Perinatal Syndromes
This chapter is divided into the following sections:
Prenatal infections (alphabetized by disease)
Exposures during pregnancy
Chronic congenital infection (“TORCH syndrome”)
Neonatal sepsis and meningitis
Miscellaneous neonatal infections (alphabetized by organ system)
Use of antimicrobial agents in pregnancy and lactation
The field of neonatal infectious diseases has expanded and is thoroughly covered in large reference books such as that edited by Drs. Remington and Klein,1 to which the reader is referred for more detailed discussion of the topics presented in this chapter.
Some neonatal infections are covered elsewhere in this book. For example, neonatal hepatitis is discussed in Chapter 13, neonatal osteomyelitis is covered in Chapter 16, and prevention of respiratory syncytial virus (RSV) in premature infants is covered in Chapter 7.
Prenatal Infections
Prenatal infection means any infection that occurs before birth and involves either the pregnant woman or the fetus, although maternal infections usually do not damage the fetus.2 Congenital infections are infections of the fetus acquired in utero and are usually manifest at the time of birth. Neonatal infections are acquired during delivery or shortly after birth; they may be evident at birth but are usually manifest later (within the first 4 weeks of life). “Natal infections” or “intrapartum infections” are expressions sometimes used to describe infections acquired during delivery; these terms constitute a subset of neonatal infections.
Possible Outcomes
Infection of the pregnant woman can lead to six possible results in the fetus. More than one of these results may be present.
Death. Abortion, stillbirth, or neonatal death can be produced by infection in the pregnant woman.
Low birth weight. Low-birth-weight infants may be a result of maternal infection because of either intrauterine growth retardation or premature onset of labor and delivery.
Teratogenic malformations. Rubella virus is the infectious agent most clearly documented as capable of producing teratologic malformation, such as pulmonic stenosis. Reports that other maternal infections can cause such anomalies in infants should be examined carefully. Intrauterine infection of a particular fetus can be confirmed by recovery of the agent, by demonstration of an antibody titer rise, or by detecting agent-specific IgM (immunoglobulin M) antibodies in the newborn infant. However, prospective studies of women with carefully diagnosed infections must be compared with findings in matched controls to determine whether the malformation is statistically associated with infection by that particular agent. Some infections produce damage without teratogenic anomalies, as described in item 5.
Active congenital infection. This infection occurs before birth with an infectious agent still present after birth. Often, the infant clearly has an illness at the time of birth. The congenital infection can be defined as chronic if the onset is at least a month before birth or if active disease continues after birth. Because the time of onset of infection in the fetus usually cannot be determined, the diagnosis of chronic active congenital infection is usually suspected on the basis of findings suggesting earlier infection, particularly low birth weight for gestational age or congenital malformation. The phrase “chronic active congenital infection” is useful to describe an active disease process, as in “active tuberculosis” or “chronic active hepatitis.” Expanded congenital rubella syndrome (CRS) and congenital cytomegalovirus
(CMV) infection are examples of chronic active congenital infections.
Organ damage. Some fetal infections produce damage to an organ, such as the brain or eye, without teratogenesis. This outcome is common in congenital toxoplasmosis.
No disease or damage. CMV and rubella virus are examples of viruses that can infect the fetus without any apparent damage.
Multiple Causal Factors
Fetal teratogenesis has multiple possible causes including genetics, exposure to drugs, and environmental exposures. The causes of most birth defects are unknown. Infection is a very uncommon cause of birth defects, and with the exception of a small number of infectious agents, infection of the fetus is not associated with teratogenesis. Several other prenatal infections can be associated with poor fetal outcomes, and are discussed in the following text.
Specific Infections
The risks of prenatal infection are discussed in the following short sections in alphabetical order, with unusual and miscellaneous infections discussed at the end. Perinatal AIDS is discussed in Chapter 20.
Appendicitis
Large studies have demonstrated that the incidence of acute appendicitis during pregnancy is approximately 1:1400, which is lower than that of the general population of the same age.3 The most common presenting symptom is right lower quadrant abdominal pain. White blood cell (WBC) count and temperature do not differentiate patients with histologically proven appendicitis from those with pseudoappendicitis.4 Rarely, the symptoms may be misinterpreted as preterm labor.5 Outcome after appendectomy, whether open or laparoscopic, is usually quite good.4,6 Fetal loss is rare in patients who present past the first trimester.7 For those who present after 24 weeks’ gestational age, premature labor is a frequent complication; fortunately, preterm delivery is uncommon.4
Babesiosis
Babesiosis is a condition not unlike malaria, in which the pathogen, Babesia microti, infects red blood cells. Successful treatment of a pregnant woman, with no adverse consequences for the fetus, has been described.8 Infantile babesiosis, without proof of intrauterine infection, was first reported in 1986.9 A more recent case describes a 5-week-old baby whose mother was bitten by a tick 7 weeks prior to delivery; the baby was pale, lethargic, and mildly icteric. The platelet count was 87,000/μl and the serum bilirubin was 9.7 mg/dL. More than 4% of the baby’s red blood cells demonstrated ring forms, and the illness resolved with quinine, clindamycin, and azithromycin. Both mother and baby mounted a serologic response to B. microti.10
Bacterial Vaginosis
As discussed in Chapter 15, bacterial vaginosis during pregnancy is associated with premature rupture of membranes, preterm delivery, and low birth weight.
Bartonella henselae
No known human cases of congenital bartonellosis exist. In an experimental feline model, bacteremic cats had difficulty conceiving; none of the offspring suffered ill effects of the maternal infection.11
Brucellosis
Brucella abortus is a well-known cause of fetal demise in farm animals. Because it had not been carefully studied, humans were thought to be protected from such effects by an unknown mechanism. However, in a report from Saudi Arabia of 92 pregnant women with brucellosis, 40 (43%) underwent spontaneous abortions during the first and second trimesters.12 There was a 2% incidence of intrauterine death in the third trimester. Antepartum antimicrobial treatment of the infection was protective against spontaneous abortion (relative risk 0.14, p <0.0001).
Candidiasis
About 20–25% of pregnant women have vaginal candidiasis, but intrauterine candidiasis is rare.13 Babies who are infected while in the womb may develop congenital cutaneous candidiasis. The name is somewhat inappropriate, because babies born prematurely with this condition often have positive blood, urine, and/or cerebrospinal fluid cultures.14 In full-term babies who are otherwise healthy, disease is almost always restricted to the skin, and the prognosis is excellent, with or without
topical antifungal therapy.14 In its most common presentation, the baby is born with a rash or develops it within the first few days of life. The rash is usually widespread, including the palms and soles, and consists of discreet macules, papules, or pustules. Over a few days, it evolves toward a more pustular or vesicular appearance, and sometimes to bullous lesions. Involvement is worst on the back and extensor surfaces of the extremities. The diaper area is usually less involved than the back and trunk. Lesions should be unroofed for microscopic examination and culture.
topical antifungal therapy.14 In its most common presentation, the baby is born with a rash or develops it within the first few days of life. The rash is usually widespread, including the palms and soles, and consists of discreet macules, papules, or pustules. Over a few days, it evolves toward a more pustular or vesicular appearance, and sometimes to bullous lesions. Involvement is worst on the back and extensor surfaces of the extremities. The diaper area is usually less involved than the back and trunk. Lesions should be unroofed for microscopic examination and culture.
Extremely low-birth-weight infants more commonly have a flat red rash that resembles a burn, or extensive areas of denudation. This rash heralds a higher likelihood of systemic infection and mortality.14
Chlamydia
Cervical Chlamydia trachomatis is transmitted to about half the babies who pass through the infected birth canal.15,16 Of these, between 10–20% develop pneumonia, and between 20–45% develop conjunctivitis.16 These infections are discussed in detail in Chapters 5 and 8. Risk factors for C. trachomatis infection include age less than 24 years at the time of pregnancy, unmarried status, and unemployment.17
There is evidence that cervical C. trachomatis infection is a risk factor for premature birth and, perhaps, low birth weight.18 Study of a cohort of 264 babies with perinatal problems resulting in neonatal intensive care unit (NICU) admission and 274 control babies without significant perinatal complications revealed that 15% of the former versus 6% of the latter had an IgM (immunoglobulin M) response to C. trachomatis.18 Average gestational age at delivery was 32 weeks for those seropositive for chlamydia, and 34 weeks for those who were seronegative. A much larger, prospective study compared outcomes in 1,110 C. trachomatis–positive but untreated women with 1,323 who were treated during pregnancy; treatment decreased the risk of premature rupture of membranes (odds ratio 0.56). There was also a trend toward increased perinatal survival in babies whose mothers were treated.19
There is no evidence that C. trachomatis infection is associated with respiratory distress syndrome or chronic lung disease.20
Cytomegalovirus
Congenital infection with this virus is represented by the C in the “TORCH” acrostic, discussed later. Primary infections and those occurring in the first trimester are most likely to lead to adverse perinatal outcomes.21,22 If the maternal infection represents a reactivation rather than a primary infection, a poor fetal outcome is unlikely.23
Diarrhea
If a pregnant woman has an apparently infectious diarrhea, she should be placed in enteric isolation, and cultures should be done. Salmonellosis probably represents the greatest risk through colonization during delivery, with a risk of neonatal meningitis developing later. Other bacterial pathogens, such as Campylobacter or Shigella, should be treated and the mother and baby isolated.
Enteroviruses
Enteroviruses rarely cause intrauterine infection that results in severe disease or death of the fetus. Echovirus 71 was found in the midbrain and liver of a stillborn baby who, by the use of ultrasound, was found to have hepatosplenomegaly, liver calcifications, and ascites at 25 weeks of gestation.24 One neonate was born with a disseminated papulovesicular, nodular, ulcerated and partially necrotic rash, and developed pneumonia, carditis, and hepatitis in the days after birth. This disease was attributed to late prenatal acquisition of coxsackievirus B3 after the virus was cultured from throat and rectal swabs of both the mother and the baby, and serologic results suggested intrauterine transmission.25 Infection early in gestation may result in an increased number of spontaneous abortions.26,27 Coxsackievirus infection can be suspected clinically in the pregnant woman because of pleurodynia or ulcerative pharyngitis, and has been proposed as a cause of congenital heart disease and digestive and
urogenital anomalies.28 Increased frequency of fetal death or prematurity also appear to follow maternal coxsackievirus infections.28 There may be an association with rare central nervous system defects after early pregnancy infections.29 Disseminated coxsackievirus disease can also occur in the early weeks of life, possibly by intrauterine or postpartum transmission.
urogenital anomalies.28 Increased frequency of fetal death or prematurity also appear to follow maternal coxsackievirus infections.28 There may be an association with rare central nervous system defects after early pregnancy infections.29 Disseminated coxsackievirus disease can also occur in the early weeks of life, possibly by intrauterine or postpartum transmission.
Epidemiologic and case-control studies suggest that intrauterine infection with enteroviruses may predispose to the development of insulin-dependent diabetes mellitus (IDDM); however, this is controversial. In one study of 55 mothers whose children developed IDDM and 55 matched controls who delivered at the same hospital and in the same month, IgM antibodies to coxsackievirus B3 were found more frequently in the case population, with an odds ratio of 2.57 (95% CI, 1.02-7.31).30 A second study of 96 pregnant mothers and matched controls revealed similar findings. These authors found the association was stronger when IDDM developed at or before age 3 years.31 There is a potential physiologic basis for this association (based on molecular mimicry), and postnatal acquisition of enteroviral infections has also been postulated as a potential risk factor for IDDM.32 However, a prospective study that examined coxsackievirus infections at delivery in 16 mothers whose children later developed islet-cell autoantibodies and in 110 HLA (histocompatibility locus antigen)-matched control mothers found no association between maternal coxsackievirus infection and IDDM in their offspring.33 Research in this area is ongoing.
Epstein-Barr virus
More than half of all women of childbearing age are seropositive for Epstein-Barr virus (EBV) and asymptomatic reactivation is common. However, in one study of 67 mother-infant pairs, only 2 of the babies were polymerase chain reaction (PCR) positive for EBV in peripheral blood lymphocytes.34 The literature contains one case of suspected intrauterine EBV infection in which the baby was born with hypotonia, petechiae, anemia, elevated liver enzymes, and thrombocytopenia. Other intrauterine infections were systematically excluded. The mother was shown to have suffered a primary EBV infection during the pregnancy.35
Ehrlichiosis
Experience with this infection during pregnancy is limited. Two pregnant women diagnosed with ehrlichiosis during pregnancy and successfully treated with rifampin have been reported. Neither of the fetuses suffered any illness.36 One reported case was strongly suggestive of intrauterine transmission, in which the mother developed fever and malaise the day after delivery and the baby was admitted at age 9 days with a fever, poor eating, and lethargy. A buffy coat smear showed 23% of the neonate’s granulocytes had morulae, and a PCR was positive. Both the mother and the baby mounted a serologic response to the pathogen.37
Gonorrhea
Intrauterine infection with fetal death can occur via aspirated contaminated amniotic fluid.38 Early diagnosis and effective treatment of gonococcal salpingitis or disseminated gonococcemia during pregnancy is likely to spare the fetus from adverse effects. In one case, a 7-day-old baby whose mother had tested negative for Neisseria gonorrhoeae during pregnancy presented with septic arthritis of the right hip with gram-negative diplococci. The mother’s sexual partner had been seen in the emergency department 10 days prior to the baby’s delivery sick with fever, malaise, and dysuria, and his urethral culture grew N. gonorrheae.39
One study of 256 pregnant women in South Africa suggests that in areas of high prevalence, untreated gonorrhea is an independent risk factor for premature delivery and low birth weight.40
Group B Streptococci
This is the most important cause of sepsis in newborns, and is discussed in detail in the section on neonatal sepsis.
Hansen Disease (Leprosy)
Very little is known about pregnancy risk in patients with Hansen disease. Pregnancy causes a relative decrease in cellular immunity, which can lead to development of primary lesions or cause reactivation of previously treated disease.41 It is thought that the principal risk to the fetus is the antimicrobial agents used to treat the disease.
Human Herpesvirus 6 and 7 (HHV-6, HHV-7)
Both of these herpes group viruses are ubiquitous, and infection usually occurs within the first two
years of life. Seroprevalence in pregnant women approaches 100% for HHV-6 and is almost as high for HHV-7. In one study of 569 women, 345 of whom were pregnant, the seroprevalence of HHV-6 was 100%; genital shedding was found in 7 (2.4%) of 297 pregnant women tested versus 8 (3.7%) of 214 nonpregnant women.42 Another study demonstrated that reactivation of latent HHV-6 occurred in more than 40% of those studied at some time during pregnancy, but HHV-6 was found in only 1% of cord blood samples.43 A study of 106 pregnant women found HHV-6 reactivating during the first trimester in 28 (25%); outcomes of these pregnancies did not differ from those of women who did not have active replication.44 Taken together, these data suggest that neither HHV-6 nor HHV-7 cause fetal harm.
years of life. Seroprevalence in pregnant women approaches 100% for HHV-6 and is almost as high for HHV-7. In one study of 569 women, 345 of whom were pregnant, the seroprevalence of HHV-6 was 100%; genital shedding was found in 7 (2.4%) of 297 pregnant women tested versus 8 (3.7%) of 214 nonpregnant women.42 Another study demonstrated that reactivation of latent HHV-6 occurred in more than 40% of those studied at some time during pregnancy, but HHV-6 was found in only 1% of cord blood samples.43 A study of 106 pregnant women found HHV-6 reactivating during the first trimester in 28 (25%); outcomes of these pregnancies did not differ from those of women who did not have active replication.44 Taken together, these data suggest that neither HHV-6 nor HHV-7 cause fetal harm.
Human Herpesvirus 8 (HHV-8)
This virus, the cause of Kaposi’s sarcoma in patients with HIV infection, can be transmitted transplacentally. Blood from 2 (2.2%) of 89 babies born to mothers seropositive for HHV-8 had HHV-8 DNA detectable within their peripheral blood mononuclear cells.45 No known adverse pregnancy outcome or disease in newborns has been associated with HHV-8.
Hepatitis A, B, C, and E Viruses
Management of these infections during pregnancy and issues of maternal-fetal transmission are discussed in Chapter 13.
Herpes Simplex Virus
The incidence of herpes simplex virus (HSV) infection in newborns in the United States is approximately 1 in 1,500–3,200.46,47
Pregnant women with genital herpes can transmit the virus to the fetus by three different pathways. Transmission to the baby as it passes through the vaginal canal (intrapartum) is by far the most common. Infection can also be transmitted via the amniotic fluid (ascending route; usually when membranes have been ruptured, but in rare cases despite membranes thought to be intact) or via the blood (transplacentally; rare). Although rare, transplacental (intrauterine) HSV infection is associated with severe manifestations present at birth, including skin lesions and scars, chorioretinitis, microcephaly, hydrocephalus, and microphthalmia. Surviving infants have severe neurologic sequelae.48
Pregnant women who develop disseminated HSV infection should be treated with intravenous acyclovir. Intravenous acyclovir also crosses the placenta. Data on outcomes from more than 1,100 prospectively followed acyclovir-exposed pregnancies (more than 700 involving first-trimester exposure) have been compiled. The findings do not show an increase in the number of birth defects identified among the prospective reports when compared with those expected in the general population. However, this sample size is insufficient to detect small increases in risk to the fetus.49
The most important consideration is that of neonatal infection acquired via intrapartum transmission. Intrapartum transmission is most common with primary infection because of the higher viral titers present in vaginal secretions and because of the lack of placentally transferred type-specific HSV antibodies in the newborn. In the absence of Cesarean birth, the infection rate of babies delivered vaginally through a primarily infected birth canal is 30–50%; the rate in cases of recurrent disease is less than 5%. A large prospective trial of women with asymptomatic shedding at the time of delivery disclosed a 33% infection rate among babies born to mothers with asymptomatic shedding and serologic evidence of primary infection, and a 3% rate among a similar group of babies whose mothers had asymptomatic recurrent disease.50
Prevention of neonatal herpes infection is a complicated process, mainly because cervical shedding of the virus can occur in either primary or recurrent disease, and often occurs in the absence of either physical signs or symptoms of maternal infection. In the past, attempts were made to predict HSV shedding using weekly cervical cultures as a guide; unfortunately, if cervical cultures are obtained more than 48 hours apart, only 20% of the culture pairs are concordant; thus, it is virtually impossible to predict viral shedding at the time of delivery by the use of cervical cultures.51 The presence of maternal antibodies against HSV at the time of pregnancy augurs a low risk of neonatal herpes infection, probably because transmission of the virus is most common from primary infection, and the presence of antibodies suggests past infection. However, it is important to note that although primary maternal infection confers the highest risk to the fetus, nearly half of all neonatal HSV infections are the result of recurrent disease in the mother. This is because
recurrent infections are so much more common than primary infections. Decision analysis suggests that screening for the presence of type-specific antibodies is not a practical method for preventing neonatal herpes infection.52 The most commonly used mechanism for prevention of disease is visual inspection at the time of labor, with cesarean delivery of babies whose mothers have clinically apparent lesions. In a recent study, cesarean delivery reduced the HSV transmission rate among women from whom HSV was isolated during labor from 7.7% to 1.2%.47
recurrent infections are so much more common than primary infections. Decision analysis suggests that screening for the presence of type-specific antibodies is not a practical method for preventing neonatal herpes infection.52 The most commonly used mechanism for prevention of disease is visual inspection at the time of labor, with cesarean delivery of babies whose mothers have clinically apparent lesions. In a recent study, cesarean delivery reduced the HSV transmission rate among women from whom HSV was isolated during labor from 7.7% to 1.2%.47
Unfortunately, this practice will not prevent all cases of transmission because of the possibility of asymptomatic shedding. A prospective study of 143 women with known risk factors for acquisition of HSV infection revealed that of 123 who were asymptomatic at the time of delivery, only 3 (2.4%) were found to be shedding the virus. In addition, 2 of 5 women who had prodromal symptoms (itching or burning) in the absence of overt herpetic lesions had positive cervical cultures.53 Therefore, the absolute magnitude of the problem (asymptomatic shedding) is not overwhelming; however, children born to women without a history of genital herpes who are asymptomatic at the time of delivery are overrepresented in the patient population of neonates with herpes infections, mainly because steps are taken to avert the infection in babies whose mothers have clinically obvious disease.
Prophylactic oral acyclovir treatment of women with recurrent genital herpes lesions decreases the percent who are shedding the virus or have active disease at the time of labor; and this, in turn, decreases the number of cesarean deliveries.54 However, because viral shedding may still occur, neonatal infection is still possible.54a In addition, this intervention would be expected to have minimal effect on the number of babies infected with HSV, however, because the risk of infection with recurrent disease is about ten-fold lower than the risk with primary infection. There are currently insufficient data to justify the routine use of suppressive therapy in pregnant women who have had genital herpes.54a
Pregnant women who deliver herpes simplex–infected babies tend to be young and nulliparous, without a significantly increased frequency of nonherpetic venereal disease. They often deliver between 30 and 37 weeks’ gestation. Acute neonatal herpes is discussed in the next section.
Influenza
Transplacental transmission of influenza virus at term in a febrile woman has been reported, without adverse effects on mother or baby.55 A prospective study showed that such transmission is certainly uncommon; of 138 babies born to women with serologically proven influenza virus infection, none had cord blood IgM antibodies, and IgG seroreversion occurred within the first 6–12 months of life in all, suggesting passive transfer of maternal antibody and no in utero infections.56 There has been much speculation in the literature about the possibility that influenza virus infection during pregnancy sets the stage for later development of schizophrenia in the offspring of those pregnancies.57 All carefully designed trials testing this hypothesis have been unable to find an association.58,59,60,61
Pregnant women are certainly at higher risk of morbidity and hospitalization from influenza, especially during the third trimester, at which time they are approximately 5 times more likely to be hospitalized for respiratory illness than are postpartum women.62 These data are the impetus for the recommendation that women who will be beyond the first trimester of pregnancy during influenza season receive the influenza vaccine.63
Leishmaniasis (Kala Azar)
Visceral leishmaniasis is transmitted by the bite of the sandfly. Transmission through other means (such as blood transfusion and organ transplants) has been documented. Vertical transmission occurs, but is rare. Parasites have been found in the placenta of a 5-month fetus.64 In other cases, the time between transmission and presentation with overt disease ranged from weeks to 16 months; in utero spread was suspected for epidemiologic reasons.65 Presentation is with fever, hepatosplenomegaly, and diffuse lymphadenopathy. Diagnosis is usually established by bone marrow biopsy, liver biopsy, or both.
Leptospirosis
Rare in the United States, leptospirosis during pregnancy can be devastating to the fetus. A review of 16 cases revealed that 8 pregnancies ended in abortion and 4 babies were born with active disease.66
Spontaneous abortion is reportedly more common when infection occurs early in the course of pregnancy.
Spontaneous abortion is reportedly more common when infection occurs early in the course of pregnancy.
Listeriosis
Listeria monocytogenes is a fastidious, intracellular gram-positive rod that contaminates foodstuffs, especially processed meats and soft cheeses. Contact with this bacterium is apparently common; about 10% of all refrigerated food is contaminated.67 An intact cellular immune system prevents infection and disease.
Unfortunately, pregnant women have a predilection for development of disease due to pregnancy-induced diminishment of cellular immunity. The risk appears to be higher for women with multiple gestations than for those with singleton pregnancies. In Los Angeles women with multiple gestations accounted for 4% of all cases of listeriosis, although they made up only 1% of the population. Infection was even more common in triplet pregnancies, in which the relative risk was estimated to be 38.68 Rates are also higher in mothers with HIV infection and AIDS.69
The infection in mothers usually presents as an indolent, “flu-like” illness, with fever, myalgias, arthralgias, and headache. Premature labor is also a common sign. In one series of 21 untreated patients, there were 5 perinatal deaths and one fetal loss at 18 weeks.70 In general, about a fifth of perinatal infections result in neonatal death or stillbirth. Prompt treatment during pregnancy considerably improves outcomes.
Babies may be born with diffuse microabscesses and granulomas and die in the first day of life from listeriosis; this presentation is known as granulomatosis infantiseptica. Usually, though, listerial infection in the newborn period resembles that of group B streptococci, with early-onset and late-onset forms. Gram staining of the meconium may provide an early clue to the diagnosis while cultures are incubating. Treatment is with ampicillin. Gentamicin is given for synergy until sterilization is documented.
General recommendations for preventing listeriosis are similar to those for other foodborne illnesses (Chapter 12), and include avoidance of unpasteurized milk and cheeses. Persons at high risk for complications from listeriosis (i.e., pregnant women and immunocompromised persons) should adhere to these additional precautions: (a) avoid soft cheeses (i.e., feta, Brie, Camembert, blue-veined, and Mexican-style cheese); (b) cook leftover foods or ready-to-eat foods (e.g., hot dogs) until steaming hot; and (c) consider avoiding deli meats.71
Lyme Disease
Lyme disease during pregnancy is evidently a rare occurrence. A serologic survey of 1,416 mothers and their 1,434 babies found a prevalence (in the mothers) of only 0.85%.72 Only 1 of the 12 women with elevated titers to Borrelia burgdorferi had active disease. There is some evidence that the spirochete is transmissible to the fetus; however, proof of adverse outcome as a result of such transmission is lacking. When 60 placentas from women with serologic evidence of Lyme disease were silver stained, 3 (5%) of 60 stained positive for spirochetes, and in two of these cases PCR was positive for B. burgdorferi.73 The babies were healthy. There is a case report in the literature of a 37-year-old pregnant woman who was bitten by a tick, developed an erythema migrans rash, was treated with penicillin for 7 days, and seroconverted to B. burgdorferi, whose baby died of unclear reasons 23 hours after birth.74 Postmortem examination demonstrated rare spirochetes in brain and liver without any accompanying inflammation. The child died of respiratory causes, and no spirochetes were found in the lung.
Lymphocytic Choriomeningitis Virus (LCMV)
This arenavirus, carried and spread by asymptomatic rodents, causes a congenital infection syndrome marked by chorioretinitis, microcephaly or macrocephaly, and periventricular calcifications.75 Long-term neurologic deficits, including cerebral palsy, mental retardation, and visual problems, are the rule in affected babies. At birth, the syndrome resembles that caused by CMV, toxoplasmosis, or rubella virus. The unique features of LCMV disease are principally that hepatosplenomegaly and hearing deficits are uncommon.75
If infection occurs early in pregnancy, fetal loss may occur.76 Occasionally, nonimmune hydrops fetalis has been reported.
A history of known or suspected rodent exposure is present in about half of cases. Diagnosis is established by serologic evaluation. An immunofluorescence-based test is commercially available and reasonably sensitive. Complement fixation–based
antibody testing is not sufficiently reliable and should not be used.
antibody testing is not sufficiently reliable and should not be used.
Malaria
Although traditionally regarded as an infection without much risk to the fetus, parasitized red blood cells tend to get sequestered in the placenta, causing decreased blood flow to the fetus,77 which may be the pathophysiologic mechanism behind the four-fold increase in low birth weight babies and the two-fold increased risk of stillbirth that has been documented in mothers with malaria.78 In areas of malaria endemicity, low birth weight increases infant mortality by 300%. Mathematical modeling suggests that malaria may, in fact, indirectly account for up to 6% of infant deaths in those areas.79
In endemic areas, congenital malaria has been thought to occur in less than 1% of newborns.80 However, two different prospective studies in sub-Saharan Africa found congenital malaria in 7% of newborns.81,82 Cases are occasionally reported in the United States.83
Congenital malaria should be considered in any infant less than 4 months of age whose mother has a suspect travel history and who presents with fever, anemia, and splenomegaly.83a Jaundice and respiratory distress are also common findings in neonates with congenital malaria.84
Appropriate antimalarial prophylaxis of the mother protects against maternal and fetal malaria, low birth weight, and perinatal death.82
Measles
Fortunately, most women of childbearing age are not susceptible to measles because of vaccine receipt. In one study of 26 pregnant women with measles, fetal mortality was 8%; the risk of fetal loss was higher if measles virus infection occurred early in pregnancy.85 Another study compared the outcomes of pregnancy in 40 women who contracted measles during gestation with 120 controls without evidence of measles virus infection. Mothers with measles were more likely to be hospitalized with pneumonia and fever; and perinatal morbidity was higher, with premature delivery, neonatal hospital admission, and neonatal length of stay significantly increased in the group that contracted measles.86 Prematurity may result from premature onset of labor.87 Congenital malformations were not documented in a small prospective study.88
Measles vaccine is contraindicated during pregnancy. However, pregnancy is listed as a situation where prevention with immune globulin is indicated for susceptible household contacts of measles patients (Table 21-9).89
Immune globulin has also been recommended for the newborn exposed just prior to birth.90
Meningococcus
Perinatal infection with Neisseria meningitidis appears to be extremely rare. In one case, a 25-year-old mother presented at 38 weeks’ gestation with sepsis, meningitis, petechiae, and purpura; her baby had Apgar scores of zero and one at 1 and 5 minutes, had a purpuric rash, and ultimately succumbed at age 72 hours. Although the neonate’s cultures were sterile, the mother’s blood and cerebrospinal fluid cultures were both positive for N. meningitidis. She had received antibiotics for 18 hours prior to delivery, which probably accounts for his negative cultures.91
Mumps Virus
In the first trimester, mumps virus infection is associated with significantly increased fetal death rates.92 Maternal mumps virus infection is not statistically associated with prematurity.87 Congenital malformations have been ascribed to maternal mumps, but the evidence is not conclusive. Hydrocephalus secondary to aqueductal stenosis can be produced in experimental fetal infections in rodents and has been observed after acquired infection in children but may have been coincidental. It has not yet been observed in a statistical study of human pregnancies.93 In a small prospective study of 19 pregnant women with mumps infection, there was no increased frequency of congenital anomalies.94 In a larger study of 117 newborns, the frequency of congenital anomalies was the same as in a control group.88
Case reports describe babies who were born severely ill and required mechanical ventilation following maternal mumps infection late in pregnancy.95,96 Mumps IgM was present in both babies; mumps virus RNA was found in cord blood by PCR in one, and postmortem examination revealed intra-alveolar multinucleated giant cells in the other. Neonatal thrombocytopenia and splenomegaly have been reported following perinatal mumps infection.97
Whether intrauterine mumps infection is a cause
of endocardial fibroelastosis has been a subject of debate. The incidence of both of these entities has decreased dramatically in recent years.98 In a study of myocardial tissue from 29 children with autopsy-proven endocardial fibroelastosis, 21 (72%) of samples were positive for mumps viral RNA by PCR as compared with none of 65 control samples.99
of endocardial fibroelastosis has been a subject of debate. The incidence of both of these entities has decreased dramatically in recent years.98 In a study of myocardial tissue from 29 children with autopsy-proven endocardial fibroelastosis, 21 (72%) of samples were positive for mumps viral RNA by PCR as compared with none of 65 control samples.99
Mycoplasmas
Low birth weight is statistically associated with prenatal cervical Mycoplasma hominis colonization.100 A very small percentage of extremely low-birth-weight premature neonates have respiratory tract colonization with M. hominis; this has not been conclusively linked to any particular clinical syndrome.
Papillomaviruses
Human papillomaviruses (HPV) cause genital warts and some are precursors for cervical cancer (Chapter 15). The idea that pregnancy increased the prevalence of cervical infections with these viruses has been around for years; some investigators even found that the prevalence increased with increasing gestational age.101 However, the largest study of its type to date found that the prevalence of HPV did not differ between 752 pregnant and 504 nonpregnant women.102
The principal disease associated with perinatal acquisition of HPV infection is laryngeal papillomatosis, an intractable cause of stridor. Intact membranes and cesarean delivery are not absolutely protective against perinatal transmission; in fact, 23103–33%104 of babies whose mothers have genital warts and who are born by cesarean delivery are PCR positive for HPV at birth. One study of 37 babies showed that the risk of being PCR positive for HPV increased with a longer duration of ruptured membranes. These investigators also showed, however, that all 11 of the “infected” babies cleared the virus, some as early as 5 weeks after delivery.105 They suggest that babies are “contaminated” rather than infected. Given the disparity between the large numbers of women that are PCR positive for these viruses during pregnancy (one-fourth to one-half of all women) and the small number of children with laryngeal papillomatosis, the concept of “contamination” (or, more palatably, transient colonization) may well be correct.
Parasites
Onchocerciasis appears to be a cause of spontaneous abortion. Gross rates of pregnancy loss in endemic areas are decreased when women of childbearing age receive ivermectin treatment every six months.106 Infection later in pregnancy can cause congenital disease. The syndrome principally consists of an intensely pruritic body rash, with or without intermittent fever. The diagnosis is established by skin snip.107,108
Parvovirus B19
Much has been made about parvovirus B19 infection in pregnancy because of the sometimes dramatic outcomes associated with this condition. The most commonly associated problem is nonimmune hydrops fetalis (NIHF). It is estimated that parvovirus infection is responsible for 15–27% of all cases of NIHF.113,114 Parvovirus infects red blood cell precursors, thus arresting their maturation. In the fetus, this can lead to severe anemia.114a The anemia, coupled (in some cases) with myocarditis, causes cardiac failure, which leads to the anasarca characteristic of NIHF. In most cases, the primary infection in the mother is asymptomatic, and the problem is discovered by routine ultrasound. In addition to NIHF, parvovirus infection during the first and second trimesters can lead to fetal loss; one study found parvovirus B19 in the placentas of 7 (15%) of 47 cases of intrauterine fetal death after 22 weeks’ gestation versus zero of 53 placentas from full-term deliveries. The virus was also found in 2 (5%) of 37 products of spontaneous abortion versus none of 29 elective abortions.115 Parvovirus B19 is not a common cause of premature labor and premature delivery.116
Although the consequences can certainly be dire, there are several facts that mitigate against hysteria when it comes to parvovirus B19 in pregnancy. First, one-half to two-thirds of women of childbearing age have serologic evidence of past infection.117,118 Second, the incidence of seroconversion (thus, primary infection) during pregnancy is approximately 1.5%119 The exact incidence of NIHF or fetal death among fetuses of mothers who undergo primary infection has not been clearly defined; however, in one prospective study, the fetuses of only 3 (8%) of 38 mothers who underwent
seroconversion developed NIHF.120 Outcomes were good in all babies. Mathematically, this works out to a risk of about 2.5 cases per 10,000 pregnancies. Seroconversion rates can be considerably higher (up to 15%) during outbreak situations, however.118 Women with occupational exposure to children (e.g., pediatric nursing and child care work) are at increased risk of acquiring infection, but this risk is lower than the presence of other children in the home.118,119
seroconversion developed NIHF.120 Outcomes were good in all babies. Mathematically, this works out to a risk of about 2.5 cases per 10,000 pregnancies. Seroconversion rates can be considerably higher (up to 15%) during outbreak situations, however.118 Women with occupational exposure to children (e.g., pediatric nursing and child care work) are at increased risk of acquiring infection, but this risk is lower than the presence of other children in the home.118,119
Counseling of pregnant women who become exposed during pregnancy can be simplified into the following: about 50% of women are susceptible, approximately 30% of exposed susceptible hosts become infected, approximately 25% of exposed fetuses become infected, and approximately 10% of infected fetuses die. Thus, the risk of fetal death in a woman with known exposure to parvovirus B19 is approximately 0.5 × 0.3 × 0.25 × 0.1 = 0.4%. If the woman is seropositive, the risk approximates zero; if she is seronegative, the risk is about 0.8%.
Outcomes for fetuses with NIHF is improved by intrauterine transfusion.121
Q Fever
The agent of Q fever, Coxiella burnetii, is an abortifacient in animals, both in the wild and in experimental situations.122 In one review of more than a thousand cases of Q fever, 15 were diagnosed during pregnancy; only 5 babies were delivered, and only 2 of these were of normal birth weight.123 Chronic infection with C. burnetii can be reactivated during subsequent pregnancies.124
Rocky Mountain Spotted Fever
There have been no documented cases of maternal-fetal transmission of Rickettsia rickettsiae, the agent of Rocky Mountain spotted fever (RMSF). Treatment of infected pregnant women is tricky, because the drug of choice, doxycycline, is contraindicated during pregnancy.125 However, because of the severe consequences of inadequately treated RMSF, the use of doxycycline in this case is probably justified.
Rubella Virus
A very high fetal death rate occurs in pregnancies complicated by first-trimester rubella virus infections.126 Transmission of the virus across the placenta reaches its maximum during the first trimester (more than 80%), goes down to less than 25% by the end of the second trimester, and then rises again during the third trimester (more than 60%).127,128
About 20% of maternal rubella infections occurring in the first trimester result in spontaneous abortion. Among live births, congenital defects occur in more than 80% of children infected during the first trimester, and in about one-third of children infected between 13 and 16 weeks’ gestation. Virtually no defects attributable to rubella are found in those infected after 16 weeks.127
The time from maternal rash to infection of the embryo is 20–30 days.128 The pathophysiology behind the teratogenic effects of rubella virus infection in embryos is not completely understood; the virus spreads through the vascular system and causes widespread vessel damage.129 A direct cytopathic effect through necrosis and apoptosis may disrupt organogenesis.130
Postnatally acquired rubella (“German measles”) is generally a mild disease. Immunization against rubella is principally aimed at preventing congenital rubella syndrome (CRS). In the United States, rubella immunization is done in childhood, during adolescent birth-control counseling, for the mother by the primary care physician when her child is immunized, and as a requirement for college entry, military service, or hospital employment. Despite widespread use of the vaccine, a small percentage of women of childbearing age remain susceptible to rubella virus infection.131 In addition, only about half the world’s population resides in areas where rubella vaccination is routinely given.132 As a consequence, CRS continues unabated in those areas. In Russia, for example, CRS accounts for 15% of all birth defects.133 Immigrants who come to the United States from countries not practicing rubella vaccination represent opportunities for possible outbreaks.134,135,136
Prematurity and intrauterine growth retardation occur. Active congenital infections and many congenital malformations can be produced and are discussed in the section on chronic congenital infections (TORCH syndrome). Patent ductus arteriosus, pulmonic stenosis, cataracts, glaucoma, and deafness deserve emphasis.
All women should know their immune status for rubella before risking pregnancy. The importance of knowing this status is, however, not sufficiently appreciated. There is a small but measurable risk
of transmitting the attenuated vaccine virus to the fetus.137 Therefore, patients who may be pregnant should not be given the vaccine, and vaccine recipients should avoid becoming pregnant within 28 days of being vaccinated.138
of transmitting the attenuated vaccine virus to the fetus.137 Therefore, patients who may be pregnant should not be given the vaccine, and vaccine recipients should avoid becoming pregnant within 28 days of being vaccinated.138
Syphilis
The outcome of maternal infection with Treponema pallidum, the causative agent of syphilis, depends on the timing and stage of the disease. A woman may (i) become pregnant while in the primary or secondary stages of the disease, or (ii) become infected during pregnancy. In the former situation, the longer a woman has had syphilis prior to conception, the less likely the fetus will be infected; chances of infection are highest when the fetus is conceived during primary or secondary stages.139 In the latter situation, morbidity and mortality are increased when the pregnant mother is infected during the first or second trimester.139 Infection of the fetus often results in stillbirth. Alternatively, the baby may be born with congenital syphilis, which may be asymptomatic, or carry the signs and symptoms outlined in the section on chronic congenital infections. Signs of syphilis in the mother may be subtle or absent. In one study, 121 (78%) of 155 pregnant women with syphilis were asymptomatic.140 Therefore, prevention of congenital syphilis must depend on serologic testing and adequate treatment of pregnant women. The risk of congenital syphilis is highest in those with less frequent prenatal visits.141 Unfortunately, outbreak investigation has shown that even when prenatal appointments are kept, many physicians do not offer appropriate screening tests.142 Of all 451 cases of congenital syphilis reported in the US in 2002, a total of 333 (74%) occurred because the mother had no documented treatment or received inadequate treatment of syphilis.142a
Because routine serologic testing of women early in pregnancy will not detect syphilis acquired later in pregnancy, a serologic test for syphilis should be repeated later in high-risk pregnancies.
Antibiotic therapy should be given to the pregnant woman with a serologic diagnosis of syphilis. A total of three shots of benzathine penicillin, given one week apart, is recommended and appears to have been effective over the years.143 Women who are allergic to penicillin should undergo desensitization and treatment with penicillin. Alternative antibiotics or alternate schedules of penicillin are not acceptable. Babies born to mothers with incomplete or improper treatment should be considered infected. Fetal infection has been observed in a few instances despite appropriate treatment with benzathine penicillin.143 The optimal penicillin treatment regimen has not really been scientifically established; a recent Cochrane database review revealed no clinical studies that randomized subjects into treatment groups.144
Toxoplasmosis
Infection with toxoplasmosis during pregnancy is usually asymptomatic, and only a rise in titer of paired sera early and later in pregnancy is a reliable method of diagnosis. Physicians who order such titers must be able to rely on the accuracy of those tests, offer further testing such as amniotic fluid PCR,145 and discuss risks and prognosis with the prospective parents. In general, infection of the fetus is most likely to occur when the mother seroconverts later in pregnancy, but the severity of the congenital illness is greater when infection occurs early.146 The transmission rate is about 6% for mothers infected in the first 13 weeks of pregnancy, but up to 72% for those infected at 36 weeks. The risk of infection and the frequency of severe consequences counterbalance, so that women who seroconvert between 24 and 30 weeks’ gestation have the highest chances of having a symptomatic congenitally infected child.146 However, it should be remembered that even babies with asymptomatic congenital infection usually go on to have adverse outcomes, especially chorioretinal lesions and learning disabilities.
If infection of the mother is confirmed during pregnancy, treatment can be offered, although the scientific basis for the efficacy of this treatment is shaky. One group attempted to do a systematic review of published studies about the utility of maternal treatment; of 2,591 published studies, none was rigorous enough to meet inclusion criteria, so the review had to be scrapped.147 In countries with a high number of toxoplasmosis infections in pregnant women (such as France), most practitioners do treat the maternal infection. Small, nonrandomized studies suggest that the rate of transmission is not affected by maternal treatment, but the severity of the illness may be lessened.148
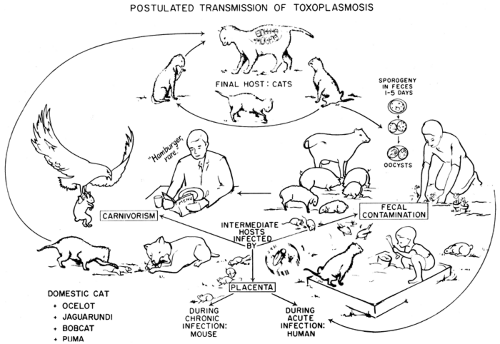
Pregnant women should avoid unnecessary exposure to possible sources of toxoplasmosis, such as cat feces or undercooked meat. (Fig. 19-1). In
the United States, physicians have done a good job of alerting pregnant mothers to the dangers of cat litter; however, the dangers of undercooked meat have not been adequately trumpeted. Overseas, where consumption of raw or undercooked meats is more common, this has become the principal risk factor for the acquisition of toxoplasmosis during pregnancy.149
the United States, physicians have done a good job of alerting pregnant mothers to the dangers of cat litter; however, the dangers of undercooked meat have not been adequately trumpeted. Overseas, where consumption of raw or undercooked meats is more common, this has become the principal risk factor for the acquisition of toxoplasmosis during pregnancy.149
Tuberculosis
Tuberculosis during pregnancy is important to diagnose, because as many as two-thirds of pregnant women with pulmonary tuberculosis are asymptomatic150,151,152 and newborns are at very high risk of disseminated tuberculosis if exposed to an active case.153,154 Congenital tuberculosis is very uncommon and is discussed later in this chapter.
All of the first-line drugs (isoniazid, rifampin, ethambutol, and pyrazinamide) have an excellent safety record in pregnancy and have not been associated with congenital malformations.155 Streptomycin is strongly associated with hearing and balance problems in children exposed in utero, and its use is contraindicated during pregnancy.155
Pregnant women with latent tuberculosis infection who have recent acquisition of infection, and those with HIV infection, may have rapid progression to active disease and should be treated promptly with isoniazid for 9 months.156 Although no harmful effects of isoniazid to the fetus have been observed, some experts delay therapy of latent infection until after the delivery in the absence of HIV infection, immunosuppression, or recent tuberculosis infection. Pregnant and nursing women on isoniazid should receive pyridoxine (vitamin B6) 25 mg daily.156
Pregnancy is associated with progressive suppression of tuberculin sensitivity (and other lymphocyte functions), which is maximal between 36 weeks’ gestation and delivery.157
Ureaplasmas
Colonization of the cervix with Ureaplasma urealyticum probably occurs in about half of pregnant
women.158 Ascending intrauterine infection is a major cause of premature labor; ureaplasmas are some of the most common of the organisms thought to be associated with early induction of labor.159 Antibiotic treatment aimed at U. urealyticum in laboring mothers does not, however, alter the rate of preterm birth.159
women.158 Ascending intrauterine infection is a major cause of premature labor; ureaplasmas are some of the most common of the organisms thought to be associated with early induction of labor.159 Antibiotic treatment aimed at U. urealyticum in laboring mothers does not, however, alter the rate of preterm birth.159
There has been controversy surrounding the possible role of U. urealyticum in the pathogenesis of chronic lung disease (“bronchopulmonary dysplasia”) in premature neonates. The hypothesis is that ascending infection from the cervix causes an increase in inflammatory mediators in the intrauterine environment, and colonizes the infant’s respiratory tract with the organism.160 After birth, ongoing inflammation due to the presence of Ureaplasma in the trachea and lung causes arrest of normal lung development and initiates fibrosis, both of which contribute to the development of chronic lung disease (CLD). In vitro studies demonstrate that U. urealyticum causes neonatal macrophages to overproduce proinflammatory cytokines.161 Some clinical studies demonstrate an increased incidence of CLD in colonized versus uncolonized babies,162,163,164 while others do not.165,166,167,168 Finally, a histopathologic study showed more interstitial fibrosis in the lungs of babies colonized by Ureaplasma.169
Prospective, placebo-controlled trials of erythromycin treatment show no benefit.168 Taken together, these data suggest that a link between Ureaplasma colonization and CLD, if it exists at all, is tenuous and of a small magnitude.
Ureaplasmas have rarely been isolated from cerebrospinal fluid specimens, sometimes in concert with pleocytosis and sometimes without. Cases of spontaneous resolution have been described.170 In the largest study to date, U. urealyticum was isolated from the spinal fluid of only 2 (0.2%) of 920 infants.168 One very low-birth-weight infant developed osteomyelitis of the right femur and had a blood culture positive for U. urealyticum.171
Urinary Infections
Preterm delivery is statistically more common in women with bacteriuria, and the mortality rate of infants is significantly higher if the infection occurs within 15 days of delivery.172
Varicella
Varicella tends to be particularly severe in pregnant women, in whom life-threatening pneumonitis may occur. Maternal varicella between 8 and 20 weeks of gestation results approximately in a 1–2% incidence of congenital varicella syndrome.173 In one study of 1,373 women with varicella during pregnancy, the risk of congenital varicella syndrome was 2% if infection occurred between 13 and 20 weeks’ gestation and 0.4% before 13 weeks’ gestation.174
The syndrome consists of limb hypoplasia, cicatricial (scar-like) rash, microcephaly, chorioretinitis or other ophthalmic defects (optic atrophy, microphthalmia, cataracts, or keratoconjunctivitis), and sometimes intracranial calcifications. Incomplete forms may exist; one report describes finding anti-varicella-zoster virus (VZC) antibodies in the cerebrospinal fluid of 4 babies who presented with seizures and mild hypotonia.175 Congenital varicella syndrome is rare for two reasons: (a) most women of childbearing age, with or without a clinical history of varicella, are immune to the disease,176 and (b) most cases of proven varicella during pregnancy do not lead to the syndrome.177 In the first trimester, maternal chickenpox is associated with slightly increased fetal death rates.92
Fatal neonatal infections can occur, especially in infants born to mothers who developed a chickenpox rash 5 or fewer days before delivery or within 2 days after delivery.178 The probable reason for the severity of primary varicella in these babies is that the mother passes the virus to the baby transplacentally before she has had time to mount an antibody response, thus leaving the baby infected and unprotected. Varicella-zoster immune globulin (VZIG) is recommended for the baby after birth (Table 21-9).179 Acyclovir may provide some additional protection; in one small prospective study, 2 of 4 babies given VZIG alone versus none of ten given both VZIG and acyclovir developed symptoms of varicella.180 Most experts withhold acyclovir unless the baby develops signs or symptoms of disease.181
Infants who develop zoster in the first few years of life without a history of preceding chickenpox182 probably had primary varicella in the womb. Most of these babies will not have any of the findings of congenital varicella syndrome, although unrecognized chorioretinitis should be sought.
About 90% of women of childbearing age who have a negative or unknown history of chickenpox have serologic evidence of past infection;176 those who are seronegative and wish to be protected can be offered varicella vaccine. Because the vaccine is a live attenuated virus, it should not be given to women who are already pregnant. There is a theoretical
risk of congenital varicella syndrome or fetal loss if it is inadvertently administered to pregnant women, but there have not been any reported cases.183
risk of congenital varicella syndrome or fetal loss if it is inadvertently administered to pregnant women, but there have not been any reported cases.183
VZIG should be administered to susceptible women exposed to active varicella during pregnancy (Table 21-9). If the mother’s immune status is uncertain, VZIG can be withheld pending serologic results. Most will be immune. However, the consequences of varicella to both the mother and fetus can be severe; therefore, VZIG should not be withheld if serologic results cannot be returned in a timely fashion.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree