) | 3 | 749 | Locally advanced, unresectable/stage IV esophageal adenocarcinoma or SCC, GEJ adenocarcinoma | Pembrolizumab + 5-FU + Cisplatin | Placebo + 5-FU + Cisplatin | PFS, OS | Median OS: 12.4 vs 9.8 months, PFS 6.3 vs 5.8 months SCC group: median OS 12.6 vs 9.8 months, PFS 6.3 vs 5.8 months PD-L1 w/CPS ≥ 10: median OS 13.5 vs 9.4 months, PFS 7.5 vs 5.5 months SCC and PD-L1 CPS ≥ 10: median OS 13.9 vs 8.8 months | 72% vs 68% |
| Second-Line Therapy | |||||||||
| KEYNOTE-180 (NCT02559687) | Pembrolizumab (Keytruda) | 2 | 121 | Locally advanced, unresectable/stage IV esophageal adenocarcinoma or SCC, GEJ adenocarcinoma that progressed after 2+ lines of therapy | Pembrolizumab | N/A | ORR | ORR: 9.9% | 12.4% |
| KEYNOTE-181 (NCT02564263) | Pembrolizumab (Keytruda) | 3 | 628 | Locally advanced, unresectable/stage IV esophageal adenocarcinoma or SCC, GEJ adenocarcinoma that progressed after first-line chemo | Pembrolizumab | Chemo | OS | 1-Year OS: 43% vs 20% CPS ≥ 10: median OS 9.3 vs 6.7 months SCC group: median OS: 8.2 vs 7.1 months | 18.2% vs 40.9% |
| ATTRACTION-3 (NCT02569242) | Nivolumab (Opdivo) | 3 | 419 | Locally advanced, unresectable/recurrent esophageal SCC that progressed after one line of therapy | Nivolumab | Chemo | OS | OS: 10.9 vs 8.4 months | 18% vs 63% |
| Adjuvant Therapy | |||||||||
| CheckMate 577 (NCT02743494) | Nivolumab (Opdivo) | 3 | 794 | Resected stage II/III esophageal adenocarcinoma or SCC, GEJ cancer after neoadjuvant CRT, surgery with residual pathologic disease | Nivolumab | Placebo | DFS | Median DFS: 22.4 vs 11.0 months | 13% vs 6% |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree