HIV Infection and AIDS
Introduction
This chapter will discuss several aspects of HIV infection and AIDS, including maternal-infant transmission, diagnostic tests, treatments, and prognosis. Detailed information about management of these children is beyond the scope of this book. Care of the HIV-infected baby, child, or adolescent is complex and rapidly evolving. It is hoped that the clinician can learn enough from this chapter to feel comfortable about the basics. Once HIV infection has been definitively diagnosed, care of these children should be done in concert with a physician who has specific training in pediatric infectious diseases and experience caring for children with HIV infection.
Modes of Transmission
Maternal-Fetal (Vertical Acquisition)
Vertical transmission remains the most common way that pediatric patients become infected with HIV. It may occur during gestation, during the intrapartum period, or in the immediate postpartum period. In the absence of any steps to prevent transmission, 20–40% of babies born to HIV-positive mothers will become infected.1,2,3 It is thought that most babies who become infected do so in the peripartum period. Earlier infection does occur, as HIV has been found by in situ PCR (polymerase chain reaction) in the tissues of aborted fetuses during the second trimester.4 Transmission to babies after they have been born usually occurs through breastfeeding. This remains a problem in the developing world, where breast milk may be the only form of nutrition available, but it has been virtually eliminated in the United States, where clean water for formula is readily available.
The single most important factor in determining the risk of transmission is the viral load in maternal plasma. In one study of 552 maternal-infant pairs, none of 57 babies whose mother’s viral load was less than 1,000 copies/mL became infected, but 26 (41%) of 64 babies whose maternal viral loads were greater than 100,000 copies/mL became infected.2 The plasma viral load has been shown to correlate with the amount of HIV in both cervical mucus and in vaginal secretions,5 which is a plausible explanation for the phenomenon. Transmission of HIV is also augmented by concomitant maternal syphilis.6
In the early 1990s, Pediatric AIDS Clinical Trials Group (PACTG) Protocol 076 demonstrated the effective prevention of maternal-infant transmission. This double-blind, prospective, placebo-controlled trial showed that the administration of zidovudine (ZDV or sometimes called azidothymidine [AZT]) to pregnant mothers during the last trimester of pregnancy, coupled with intravenous AZT in the immediate peripartum period and six weeks of oral AZT therapy for their neonates decreased maternal-infant transmission from 28% to 8%.3 Maternal-infant transmission decreased greatly after this standard was adopted. Of course, the success of this form of prophylaxis depends upon knowing which mothers are HIV infected. This, in turn, relies upon prenatal counseling and universal HIV testing of pregnant women. When testing is offered to pregnant women, the majority accepts it. In one exemplary early program, counseling and testing increased from 21% to 95% of pregnancies, and the perinatal HIV transmission rate in North Carolina was documented to decrease from 31% to 3.1% statewide.7 Testing pregnant women for HIV infection should be routine, similar to testing for syphilis or rubella immunity.
At any plasma viral load, adherence to the 076 AZT protocol decreases transmission rates. Factors that decrease the chances that a mother-infant pair will receive all three components of the protocol include older maternal age, CD4 counts greater than 500/μL, and cocaine or heroin use.8 Because following all three parts of the 076 protocol is cumbersome and expensive, it has been difficult to implement in developing countries. Trials in which the maternal portion, the neonatal portion, or both
were truncated suggest that a longer maternal component is more important than a longer neonatal component.9 In these trials, the intrapartum intravenous AZT was replaced with frequent oral AZT. In another attempt at simplification, mothers and their infants were each given a single dose of nevirapine.10 This regimen decreased transmission significantly only in mothers not already receiving standard antiretroviral therapy.11 In one open-label trial, the combination of AZT and lamivudine (3TC) was also shown to be effective in preventing transmission. However, a common 3TC resistance mutation was demonstrated in 52 (39%) of 132 neonates, and adverse events, including severe anemia and thrombocytopenia, were common. Two children who were subsequently proven to be uninfected with HIV died before the age of 12 months from mitochondrial toxicities thought to be related to the combination therapy.12
were truncated suggest that a longer maternal component is more important than a longer neonatal component.9 In these trials, the intrapartum intravenous AZT was replaced with frequent oral AZT. In another attempt at simplification, mothers and their infants were each given a single dose of nevirapine.10 This regimen decreased transmission significantly only in mothers not already receiving standard antiretroviral therapy.11 In one open-label trial, the combination of AZT and lamivudine (3TC) was also shown to be effective in preventing transmission. However, a common 3TC resistance mutation was demonstrated in 52 (39%) of 132 neonates, and adverse events, including severe anemia and thrombocytopenia, were common. Two children who were subsequently proven to be uninfected with HIV died before the age of 12 months from mitochondrial toxicities thought to be related to the combination therapy.12
Ultimately, antiretroviral therapies that best treat the mother’s disease probably result in the best possible outcomes for both mother and baby.13 The standard of care for pregnant women with HIV infection now includes use of multiple-drug therapy, at least one of which should be AZT. Fears that there would be long-term adverse outcomes in HIV-uninfected babies whose mothers received AZT during pregnancy have been largely assuaged. One study showed a slightly increased risk of first febrile seizure in these children,14 but another study that followed, for a mean of 4.2 years, HIV-uninfected children whose mothers were enrolled in protocol 076 showed no significant difference in growth, cognition, or development in those whose mothers received AZT compared with those whose mothers received placebo.15
Other factors shown to be independently associated with increased risk of maternal-infant transmission include maternal obesity and procedures that cause the baby to contact maternal blood or cervical secretions.16 In light of this finding, it is not surprising that elective cesarean delivery prior to rupture of membranes decreases transmission by about half.17 However, the efficacy of cesarean delivery in the era of highly active antiretroviral therapy (HAART) has not been evaluated. Enthusiasm for elective cesarean birth has been waning, due, in part, to the fact that transmission rates are already less than 2% in many populations, and that cesarean delivery is not without risk, especially because wound healing may be poor in patients with advanced HIV disease.
Postnatal transmission via breast milk occurs in about 15% of babies who are exclusively breastfed.18 Thus, HIV-positive mothers should not breastfeed their babies unless there is no possible alternative source of nutrition.
Sexual and Parenteral Transmission (Horizontal Transmission)
Horizontal transmission of HIV is usually from either sexual contact or through intravenous drug use. In recent years, there has been an increase in the number of teenagers being infected, usually through unprotected sex. It has been estimated that at least half of all new HIV infections in the United States occur among people below the age of 25, and that the majority of these young people are infected through sexual activity.19 The message of so-called safe sex (more appropriately termed “safer sex”) has been preached to a large number of people at risk. Unfortunately, adherence to this idea has been declining as improved therapies for HIV have become widely used.20 Some of this laxity is due to the perception that persons on HAART are unlikely to transmit the infection. Remarkably, surveys reveal that the relaxed attitude toward HIV transmission is largely attributable to the perception that HIV infection is now a manageable disease. In one survey of 80 women, whereas only 5% of respondents believed that safer sex was no longer important, 15% said that they no longer insist on condom use, and 40% agreed that “AIDS is now a less serious threat.”21 Many teenagers, in particular, engage in high-risk behavior even though they understand the risk factors for HIV infection. This may be caused by the tendency of teenagers to feel a sense of immortality.
Presenting Features of HIV Infection
Most perinatally-infected babies are born to known HIV-positive mothers. Testing pregnant women for HIV during prenatal care identifies the risk to the fetus early, and mothers are given antiretroviral therapy. Babies born to these mothers are followed very closely and serially tested for the presence of
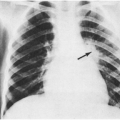
the virus. Management of these babies is outlined in Box 20-1. With the exception of babies infected in utero, who may be born prematurely and develop severe signs and symptoms early in life, most babies with known exposure who prove to be infected are not symptomatic at the time of their diagnosis. There is a tendency for HIV-infected babies to be born slightly prematurely, and to be small for gestational age.24 Other early signs, if present, include generalized lymphadenopathy, eczema, hepatosplenomegaly, recalcitrant thrush, chronic diarrhea, failure to thrive, spasticity/hypertonicity, or recurrent bacterial infections. Dramatic presentation in the first months of life with life-threatening Pneumocystis jiroveci (formerly carinii) pneumonia is now uncommon.
the virus. Management of these babies is outlined in Box 20-1. With the exception of babies infected in utero, who may be born prematurely and develop severe signs and symptoms early in life, most babies with known exposure who prove to be infected are not symptomatic at the time of their diagnosis. There is a tendency for HIV-infected babies to be born slightly prematurely, and to be small for gestational age.24 Other early signs, if present, include generalized lymphadenopathy, eczema, hepatosplenomegaly, recalcitrant thrush, chronic diarrhea, failure to thrive, spasticity/hypertonicity, or recurrent bacterial infections. Dramatic presentation in the first months of life with life-threatening Pneumocystis jiroveci (formerly carinii) pneumonia is now uncommon.
Sometimes babies are born to mothers whose HIV status is unknown, either because they received no prenatal care or because they refused or were not offered HIV testing during pregnancy. Others test negative early but become infected with HIV during the course of the pregnancy. Rapid oral tests for HIV infection have been developed and may become important in the management of these high-risk mothers and their babies.
BOX 20-1 Management of the Known HIV-exposed Baby
| In the hospital: Thorough physical examination Careful maternal history (maternal viral load, CD4 cell count, antiretroviral therapy, receipt of intrapartum IV AZT, other blood borne or sexually transmitted diseases) Begin oral AZT, 2 mg/kg per dose,a every 6 hours; first dose within 4–6 hours of birth HIV DNA PCR at time of hospital discharge (never use cord blood) Ensure that mother seeks a general pediatrician to assume primary care |
| After discharge: Follow-up visits with both a general pediatrician and an infectious diseases specialist Thorough physical examinations, with special emphasis on growth, presence or absence of lymphadenopathy and hepatosplenomegaly, skin condition, and neurologic exam Recalculate AZT dose, as rapid growth may lead to inadequate dosing Repeat DNA PCRs, preferably at ages 2–4 weeks and more than 4 months (some experts recommend one RNA PCR at some point during the first 4 months, as limited data suggest it may be slightly more sensitive) Stop AZT at 6 weeks of age At 6 weeks, babies whose growth, development, and physical examinations are entirely normal and who have two negative PCR tests have a posttest probability of HIV infection that approaches 1/10,000. Therefore, trimethoprim-sulfamethoxazole prophylaxis is probably not necessary Any baby who does not fulfill all of the above criteria should be started on trimethoprim sulfamethoxazole until the post-4 month PCR is obtained and proved to be negative When the baby is at least 4-months-old, has had at least 3 negative PCR tests, and is clinically well, he or she can be discharged from the infectious Diseases clinic with a “clean bill of health.” Documenting seroreversion is not necesary |
| aAnedotally, 3 mg/kg taken orally every 8 hours is functionally equivalent to 2 mg/kg every 6 hours, easier on family. For term babies requiring IV AZT because they cannot take by mouth for any reason, the dosage is 1.5 mg/kg IV every 6 hours. For premature babies (less than 34 weeks) able to take medicines orally, the experimental dosage is 1.5 mg/kg every 12 hours for 2 weeks, then 2 mg/kg every 8 hours thereafter until they are the size of a normal newborn baby. Source: Modified from: Benjamin DK Jr, Miller WC, Fiscus SA, et al. Rational testing of the HIV-exposed infant. Pediatrics 2001;108:e3, with permission. |
The natural course of HIV infection in vertically infected infants varies greatly. Most develop very high viral loads early in life, which may spontaneously drop within the first few months, but may plateau at a steady state that is still quite high (usually greater than 100,000/mL). Failure to thrive is a frequent problem. Linear growth is decreased within the first 6 months of life in HIV-infected infants, and the severity of the problem correlates with the magnitude of the viral load.25,26 Neurodevelopmental delays are also common.25,26 Decreased growth patterns are sustained through at least age five years. In a study that compared 95 infected children with 439 uninfected controls, poor growth was associated with history of pneumonia (relative risk [RR] 9), maternal cocaine use (RR 3), low CD4 cell counts (RR 2), and receipt of
antiretroviral therapy by age 3 months (RR 3). After adjusting for pneumonia and antiretroviral therapy, the child’s viral load remained associated with failure to thrive.27
antiretroviral therapy by age 3 months (RR 3). After adjusting for pneumonia and antiretroviral therapy, the child’s viral load remained associated with failure to thrive.27
Classification
The Centers for Disease Control and Prevention (CDC) classification scheme for infants and children with HIV infection has changed several times. The scheme currently in use is quite straightforward. Patients are classified based on symptoms designated by letters—N, for no symptoms; A, for mild symptoms; B, for moderate symptoms; and C, for severe symptoms—and immune suppression designated by numbers: 1, for no suppression; 2, for moderate suppression; and 3, for severe suppression. The type of clinical conditions and degree of immune suppression that qualify a patient for the various designations is shown in Box 20-2.
BOX 20-2 CDC Classification Scheme for Patients with HIV Infection
| Letter Designations N. No symptoms, or only one of the symptoms from A, below. A. Mild symptoms. Two of more of the following, without any symptoms from B or C, below. Lymphadenopathy, hepatosplenomegaly, dermatitis, parotitis, recurrent or persistent upper respiratory tract infections, sinusitis, or otitis media B. Moderate symptoms. Anemia, neutropenia, or thrombocytopenia; invasive bacterial infection (sepsis, pneumonia, meningitis); persistnt trush at age greater than 6 months; cardiomyopathy; recurrent or chronic diarrhea; herpes group virus infections (CMV prior to age 1 month; recurrent HSV stomatitis; HSV bronchitis, pneumonitis, or esophagitis before age 1 month; two episodes or two-dermatomal herpes zoster [shingles]; complicated chickenpox); LIP; leiomyosarcoma; nephropathy; nocardiosis; toxoplasmosis before 1 month of age; fever for greater than 1 month. C. Severe symptoms. Multiple or recurrent invasive bacterial infections; esophageal or pulmonary candidiasis; disseminated fungal infections (coccidioidomycosis, histoplasmosis, or extrapulmonary cryptococcosis); severe herpes group virus infections (persistent mucocutaneous ulcer; bronchitis, pneumonitis, or esophagitis in child greater than 1 month of age; CMV disease anywhere but liver, spleen, or lymph nodes, onset after 1 month of age; Kaposi sarcoma); diarrhea for greater than one month caused by protozoans (cryptosporidiosis, isosporiasis); PCP; PML (progressive multifocal leukoencephalopathy); severe mycobacterial infection (extrapulmonary TB, disseminated nontuberculous mycobacterial infection); recurrent Salmonella sepsis; toxoplasmosis of the brain after age 1 month; oncologic problem (primary brain lymphoma, burkitt lymphoma, large-cell lymphoma); encephalopathy; wasting syndrome. | ||||||||||||||||||||||||||||||||||
Number designations
| ||||||||||||||||||||||||||||||||||
| Abbreviations: CMV, cytomegalovirus, HSV, herpes simplex virus; LIP, lymphoid interstitial pneumonitis (see text); PCP, Pneumocystis jiroveci pneumonia (see text); PML, progressive multifocal leukoencephalopathy (rare in children); TB, tuberculosis. | ||||||||||||||||||||||||||||||||||
Treatment Options
Antiretroviral Therapy
An introduction to the various classes of antiretroviral agents, their uses, and their side effects and limitations follows. The actual clinical approach to using these agents is discussed briefly thereafter.
Nucleoside Analogue Reverse Transcriptase Inhibitors (NRTIs)
The first class of effective antiretrovirals was the nucleoside analogues. These agents work by blocking
the action of reverse transcriptase. Reverse transcriptase is an enzyme that the virus uses to change its genomic RNA into DNA. Once DNA is formed, it is integrated into the host cell genome. When reverse transcription is inhibited, RNases (ribonucleases) in the cytoplasm are able to destroy the viral RNA.
the action of reverse transcriptase. Reverse transcriptase is an enzyme that the virus uses to change its genomic RNA into DNA. Once DNA is formed, it is integrated into the host cell genome. When reverse transcription is inhibited, RNases (ribonucleases) in the cytoplasm are able to destroy the viral RNA.
AZT is the prototype nucleoside analogue drug. Many others have been developed (see Box 20-3). The virus is able to develop resistance to these agents very rapidly; thus, they should never be used alone for treatment. The combination of AZT and 3TC has been used extensively because resistance to AZT tends to confer susceptibility to 3TC, and vice versa.28 Because they are analogues of the same nucleoside (thymidine), AZT and d4T (stavudine) compete with each other and should never be used together.
In a typical regimen, a pair of nucleoside analogues is coupled with a non-nucleoside reverse transcriptase inhibitor or with a protease inhibitor. These types of combination or “cocktail” therapies have been called highly active antiretroviral therapy (HAART).
BOX 20-3 Antiretroviral Agents
| Nucleoside reverse transcriptase inhibitors AZT (azidothymidine, ZDV, zidovudine, Retrovir) 3TC (lamivudine, Epivir) d4T (stavudine, Zerit) ddI (didanosine, Videx) ddC (zalcitabine, dideoxycytidine, Hivid) abacavir (Ziagen) emtricitabine (Emtriva) tenofovir (Viread, technically a nucleo TIDE RT inhibitor) |
| Non-nucleoside reverse transcriptase inhibitors Nevirapine (Viramune) Efavirenz (Sustiva) Delavirdine (Rescriptor) |
| Protease Inhibitors Nelfinavir (Viracept) Saquinavir (Fortovase, Invirase) Ritonavir (Norvir) Indinavir (Crixivan) Amprenavir (Agenerase) Lopinavir/ritonavir (Kaletra) Fosamprenavir (Levitra) Atazanavir (Reyataz) |
| Fusion Inhibitors T-20 (enfuvirtide, Fuzeon) |
Non-nucleoside Reverse Transcriptase Inhibitors (NNRTIs)
These drugs also inhibit reverse transcriptase, but do it by a different mechanism. Non-nucleoside agents are much more potent than nucleosides. The combination of two nucleoside agents and a non-nucleoside has been shown to be just as effective as two nucleosides and a protease inhibitor.29,30 Nevirapine, a non-nucleoside agent, is given twice a day. It has been associated with severe and even fatal hepatotoxicity. Rarely, nevirapine has been associated with untoward events in pregnant women, particularly in those with CD4 cell counts greater than 250/μL.
Efavirenz is the other commonly used non-nucleoside agent. Its principal toxicity involves the central nervous system (CNS). Patients taking efavirenz may experience dysphoria and complain of feeling “spaced out,” and dreams have been reported to be vivid and sometimes frightening. This side effect is much more common in adults than in children and tends to subside with continued therapy. There are concerns of teratogenicity based upon animal studies. Therefore, efavirenz should never be used in pregnant patients or in adolescents in whom the possibility of becoming pregnant cannot be eliminated.
Protease Inhibitors (PIs)
During replication, HIV produces a polyprotein (a long chain of amino acids that includes multiple protein molecules linked together). This polyprotein is not functional and must be broken up into its constituent proteins by a viral enzyme called protease. Protease inhibitors act by preventing this enzyme from cleaving the polyprotein into usable protein molecules. These drugs are highly effective when used in combination with other antiretrovirals. If used alone or with only one other drug, resistance develops rapidly.
Of all the protease inhibitors currently available (Box 20-3), nelfinavir is the one that has the longest track record in the treatment of childhood HIV infection, mainly because it was the first to be available in liquid form. Ritonavir, difficult to tolerate as the only PI, is now being used in combination with other PIs because it boosts levels by its strong inhibition of cytochrome P450 in the liver.
Side effects of protease inhibitors are numerous. Perhaps most troublesome are hypertriglyceridemia, hypercholesterolemia, and lipodystrophy (discussed follows).
New Classes of Antiretroviral Agents
Although other targets have been identified, such as integrase, development of agents to suppress targets other than reverse transcriptase and protease has been difficult. Enfuvirtide (T-20, Fuzeon) is a new drug whose function is to inhibit fusion of the viral envelope with cell membranes. It has recently been FDA-approved for use in adults and children aged 6 years and older. Inhibition of fusion inhibits cell-to-cell spread of the virus as well as slowing the penetration phase of viral replication. In highly antiretroviral-experienced patients, the addition of enfurvitide versus placebo to an optimized background regimen improves viral control, and concomitant increases in CD4 cell counts are also seen.31 As with other classes of antiretrovirals, resistance develops very quickly if they are used alone. T-20, the prototype of the class, must be administered by subcutaneous injection twice a day, which can be a major impediment for some patients. Injection site reactions (including subcutaneous nodules, itching, redness, swelling, and rarely cellulitis) were seen in 98% of participants in one clinical trial, although the majority of these were manageable and not dose limiting.31
Highly Active Antiretroviral Therapy (HAART)
Optimally, antiretroviral therapies are given in combination. The principal reason for this is that resistance emerges very quickly when they are used alone. Beyond this simple concept, the clinical work of initiating, monitoring, maintaining, and changing antiretroviral therapies is vastly complicated and generally not the subject of much consensus; thus, a thorough discussion is beyond the scope of this book.
Increasingly, physicians are realizing that antiretroviral therapy is probably best when individualized. Much of the success of any regimen is dependent on the patient’s adherence to it. A study in adults demonstrated a striking difference in rates of virologic failure (defined as more than 400 viral copies/mL a median of 6 months later) between those with 95% adherence or more (22% had virologic failure) and those with 80–94.9% adherence (61% had virologic failure).32 Eighty percent of those who took less than 80% of the prescribed doses experienced virologic failure.32 This problem is compounded somewhat in pediatrics, because adherence is often the job of someone other than the patient, usually the primary caregiver. Primary caregivers may also be HIV infected, and are sometimes ill or even suffer from dementia associated with HIV infection. They may not be well organized and may not be able to remember each dose. In addition, young children (particularly from about age 18 months to 4 years) may refuse to take their medications. In these children, consideration should be given to the placement of a gastrostomy tube for medication administration.33
The physician caring for children with HIV infection is faced with many difficult decisions. HIV infection should be conceptualized as a “decades-long” disease, and the approach to therapy tempered by that concept. In other words, sometimes one most forego the “optimal” short-term regimen in favor of one the patient is more likely to adhere to and tolerate. In pediatric HIV, especially, it is distressingly easy to “burn” regimens and entire classes of antiretroviral drugs, leaving the patient with extremely limited options for future treatment. The complexities involved in deciding exactly when and how to switch ARV treatment regimens are beyond the scope of this chapter. These decisions need to be made by (or in concert with) a physician with appropriate training and clinical experience. Even if adherence is perfect, antiretroviral therapies often cause side effects and complications that can become dose limiting and affect the patient’s quality of life. Some of these complications are discussed in the following text. General ideas about appropriate antiretroviral regimens are shown in Box 20-4.
Complications of Antiretroviral Therapy
Lipodystrophy
As more experience with HAART is gained, more long-term side effects of therapy are discovered. One of the more troubling of these is called lipodystrophy, the essence of which is fat redistribution. There are many different forms of the condition: some patients have peripheral fat wasting, some have truncal fat accumulation, some have dyslipidemia and hypercholesterolemia, and some have insulin resistance. In most cases, patients have a combination of two or more of these problems.
BOX 20-4 Combination Antiretroviral Therapies
| 2 NRTIsa plus 1 PIb 2 NRTIs plus 1 NNRTI 3 NRTIs (AZT/3TC/abacavir)c 1 or 2 NRTIs plus 1 NNRTI plus 1 PIb |
| Abbreviations: NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor. aThe following NRTI combinations are not recommended: AZT plus d4T or 3TC plus FTC (competitive inhibition), ddI plus d4T (too much toxicity), tenofovir plus either ddI or abacavir, ?{AQ4} ddI plus abacavir. bPIs (saquinavir, indinavir, and amprenavir but not nelfinavir) can be boosted with low-dose ritonavir). cRecently shown to be less effective than PI-containing or NNRTI-containing regimens. |
Lipodystrophy occurs with distressing frequency. Large surveys and prospective studies have documented that up to 41% of patients on HAART regimens experience increased abdominal girth,34 32% have decreased facial fat, and 57% have hypercholesterolemia.35 In one small study of children with HIV infection, even the 28 children without clinically apparent lipodystrophy had increased central fat and peripheral lipoatrophy documented by dual-energy x-ray absorptiometry (“dexa”) scanning.36 Risk factors for the development of lipodystrophy include advanced disease,37 duration of HIV positivity,34 use of stavudine (d4T) for longer than a year,37 and use of indinavir or ritonavir.34
Long thought to principally be a complication of protease inhibitor therapy, it has now been clearly established that nucleoside reverse transcriptase inhibitors (especially stavudine and didanosine) can also lead to lipodystrophy. One hypothesis is that a combination of these NRTIs and the protease inhibitors leads to the full syndrome of lipodystrophy, with the NRTI affecting the mitochondrial function of the adipocytes and the protease inhibitor causing insulin resistance and impaired adipocyte maturation.38
Patients with lipodystrophy say that their quality of life is impaired; self-esteem issues are the primary determinant of this problem.37 Adherence to therapy may also suffer, as patients often stop taking the medications in an effort to treat the syndrome.39 Unfortunately, lipodystrophy is not that easily reversed. An exercise program combined with a moderate-fat, low-glycemic-index, high-fiber diet, however, has been shown to improve several aspects of lipodystrophy.40 Interestingly, even though many patients with lipodystrophy syndrome have elevated levels of triglycerides and cholesterol, lipodystrophy in patients with HIV does not present as large a risk factor for atherosclerotic coronary vascular disease as similar cholesterol and triglyceride levels would in the HIV-uninfected patient.41
Mitochondrial Toxicity
The principal toxicity of the nucleoside analogue drugs occurs in the mitochondria, and is a direct extension of the pharmacologic activity of these agents. Mitochondria contain reverse transcriptases (principally polymerase gamma and telomerase), and these are inhibited to varying degrees by reverse transcriptase inhibitors. Any drug of this class can produce mitochondrial toxicity, but ddC (dideoxycytidine), ddI (didanosine), and d4T are more likely to do so than are 3TC and AZT. Abacavir is the least mitochondria-toxic of all the NRTIs. When suppression of mitochondrial polymerases occurs, tissues with high metabolic needs are the most likely to be affected. Clinically, the syndrome usually presents with vague symptoms, such as gastrointestinal upset, dyspnea, and fatigue. Occasionally, it manifests as severe myalgias and/or myocarditis. Laboratory studies show mildly elevated aminotransferase levels and elevated lactate. Obesity, female gender, and preexisting liver disease are risk factors for the development of symptomatic mitochondrial toxicity states. Micronutrient deficiencies may increase the risk of developing the syndrome. Symptoms of mitochondrial toxicity syndrome usually resolve when the offending nucleoside is discontinued.42
Immune Reconstitution Inflammatory Syndrome (IRIS)
Soon after the advent of HAART, it became clear that some patients were developing worsening symptoms of illness as their immune systems were recovering function. The symptoms are based upon the newfound ability of the immune system to
mount an inflammatory response to a variety of antigens. This phenomenon has been seen with a number of infectious agents, but is most frequently described with Mycobacterium avium complex (MAC) infection.43 When symptoms of MAC develop in patients on HAART, they usually do so within a month of effective antiretroviral therapy.43 Disease tends to be more localized, and may include caseating granulomas.44 In one study, 12 (36%) of 33 adult patients co-infected with Mycobacterium tuberculosis and HIV developed “paradoxical worsening” of symptoms when HAART was begun.45 Subclinical CMV (cytomegalovirus) infection of the eye may turn into severe, sometimes sight-threatening inflammation, termed “immune recovery uveitis.”46 Most cases of IRIS have been described in adults, but the phenomenon also occurs in children.
mount an inflammatory response to a variety of antigens. This phenomenon has been seen with a number of infectious agents, but is most frequently described with Mycobacterium avium complex (MAC) infection.43 When symptoms of MAC develop in patients on HAART, they usually do so within a month of effective antiretroviral therapy.43 Disease tends to be more localized, and may include caseating granulomas.44 In one study, 12 (36%) of 33 adult patients co-infected with Mycobacterium tuberculosis and HIV developed “paradoxical worsening” of symptoms when HAART was begun.45 Subclinical CMV (cytomegalovirus) infection of the eye may turn into severe, sometimes sight-threatening inflammation, termed “immune recovery uveitis.”46 Most cases of IRIS have been described in adults, but the phenomenon also occurs in children.
Evaluation of the Pediatric HIV-infected Patient with Fever
The workup of any HIV-infected child who presents with fever begins with an assessment of the child’s immune status. Causes of fever in children with category 1 disease (i.e., CD4 count 25% or greater) do not differ significantly from those seen in non-HIV-infected children. They are likely to have common community-acquired infections, as would any child with fever. The exception would be that HIV-infected children have a much higher incidence of pneumococcal infection than their uninfected peers (see text that follows). Children in category 2 (i.e., CD4 cell count 15–24%) are still more likely to acquire common diseases than opportunistic infections. Patients with low absolute lymphocyte counts, those in the lower end of CDC class 2, may have relatively compromised immunity, which might predispose to a broader array of infections. In all children with HIV and fever, if an obvious local source cannot be found, or if the source is pneumonia, a set of blood cultures should be obtained prior to administration of any antibiotic agent. Selected antibiotics should have good activity against Streptococcus pneumoniae, the most common pathogen. As with infections in other immunocompromised patients, the intravenous route may be necessary initially and duration of therapy may need to be longer than in the healthy host.
For patients with category 3 immune suppression (CD4 less than 15%), infection with a wide variety of pathogens is possible (including concomitant infection with more than one pathogen). As immunity is severely suppressed, signs and symptoms of disease may be mild. Evaluation needs to be thorough, and, when febrile, these patients often require admission to the hospital. It is prudent to involve an infectious-diseases trained pediatrician from the outset. Workup is more extensive than expected by the severity of symptoms; for example, intermittent or persistent headache with low-grade fever in this type of patient might be an indication for a computed tomography (CT) scan and a lumbar puncture. Blood cultures, including special cultures for Mycobacterium avium complex (see following text), should be obtained. If any localizing signs or symptoms are found, investigation can begin by focusing on those areas. Many of the problems children with HIV and AIDS may encounter are discussed in the remainder of this chapter.
Common Problems in Patients with HIV Infection
Central Nervous System (CNS) Problems
Encephalopathy
HIV enters the CNS of babies early during the course of infection.47 In some patients, this gives rise to HIV encephalopathy, a condition that usually manifests as peripheral spasticity, followed or accompanied by developmental delay or loss of developmental milestones and cognitive decline, with or without microcephaly.48 The course may be progressive or it may plateau and become static. It may resemble, and may be difficult to differentiate from, cerebral palsy. The severity of HIV encephalopathy is related to the cerebrospinal fluid (CSF) viral load.49 CSF viral load does not necessarily correlate with plasma viral load, is not readily measurable, and is seldom used clinically.
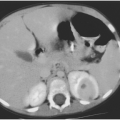
Clinical changes consistent with HIV encephalopathy are usually accompanied by neuroradiographic changes, including cerebral atrophy, white matter lesions, and basal ganglia calcifications. Of these, atrophy is most closely related to the clinical findings of HIV encephalopathy.50 In general, baseline CD4 cell count, viral load, and clinical examination correlate with the severity of atrophy on head CT scanning.50,51 Serial routine neuroimaging, therefore, is usually redundant. In one study of 58 children with HIV infection, treatment was not altered by follow-up CT scanning in any patient.52 A
thorough neurologic and developmental evaluation is probably the most sensitive indicator of cortical dysfunction associated with HIV encephalopathy.
thorough neurologic and developmental evaluation is probably the most sensitive indicator of cortical dysfunction associated with HIV encephalopathy.
Much of the literature on HIV encephalopathy concerns this condition in the pre-HAART era. Treatment of HIV infection with HAART probably has had some effect on both the incidence and severity of HIV encephalopathy. The case of an 8-year-old boy with rather severe encephalopathy who recovered much of his brain function after effective treatment of his HIV infection has been published.47 Anecdotally, the percentage of our HIV-infected patients who suffer from moderate to severe neurodevelopmental problems has decreased over the past several years.
Cryptococcal Meningitis
Cryptococcal meningitis is a disease that occurs only in patients with advanced immunosuppression. It is more common in adults with AIDS than it is in children. Of 1,478 pediatric AIDS cases reported to one registry from 1985–1994, 20 (1.4%) were diagnosed with cryptococcosis.53 In the largest pediatric series published to date, 19 (63%) of 30 cases occurred in children between the ages of 6 and 12 years. The median CD4 cell count within three months of cryptococcal meningitis was 54/μL, and more than half of these children had a prior AIDS-defining illness.53
The diagnosis should be suspected in any patient with advanced AIDS who has headache and fever, even if both of these symptoms are subtle. Cryptococcal meningitis can have a very indolent course. Altered mental status, focal neurologic signs, or seizures are uncommon, and indicate a poor prognosis.54 The inflammatory response to cryptococci in the CSF is generally not impressive. Therefore, normal CSF white blood cell counts, glucose, and protein levels should not deter the clinician from pursuing the diagnosis with India ink staining, serum and CSF cryptococcal antigen testing, and culture.
Amphotericin B, with or without flucytosine, is the treatment of choice. Mortality is higher in patients in whom clearance of the pathogen from the CSF is delayed.55 Critical elevation of intracranial pressure may ensue, which sometimes necessitates ventriculoperitoneal shunting.56 Serial cryptococcal antigen testing of the CSF may be used to monitor response to treatment; antigen levels decrease with proper therapy. The exact duration of therapy for cryptococcal meningitis has not been established; most experts recommend 0.7–1.0 mg/kg per day of amphotericin B for 14 days, followed by 8 weeks of daily oral fluconazole or itraconazole, followed by suppressive therapy with daily fluconazole at half the treatment dose. Suppressive therapy is usually maintained for life. Although prophylaxis against other opportunistic infections can usually be safely discontinued after the patient experiences immune reconstitution (usually defined as CD4 cell count greater than 200/μL for greater than 6 months), data regarding this strategy in cryptococcal meningitis are limited and, as yet, unconvincing.57 Signs and symptoms of cryptococcal meningitis are often worsened by immune recovery (see section on Immune Reconstitution Inflammatory Syndrome [IRIS]).
Cerebral Toxoplasmosis
Cerebral toxoplasmosis generally represents reactivation of latent toxoplasmosis disease. It is more common in adults than in children. All HIV-positive children outside the infant age group should be tested for antibodies against Toxoplasma gondii. If immunoglobulin IgG is measurable, past infection has occurred, meaning the patient will be at risk for disease when immune suppression ensues. Cerebral toxoplasmosis is usually seen in patients with advanced HIV disease.
The incidence of toxoplasmosis has decreased in the HAART era,58 but not as convincingly as other opportunistic diseases, including Pneumocystis jiroveci pneumonia (PCP) and cryptococcal meningitis.59 Fortunately, TMP-SMX (trimethoprim-sulfamethoxazole), given for PCP prophylaxis, is also an effective prophylaxis against cerebral toxoplasmosis.
The diagnosis is considered when a patient with advanced HIV disease has fever and headache, sometimes accompanied by altered level of consciousness or focal seizures. Differentiating toxoplasmal encephalitis from other conditions of the CNS, especially lymphoma, can be difficult. Single photon emission-computed tomography (SPECT) scanning is not always reliable.60 Most experts advise treating presumptively for toxoplasmosis in HIV-positive patients who have both positive serology and multiple cerebral lesions by CT scan.61 Treatment is with pyrimethamine, sulfadiazine, and folinic acid for 3–6 weeks. Follow-up imaging is done in 2 weeks to assess the efficacy of treatment.
Tissue diagnosis should be obtained in patients without AIDS, those with solitary lesions, or those with multiple lesions that are nonresponsive to a trial of therapy.61 Newer diagnostic modalities, including blood and spinal fluid PCR testing, are promising.62
Tissue diagnosis should be obtained in patients without AIDS, those with solitary lesions, or those with multiple lesions that are nonresponsive to a trial of therapy.61 Newer diagnostic modalities, including blood and spinal fluid PCR testing, are promising.62
In the past, suppressive antibiotics were recommended for life. In a newer study, suppressive therapy was interrupted in 75 patients with a history of proven toxoplasmosis. These patients had undergone immune reconstitution secondary to HAART therapy. In more than 119 person-years of follow-up, only one relapse of toxoplasmosis occurred.57 Prevention of toxoplasmosis is discussed in the prevention section.
Hematologic Abnormalities
Anemia
Anemia is the most common hematologic abnormality in patients with HIV infection. By the second month of life, hemoglobin levels are lower in infected than in HIV-exposed but uninfected babies, and the levels diverge throughout the first year of life.63 The incidence of anemia increases with advancing disease.64 There are many possible causes of anemia in HIV. Most cases are attributable to anemia of chronic disease,65 but nutrient deficiencies, autoimmune hemolysis, medication side effects, and persistent parvovirus B19 infection have all been described.66 AZT is particularly likely to cause broad myelosuppression.67
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree