The Child with Frequent, Severe, or Unusual Infections: Congenital Immunodeficiency Syndromes
Overview
It is impossible to pass through life without acquiring a large number of infections. Most of these infections occur during early childhood, a time when there is considerable immunologic naiveté. The child’s immune system begins to build up defenses against infection by being exposed to various pathogens.
Not so long ago, children stayed at home throughout their toddler years. When these children attended kindergarten, they began to develop frequent respiratory infections. Absenteeism was high. These days, many children are in day care centers from infancy; thus, the time of frequent infection has been switched from early school age to infancy and the toddler years. Pediatricians offices are bombarded with many children who are experiencing yet another in a long series of infections.
Frequency of Infection
The important question is: How many infections is too many? Dr. Wald and colleagues performed an observational study that looked at the number of respiratory infections acquired by otherwise normal children in day care and preschool situations.1 They found that the average child experienced 4 or 5 respiratory infections a year, and that some children experienced as many as 12 per year. When you consider that a common cold lasts approximately 10–14 days in a child of this age, and that most of these infections are clustered in the winter months, it becomes clear why some parents state that their child is “always sick.”
Differentiation Between Normal and Immunodeficiency States
Given that even a child with a normal immune system can frequently be ill, how does one differentiate between a normal child who has a naive immune system and frequent exposure to infectious agents, and a child who has a faulty immune response to infection? In general, the normal child with frequent infections has normal growth and development, no family history of immune deficiency states or early childhood death, and appears well between episodes of sickness. It is rare to find a severe immunodeficiency in a child who is normally grown and developed and who has an entirely normal physical examination apart from the signs of the current disease. Children with primary immunodeficiency states, on the other hand, are more likely to have: (a) growth failure, (b) abnormal development, (c) severe or invasive infections, (d) family history of immune deficiency or early childhood demise, and (e) infections with opportunistic or unusual organisms (i.e., pathogens not normally considered virulent).
The history and physical examination is often enough, therefore, to determine which children should receive evaluation of immune system function, and what laboratory tests should be ordered.
Frequent Respiratory Tract Infections
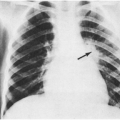
The type and severity of infections should be carefully documented. Chest radiographs should be reviewed to verify the diagnosis of pneumonia and to determine whether single or multiple lobes have been involved. Foreign body aspiration is a common cause of recurrent unilobar pneumonia.
The child’s risk for recurrent respiratory infection should also be explored. Children who attend day care or group child-care settings have a higher incidence of respiratory infections.1,2 Exposure to environmental tobacco smoke in the first 2 years of life has been shown in several studies to increase
the rate of respiratory illness by one-and-a half- to two-fold.2,3 Otherwise healthy infants with frequent infections are also more likely to be bottle-fed than breast-fed.4
the rate of respiratory illness by one-and-a half- to two-fold.2,3 Otherwise healthy infants with frequent infections are also more likely to be bottle-fed than breast-fed.4
Children with normal immune function usually acquire common cold-like illnesses, whose duration is usually less than 2 weeks and from which recovery is complete. Acute otitis media (AOM) may frequently accompany viral respiratory tract infections even in the normal host, because of the fact that the pathogenesis of AOM is often related to eustachian tube dysfunction and other anatomic realities of early childhood (Chapter 2). Normal children may have frequent AOM but individual episodes usually respond to appropriate antimicrobial therapy. Because true bacterial sinusitis is less common than AOM, multiple episodes of properly diagnosed sinusitis is more suggestive of either immune deficiency or problems of mucociliary clearance such as cystic fibrosis (Chapter 22) or primary ciliary dyskinesia. Unfortunately, bacterial sinusitis can be difficult to diagnose (Chapter 5).
An isolated episode of pneumonia, either with a clinical history suggestive of viral infection or an atypical pneumonia pathogen, or with a confirmed common cause of pneumonia such as Streptococcus pneumoniae is not suspicious for immune deficiency. In addition, keep in mind that some parents will report that their child has had “recurrent pneumonia,” when in fact the history is more compatible with multiple episodes of reactive airways disease. However, multiple episodes of radiographically documented pneumonia, pneumonia requiring hospitalization on more than one occasion, or pneumonia in concert with frequent sinusitis or episodes of otitis media should raise “red flags” in the clinician’s mind. A combination of that type of history and growth failure or developmental delay is highly suggestive of immune deficiency.
Diagnostic Possibilities in the Child with Frequent Respiratory Tract Infections
Normal Child
As previously discussed, many immunologically normal children experience frequent, transient, nonsevere respiratory tract infections, occasionally in tandem with AOM. Although there are no scientific data to buttress this claim, our practice experience suggests that many of these children are fair-skinned, blue-eyed, and blond. If the child has had no invasive infections, is well between episodes, has a history suggestive of frequent exposure to infectious agents, is exposed to tobacco smoke in the home, has normal growth and development, has a normal physical examination, and/or has never been hospitalized for infection, the parents may be reassured that the child does not have an immune deficiency. Frequency of infections may be decreased by common sense behaviors such as frequent handwashing, decreasing exposure to environmental tobacco smoke, and removal from day care or placement in a day care with fewer children. As these children grow, their immune system strengthens and the frequency of infection naturally decreases. In general, infection and illness are less frequent in the summer months.
In addition to the modifiable factors listed above, bad luck is frequently the stated explanation for why some children experience “more than their share” of respiratory infections. However, it is likely that certain genetic polymorphisms play a role in the risk for infection. For example, mannose binding lectin (MBL) is an acute phase protein that is secreted by hepatocytes. Part of the innate immune system, it is able to activate complement via the classic pathway. Several mutant alleles in the MBL gene have been described, and about 5% of the population is homozygous for these mutant alleles; thus, they have very low levels of MBL in serum.5 A population-based, prospective cohort study from Greenland showed that among children aged 6–17 months, MBL-insufficient children experienced 2.9 times more acute respiratory infections (95% CI, 1.8–4.8) than MBL-sufficient children.6 There was no effect among children aged 18–23 months, suggesting that once the adaptive immune system matures, the presence of MBL is less important.
Polymorphisms in other genes that encode proteins involved in immune function are also relatively common. Examples include the 4th component of complement (C4) and the Fcγ receptor on phagocytic cells (a receptor necessary for bacteria opsonized with Ig [immunoglobulin] G to be ingested by phagocytes). Patients with either of these defects (partial C4 deficiency or the presence of an Fcγ receptor with decreased affinity for IgG-coated bacteria) appear to be at increased risk for various infections.7
Cystic Fibrosis (CF)
Children with CF often present in the first few years of life with a history of frequent respiratory
infections and poor growth. They may have a history of slow passage of meconium in the newborn period. Parents may also state that kissing the child leaves a salty taste in the mouth. Because CF is common (prevalence ∼1 in 2,500), easily diagnosed, and requires complex medical care, a sweat test for this condition should be ordered whenever the diagnosis is considered (Chapter 22).
infections and poor growth. They may have a history of slow passage of meconium in the newborn period. Parents may also state that kissing the child leaves a salty taste in the mouth. Because CF is common (prevalence ∼1 in 2,500), easily diagnosed, and requires complex medical care, a sweat test for this condition should be ordered whenever the diagnosis is considered (Chapter 22).
Primary Ciliary Dyskinesia (PCD)
This is the preferred term for a group of disorders characterized by abnormal ciliary structure or function. About 50% of patients with PCD have situs inversus, in which case the term Kartagener syndrome is used. Like CF, PCD is usually transmitted in an autosomal recessive fashion. It is much less common than CF; the estimated prevalence is 1 in 20,000. However, symptoms are milder than in patients with CF, and it is probably underdiagnosed.8 In one series of 55 children with PCD, 37 (67%) had a history of neonatal respiratory distress, 38 (69%) had situs inversus, and 42 (76%) had a history of early-onset persistent rhinorrhea. The mean age at diagnosis was 4.4 years, which is considerably younger than in most series.9 Bronchiectasis and nasal polyps are common but do not usually manifest until the second decade of life. One group has proposed the following criteria for investigating the possibility of PCD: (a) patients with chronic otitis, rhinosinusitis, and bronchitis in whom other entities have been excluded (CF, allergy, immunologic disorders, and α1-antitrypsin deficiency); (b) term infants with neonatal respiratory distress syndrome of unknown cause; and (c) patients with situs inversus and recurrent airway infections.10
The diagnosis is usually made by epithelial cell brushing from the nasal turbinates or bronchi. Ciliary beat frequency is evaluated by phase-contrast microscopy and ultrastructural changes are detected by electron microscopy. Concurrent bacterial infection can cause secondary ciliary dyskinesia, so ciliary brushings should be obtained at least 4–6 weeks after resolution of a respiratory infection.
Structural Abnormalities of the Lung
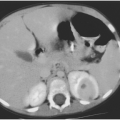
A child who has recurrent pneumonia, especially if all episodes occur in the same lobe, may have a structural abnormality that predisposes to the development of infection, such as congenital cystic adenomatoid malformation or pulmonary sequestration (Chapter 8). These conditions are best diagnosed by fine cut computed tomography.
Transient Hypogammaglobulinemia of Infancy (Transient Hypogammaglobulinemia of Early Childhood)11
Perhaps the most common of the usually symptomatic humoral immune deficiency syndromes, transient hypogammaglobulinemia of infancy may be just an exaggeration and prolongation of the physiologic gamma globulin nadir that all infants experience.12 These children are typically well during the first 3–6 months of life, when passive antibody from their mothers provides protection against most common pathogens. As this pool of passive antibody disappears, however, these patients begin to experience recurrent respiratory tract infections. Common cold syndrome and AOM are the most common syndromes, but sinusitis, bronchiolitis, and pneumonia may also occur. Recurrent gastroenteritis or “formula intolerance” has also been described.12 Generally, babies with this syndrome do not become infected with atypical pathogens, nor do they experience severe or life-threatening infections. In contrast to patients with X-linked agammaglobulinemia (discussed in the following text), patients with transient hypogammaglobulinemia of infancy have normal levels of circulating B cells and mount normal responses to diphtheria and tetanus immunizations.13 Although IgG is the principal antibody isotype affected, levels of other antibodies may also be decreased. In one series of 40 patients, IgG levels were low in 30 (75%), IgA levels were low in 17 (43%), and IgM levels were low in 10 (25%). Physical examination should reveal the presence of lymphoid tissue, without lymphadenopathy. Immunoglobulin levels eventually normalize; occasionally, patients will require IVIG therapy for a few months to a year.12,14 In most patients, immunoglobulin levels are normal by age 3 years, but in some they may be subnormal until 5 years.14 In a few, transient hypogammaglobulinemia of infancy is a precursor of a persistent immunoglobulin problem, usually IgA deficiency.11
X-Linked Agammaglobulinemia (XLA, Bruton’s Disease)
XLA is due to a B-cell maturational defect.15 It is caused by a mutation in the gene encoding Bruton tyrosine kinase (BTK), a cytoplasmic protein required
for the growth and development of B-cell precursors. Without it, B-cells never become antibody-secreting cells; therefore, antibody is scant to absent. Circulating B cells in the blood are similarly missing. This disorder presents similarly to transient hypogammaglobulinemia of infancy, previously described, in that the first few months of life are uneventful, attributable to passive maternal antibody. Thereafter, the child develops frequent sinopulmonary infections, mostly due to encapsulated gram-positive organisms, especially S. pneumoniae. Most of these bacterial infections can be readily treated with antibiotics; their frequency, however, can lead to destructive processes in the lungs and sinuses. Patients with agammaglobulinemia are also subject to severe or prolonged infections with nonenveloped viruses, especially the enteroviruses. Chronic enteroviral meningitis is an especially interesting syndrome seen almost exclusively in patients with XLA.16 Progressive viremia and paralytic poliomyelitis secondary to live attenuated oral polio vaccine (no longer available in the United States) has also been described in these patients. T-cell function is normal or nearly so. Therefore, patients with this disorder do not suffer from fungal infections or prolonged infection with enveloped viruses, such as respiratory syncytial virus (RSV) or influenza virus. The gene defect for XLA has been located to the X chromosome at position Xq21.2-22. Carrier females are immunologically normal. Physical examination of these children is generally unremarkable except that lymphoid tissue is scant. If a patient with a compatible clinical history has no visible tonsils and no palpable lymph node enlargement, the diagnosis of XLA should be strongly considered.
for the growth and development of B-cell precursors. Without it, B-cells never become antibody-secreting cells; therefore, antibody is scant to absent. Circulating B cells in the blood are similarly missing. This disorder presents similarly to transient hypogammaglobulinemia of infancy, previously described, in that the first few months of life are uneventful, attributable to passive maternal antibody. Thereafter, the child develops frequent sinopulmonary infections, mostly due to encapsulated gram-positive organisms, especially S. pneumoniae. Most of these bacterial infections can be readily treated with antibiotics; their frequency, however, can lead to destructive processes in the lungs and sinuses. Patients with agammaglobulinemia are also subject to severe or prolonged infections with nonenveloped viruses, especially the enteroviruses. Chronic enteroviral meningitis is an especially interesting syndrome seen almost exclusively in patients with XLA.16 Progressive viremia and paralytic poliomyelitis secondary to live attenuated oral polio vaccine (no longer available in the United States) has also been described in these patients. T-cell function is normal or nearly so. Therefore, patients with this disorder do not suffer from fungal infections or prolonged infection with enveloped viruses, such as respiratory syncytial virus (RSV) or influenza virus. The gene defect for XLA has been located to the X chromosome at position Xq21.2-22. Carrier females are immunologically normal. Physical examination of these children is generally unremarkable except that lymphoid tissue is scant. If a patient with a compatible clinical history has no visible tonsils and no palpable lymph node enlargement, the diagnosis of XLA should be strongly considered.
Common Variable Immunodeficiency (CVID)
CVID is a complex and heterogeneous immune dysregulation syndrome characterized by hypogammaglobulinemia, recurrent bacterial infections, and a variety of immunological abnormalities. In addition to recurrent infections (primarily upper and lower respiratory infections), patients with this syndrome are also at increased risk for autoimmune disease and malignancy.17 The incidence is approximately 1 in 100,000. The exact genetic defect is unknown, and it is likely that several different defects can result in the CVID phenotype. CVID can occur at any age; in most patients the disease does not become clinically apparent until the second or third decade of life. Its presentation can mimic that of cystic fibrosis. Most cases are sporadic but sometimes there is a family history of CVID (or of selective IgA deficiency). Unlike patients with XLA, circulating numbers of B cells are usually normal. However, IgG, IgM, and IgA levels are all usually decreased. In at least some cases of CVID, the fundamental immunologic abnormality is probably due to a defect in T cell help.18
Like patients with XLA, patients with CVID experience recurrent infections (especially sinusitis, otitis media, and pneumonia) with encapsulated organisms. Diarrhea, particularly due to Giardia, is common. Persistent enteroviral meningitis has also been described but is less common than in patients with XLA.19 Some patients with CVID also develop infections typical of patients with T-cell defects, such as Pneumocystis pneumonia.
About 20% of patients with CVID develop an autoimmune disease. Hemolytic anemia and immune thrombocytopenic purpura are the two conditions most commonly seen. Lymphoproliferation occurs in about 30% of patients and is manifested by adenopathy and splenomegaly. Some patients develop malignant lymphoma.
Hyper-IgM Syndrome
Two boys with a clinical syndrome resembling that of X-linked agammaglobulinemia who had grossly elevated levels of IgM and low to absent IgG and IgA were the first case reports of the syndrome now called Hyper-IgM syndrome. Subsequently, female cases were also reported. The phenotype is similar to that of XLA, except that patients with Hyper-IgM syndrome are also prone to some opportunistic infections, especially Pneumocystis jiroveci pneumonia. Patients with this syndrome also have a high incidence of autoimmune hematologic problems, including hemolytic anemia, thrombocytopenic purpura, and, especially, neutropenia. The disorder is caused by the inability to “class switch.” Resting B cells express IgM; antigenic stimulation, therefore, produces IgM first. In order to produce IgG or IgA, class switching must take place. In the absence of the ability to class switch, IgM levels become elevated. Although most patients have very elevated total IgM levels (more than 1,000 mg/dL), some patients have levels in the normal range. IgG levels are usually less than 150 mg/dL and IgA is not detectable. Class switching requires two signals, one
of which is the interaction of CD40 (expressed on B cells) and CD154 (CD40 ligand, expressed on T cells). Patients with the X-linked form of Hyper-IgM syndrome are incapable of producing functional CD154. This defect can be detected in utero.20 Patients with the less common autosomal recessive variant have a different genotype. In these patients, class switching does not take place, despite appropriate cross-linking of CD40, suggesting a downstream signaling defect.21
of which is the interaction of CD40 (expressed on B cells) and CD154 (CD40 ligand, expressed on T cells). Patients with the X-linked form of Hyper-IgM syndrome are incapable of producing functional CD154. This defect can be detected in utero.20 Patients with the less common autosomal recessive variant have a different genotype. In these patients, class switching does not take place, despite appropriate cross-linking of CD40, suggesting a downstream signaling defect.21
Selective IgA Deficiency
IgA is the most plentiful immunoglobulin in the body. Much of it is locally produced and secreted, and the presence of specific IgA on mucosal surfaces has been shown to be protective against a variety of infections that begin with replication at those sites. These two facts, taken together, would suggest that IgA deficiency would predispose to frequent and severe infections of the respiratory and gastrointestinal tracts. However, most people with IgA deficiency do not know they are IgA deficient, as they are entirely asymptomatic.22 It has been estimated that from 1 in 300 to 1 in 700 individuals lacks appreciable IgA, which makes IgA deficiency the most common immunoglobulin deficiency.
IgG subclass Deficiency
IgG can be divided into four different subclasses. Some people have a deficiency of one or more of these subclasses, sometimes even in the face of a normal total IgG. There is considerable controversy regarding the role of IgG subclass deficiency in patients with frequent sinopulmonary infections. Patients with frequent, nonsevere infections who have deficiencies of one or more of the IgG subclasses have certainly been described. However, subclass deficiencies can also be found in healthy control subjects, particularly children in the first 4 years of life.
The fundamental question is whether there is a difference in the function of the different IgG subclasses; if there is not, then as long as total IgG levels are normal, subclass deficiency would not be expected to cause any problems. When subclass deficiencies were first described, there were reports that IgG2 was more important in the response to polysaccharide antigens. A failure to respond well to polysaccharide antigens would certainly predispose one to the type of infections generally seen in antibody-deficient patients. However, this was an in vitro finding, and it was not supported by subsequent in vivo experiments. Many papers describing frequent and severe infections in patients who have a combination of IgA deficiency and IgG subclass deficiency have been published.23,24 Despite this, it is not clear how much of the problem stems from the IgA deficiency, and how much, if any, is attributable to the associated IgG subclass deficiency. It is probably fair to say that IgG subclass deficiency by itself is rarely a cause of significant recurrent infectious problems. A small percentage of patients with IgG subclass deficiency later go on to develop common variable immunodeficiency. In addition, in some families, one individual will have selective IgA deficiency, another will have IgG subclass deficiency, and yet another will develop CVID. Thus, these conditions may represent a spectrum of abnormalities of B-cell maturation.
One expert has suggested the following criteria for diagnosis of clinically significant IgG subclass deficiency: (a) a history of recurrent bacterial infections, primarily respiratory; (b) impaired response to immunization with protein and/or polysaccharide antigens; (c) significant reductions in serum concentrations of one or more IgG subclasses; and (d) age 4 years or older.25
Failure to Respond to Polysaccharide Antigens
Antibodies to protein antigens and antibodies to polysaccharide antigens are made through different pathways. Antibodies to polysaccharides are made by thymus-independent (T-independent) pathways. These pathways are generally not well developed in children until about the age 2 years. This explains why babies do not make good responses to polysaccharide vaccines (like the 23-valent polysaccharide pneumococcal vaccine or the quadrivalent meningococcal vaccine). Some patients never really fully develop the ability to mount responses to polysaccharide antigens.26 These patients may be plagued by recurrent infections with encapsulated bacteria, especially S. pneumoniae. Their immunoglobulin levels may be normal, and IgG subclasses may also be normal, although low levels of IgG2 are not uncommon.
Human Immunodeficiency Virus infection (HIV)
Early Complement Component Deficiency
Complement deficiencies are considerably less common than immunoglobulin deficiencies. Deficiency of one of the early components (especially C1q), however, closely resembles immunoglobulin deficiency. These patients generally suffer recurrent sinopulmonary infections with encapsulated bacteria (S. pneumoniae is the most common pathogen).27 As a secondary effect, serum IgG may be decreased; IgG2 and IgG4 are almost always low.28
Patients with C3 deficiency are generally more severely affected and suffer more frequent recurrences of infection. They are also more likely to have systemic infections such as bacteremia or meningitis. About 80% of these children also develop autoimmune disorders.27
Evaluation
Evaluation of the immune system should be undertaken when the child exceeds the expected number, type, or severity of infections. Often, the history and physical examination alone will strongly suggest that the child’s immune system is normal; in that case, reassurance should be provided. The following are elements that may be included when a work-up seems indicated.
Evaluation Elements
Complete Blood Count (CBC)
Though nonspecific, much useful information can be obtained from the CBC, including the presence or absence of anemia, neutropenia, neutrophilia, eosinophilia, and lymphopenia.
Peripheral Blood Smear
The presence of Howell-Jolly bodies suggests functional or anatomic asplenia.
Immunoglobulin Levels
Most laboratories are able to provide total immunoglobulin levels in a reasonable amount of time. Generally, IgG, IgA, and IgM levels are reported unless others are specified. When hyper-IgE syndrome (discussed later) is suspected, it is advisable to ask for IgE levels as well. It is not necessary to ask for IgG subclass levels on a routine basis. Immunoglobulin levels do not provide information about the production of specific immunity, but this is still a reasonable first screening test.
Specific Antibody Titers
It is often useful to know whether the patient is able to mount an antibody response to an antigen to which he or she is known to be exposed. Most patients have had some immunizations; therefore, antibody levels against one or more of these immunogens can be obtained. Antitetanus and antidiphtheria antibody titers are commercially available. These antibodies are of the T-dependent type. To test T-independent responses, one can administer the 23-valent polysaccharide pneumococcal vaccine and measure the type-specific antibody response 1 month later. Ideally, prevaccination and postvaccination titers are measured simultaneously. If the child has previously received the 7-valent conjugated pneumococcal vaccine, one cannot ascribe a response to those 7 serotypes (4, 6, 9, 14, 18, 19, and 23) as indicative of a functional response to polysaccharide antigens. Also, recall that children younger than about 2 years are expected to have a very poor immune response to polysaccharide antigens, no matter how they are tested.
If the child has a protective antibody titer to tetanus but a low titer to diphtheria, the child should be given a booster vaccine, and the level rechecked in 2–3 weeks. If it boosts into the normal range, the patient is able to mount appropriate T-dependent antibody responses.
HIV Antibody
Unless it can be specifically proven that the child could not possibly have HIV infection, HIV antibody titer should be obtained as part of the workup of any child with frequent, recurrent, or recalcitrant infections. Infection with an opportunistic pathogen is even more suggestive of the possibility of HIV infection.
Sweat Chloride Measurement
Examination of Ciliary Structure and Function
If more common causes of recurrent respiratory infections have been ruled out, an ENT (ear, nose and throat) physician can be consulted to perform a ciliary biopsy.
Lung Imaging
A chest radiograph should be obtained to look for evidence of chronic lung disease or bronchiectasis. If the patient has a suspected anatomic abnormality or foreign body, a fine-cut chest CT (computed tomography) should be obtained.
CH50 (Total Hemolytic Complement)
A CH50 is a reasonable screening test for patients in whom early complement component deficiency is suspected.
Asthma and Allergy Testing
If suspected based on history and physical examination, pulmonary function testing in children more than 6 years old or a trial of bronchodilator therapy (children younger than 6 years) should be considered. Referral to an allergist for skin testing may be indicated if the child has symptoms suggestive of allergies (such as sneezing, itching, or a prominent seasonal component).
Management
Because the infections are not severe, patients with transient hypogammaglobulinemia of infancy usually do not require intravenous immunoglobulin replacement therapy (IVIG). They can be followed every 3–6 months for serial evaluation of their immunoglobulin status. Recovery of serum levels to normal usually occurs spontaneously sometime before the second birthday. The prognosis is excellent.
As patients with XLA are incapable of mounting antibody responses, standard vaccines need not be given. Lifetime replacement with IVIG is required. With IVIG replacement, many patients are able to lead essentially normal lives.
Unlike XLA, not all patients with CVID require monthly IVIG infusions. The decision to treat patients with IVIG should be based on measurement of the patient’s functional antibody response and on the frequency and severity of recurrent infections. For patients in whom IVIG is indicated, 200–400 mg/kg is given every 3–4 weeks to maintain trough serum levels of IgG greater than 400 mg/dL.29
Treatment of Hyper-IgM syndrome with IVIG corrects the immune deficiency and usually also corrects neutropenia, if it is present. Some patients may require G-CSF as well.
One must be careful about the use of IVIG as a “diagnostic trial” in the patient with recurrent infections and IgG subclass deficiency. IVIG provides very effective passive immunity to many of the pathogens normally encountered in the first few years of life. For example, boys with XLA who are placed on monthly IVIG in the first few months of life have far fewer than the 6–8 colds per year that the normal child experiences.25 This same benefit is obtained in healthy children, for whom this intervention is obviously not indicated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree