TETANUS
ITZHAK BROOK
Tetanus is a serious but preventable nervous system disorder caused by the toxin produced by Clostridium tetani. Although the disease is rare in the industrial nations, it is still rampant in the developing world. The isolations of the causative organism and its toxin were cornerstones in the history of microbiology. Tetanus was one of the first bacterial conditions to be prevented by immunization, representing a triumph in the application of research and public health measures.
Tetanus is an intoxication manifested mainly by neuromuscular dysfunction expressed as muscle spasms and caused by tetanal exotoxin (tetanospasmin). Tetanus can present in one of four clinical forms: generalized, local, cephalic, and neonatal. The goals of treatment are interruption of the production of toxin, neutralization of the unbound toxin, controlling muscle spasms, management of autonomic dysfunction, and appropriate supportive management. Active immunization with tetanus toxoid is the most effective mean of protection.
HISTORICAL ASPECTS
Ancient physicians recognized the relationship between wounds and a disease producing spasticity, violent movements, and death. Case 7 in the Edwin Smith Surgical Papyrus discusses a patient with a penetrating skull wound who experiences trismus and nuchal rigidity (1). These findings were well known to the Egyptian physician who used them to help formulate the prognosis. This is commonly accepted as the earliest recorded description of tetanus. Hippocrates described the disorder clearly; and his relative contemporary Aretaeus observed that these manifestations were “apt to supervene on the wound of a membrane, or of muscles, or of punctured nerves, when, for the most part, the patients die; for, ‘spasm from a wound is fatal’” (2). Galen noted that cutting a nerve in tetanus stopped the movement but paralyzed the innervated part.
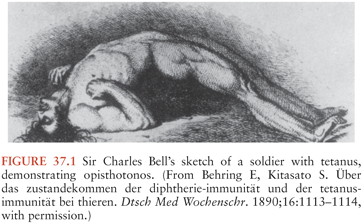
The ensuing millennia saw some refinements in clinical observation. John of Arderne (1307 to 1380), often thought to be the first English surgeon-author, described a case of tetanus in which trismus (“taken with the cramp on his cheeks”) began 11 days after a gardening injury. In the eighteenth century, tetanus was thought to be a consequence of nerve injury. However, the spasms of generalized tetanus were frequently confused with the convulsions of epilepsy. Sir Charles Bell, a noted illustrator and a surgeon, included a patient with tetanus infection in his 1824 text (3) (Fig. 37.1).
Neonatal tetanus (NT) was called the “7-day disease” in the Americas and was known as the “9-day fits” in Dublin. In 1846, Sims proposed the “congestive” theory of neonatal tetanus. He thought that this condition resulted from placing infants on their backs, which compressed the occiput and occluded the veins of the medulla. Beumer determined that the umbilicus was the portal of entry for NT in 1887.
The clinical advances of the nineteenth century culminated in this description by Sir William Gowers (4):
Tetanus is a disease of the nervous system characterized by persistent tonic spasm, with violent brief exacerbations. The spasm almost always commences in the muscles of the neck and jaw, causing closure of the jaws (trismus, lockjaw), and involves the muscles of the trunk more than those of the limbs. It is always acute in onset, and a very large proportion of those who are attacked die.
Gowers, and many of his contemporaries, felt that nontraumatic tetanus accounted for up to 20% of cases; he blamed these on a sudden chill or a frightening episode. Although he recognized the similarity between tetanus and strychnine poisoning, he disparaged the notion of pathophysiologic similarity. In one of his few failures of insight, Gowers dismissed Nicolaier’s first report of a strychnine-like toxin isolated from anaerobic soil bacteria (5). Six years later, Behring and Kitasato (6) proved that immunization with an inactivated derivative of this bacterial extract prevented tetanus.
Effective therapeutic suggestions for established tetanus also date from the nineteenth century. In 1829, Ceroli described the use of morphine as a treatment for tetanus. Based on the observations of Claude Bernard, curare was employed with some rare successes (and some dramatic failures) in France (7), Germany (8), the United States, and England. The lessons in long-term mechanical ventilation learned during the poliomyelitis epidemics of the 1950s finally made treatment with neuromuscular blockade feasible. Hutchinson and Jackson (9) of the National Hospital at Queen Square discussed the use of ether in tetanus in 1861. Meltzer and Auer (10) employed magnesium salts to treat tetanus patients at the dawn of the twentieth century. Gowers (4) gave the following description of autonomic dysfunction in tetanus in 1888: “The pulse is increased in frequency, especially during the paroxysms, and is often very small. There is some reason to believe that the small size of the pulse is due to generalized vasomotor spasm.” However, widespread recognition of the hypersympathetic state did not occur until the prolongation of survival of tetanus patients made possible by ventilatory management.
EPIDEMIOLOGY
Risks for acquired tetanus include infected wounds, infected surgical sites (include contaminated sutures, dressings, or plaster), burns and abscesses contaminated by C. tetani, animal-related injuries (bites and wounds), lack of active immunization against C. tetani, umbilical stump infections (tetanus neonatorum) when mud or feces are applied to the umbilical stump, and puerperal infections when nonsanitized instruments are used (11).
C. tetani has a worldwide distribution and has been recovered from diverse sites, including soil, feces, house dust, and contaminated heroin. It is one of the most common fatal infectious diseases throughout the world and in developing countries; it is an important cause of neonatal death. Natural immunity in communities that are not immunized is about 30% and increases with age (12). The attack rate and age-related mortality rate after the neonatal period is higher in males.
The illness is frequent in countries or in ethnic groups who are less likely to be immunized. In the United States, inadequate tetanus protection in rural elderly individuals was more common as compared to the entire population (13). Tetanus is still a major cause of mortality in those areas of the world with inappropriate hygiene and immunization programs. In 2006, an estimated 290,000 people worldwide died of tetanus, most of them in Asia, Africa, and South America (14).
Published mortality figures underestimate the number of deaths but probably represent the most reliable data for much of the world. In the United States, reported cases per 100,000 populations fell from 0.28 in 1955 to 0.02 in 2001 (15). Reported mortality has declined from about 65% in the 1940s to about 20% in the 1990s, reflecting improvements in critical care. During 2001 to 2008, a total of 233 cases were reported in the USA, and the case-fatality rate was 13.2% among the 197 cases with known outcomes. Average annual incidence during that period was 0.10 per 1 million population overall and 0.23 among persons aged 65 years and older (Fig. 37.2). Case fatality was higher among persons older than age 65 years, diabetics, and among unvaccinated persons or those not up-to-date with vaccination (16). An average of 29 cases was reported each year (range: 19 to 40).
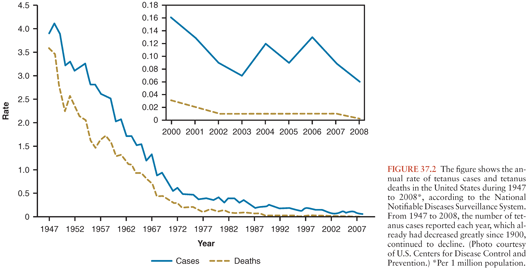
Sutter et al. (17) concluded that only 40% of cases are reported to the Centers for Disease Control and Prevention (CDC), 60% are reported to the National Center for Health Statistics, and almost 25% are reported to neither. As the disease becomes less common, more cases are likely to elude recognition, causing an artifactual decline in incidence. Most reported cases occur in patients older than 60 years (18), confirming that waning immunity is a serious problem in this population. Table 37.1 summarizes conditions before onset of tetanus among 130 reported U.S. cases (19).
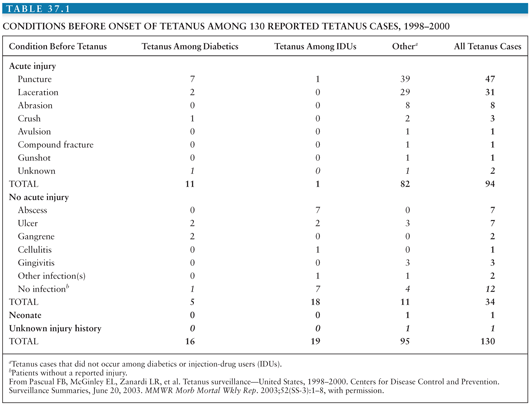
About three fourth of cases of tetanus in the United States follow injuries. Infected wounds (both traumatic and surgical), abscesses, surgical wounds, major trauma, parenteral drug abuse, and animal-related injuries account for about 25% of the tetanus-associated injuries; about 20% of wounds are due to unknown causes, and in 5%, no source can be found (16).
In the developed world, about 25% of tetanus cases stem from occupational accidents, most frequently agricultural or sylvan. Immigrants to the United States were more than three times less likely to have been immunized than native New Yorkers in one study (20).
NT accounts for about half of all cases worldwide and has a 90% mortality (11). NT is rare in the United States. This is due to the efficacy of the intensive immunization program. Unhygienic childbirth, nonmedical abortion practices, inadequate immunization of mothers, and the lack of care of penetrating wounds explain most cases of neonatal and adult tetanus in the developing world. Furthermore, climate and soil pH in the tropics may contribute to the increased prevalence of C. tetani and its availability to contaminate wounds (11).
A recent estimate of worldwide causes of child mortality between 2000 and 2010 found that NT decreased from 146,000 to 58,000 at 9.5% per year (11). The incidence of NT and factors associated with NT mortality was evaluated in 416 cases seen in a district in rural Pakistan between 1993 and 2003. The overall case-fatality rate (CFR) for NT was 30.1% and fell from 42% in 1993 to 29% in 2003 (p = 0.377). NT incidence decreased from 0.90 per 1,000 livebirths (LB) in 1994 to 0.18 per 1,000 LB in 2003. Multivariable analysis showed that age at admission of 8 days or less with or without low birth weight was the strongest predictor of mortality. The rate of decline of NT incidence and case fatality was attributable to routine and supplementary immunization activities (21).
A study of neonatal mortality in Bangladesh revealed that tetanus caused 112 of 330 deaths (22). There is great geographic variability in incidence of and mortality from the neonatal form of the disease, with an inverse relationship between the extent of maternal immunization and incidence. In 1999, the World Health Organization (WHO) reported that Somalia had the highest reported rate, with 16.49 neonatal tetanus deaths per 1,000 livebirths (23). Poor prognostic factors include age younger than 10 days on admission, symptom duration of less than 5 days on admission, risus sardonicus, and fever (24). About 30% of neonatal tetanus cases occur in babies born to mothers who have already had at least one affected child; this dramatizes the failure to immunize mothers as a major contributor to this condition, because these women are known to lack immunity (25). Immunization programs are clearly effective in decreasing the mortality attributable to neonatal tetanus. Interestingly, tetanus appears to be rare at high altitudes (26).
ETIOLOGY
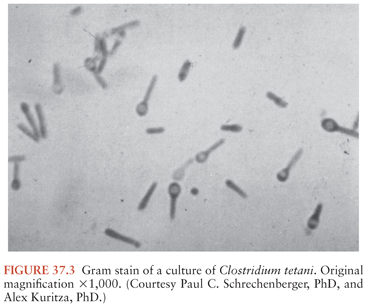
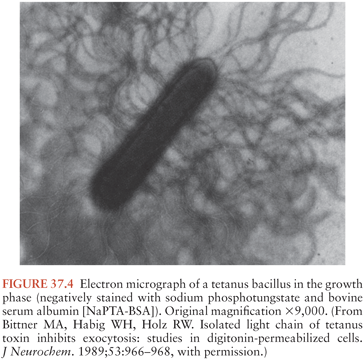
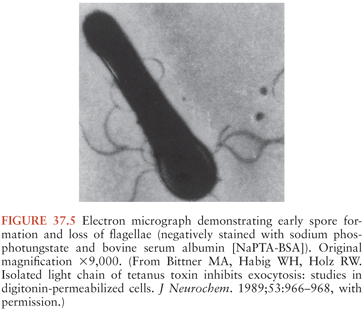
C. tetani (Fig. 37.3) is a slender, obligatively anaerobic bacillus measuring 0.5 to 1.7 µm by 2.1 to 18.1 µm (27). Usually classified as gram positive, it may stain variably, especially in tissues or in older cultures (28). Most strains are sluggishly motile and have abundant peritrichous flagellae during growth (Fig. 37.4). The mature organism loses its flagellae (Fig. 37.5) and forms a spherical terminal spore (29), producing a profile like that of a squash racket (Fig. 37.6). The spores resist extremes of temperature and moisture and are stable at ambient oxygen tension; in addition, they survive indefinitely. They are viable after exposure to ethanol, phenol, and formalin, but they are killed by iodine, glutaraldehyde, or hydrogen peroxide. Strains vary in resistance; exposure to 100°C for 4 hours or autoclaving at 121°C and 103 kPa (15 psi) for 15 minutes is necessary to ensure sterility. They can survive in soil for years and can be found in house dust, soil, salt, and fresh water (30).

Spores can be isolated from the feces of many animals and in small numbers are ubiquitous in soil and on carpets. Hence, any breach in skin defenses (e.g., wounds, burns, animal or human bites, or even insect bites) may result in inoculation. Injuries that favor growth conditions for anaerobic bacteria may lead to the development of tetanus. Tetanus-prone wounds include those contaminated with dirt, feces, or saliva; punctures (including the use of unsterile needles, or injections through unprepared skin); scratches, bites, missile wounds; burns; frostbite; avulsions; and crush injuries. Penetrating ocular injuries are considered tetanus prone based on experimental evidence, whereas nonperforating injuries are not (31). Sometimes, no clear portal of entry can be ascertained. Unusual infection sources include the alimentary tract, tonsils, ear lesions, and contaminated vaccines, sera, and catgut (32,33). Fatal tetanus has occurred in a patient with Stevens-Johnson syndrome. Other unusual sources of tetanus have been described (34). The importance of these observations lies not in their rarity, but in the much higher mortality rate associated with unusual portals of entry. This probably reflects a longer delay in considering the diagnosis. Between 7% and 21% of tetanus cases are cryptogenic (35).
In culture, growth occurs best at 37°C. C. tetani grows on several media if oxygen is excluded. When cultured on a solid medium such as blood agar, the organisms usually form a thin film over the entire surface (“swarming growth”). Routine anaerobic isolation techniques are sufficient if the tissue sample is rapidly placed in an anaerobic transport system. Clinical decisions should not be based on culture results, because (a) cultures are commonly negative in patients with tetanus, (b) isolation of the organism is of no consequence in an immune host, and (c) routine bacteriologic studies will not indicate whether a strain of C. tetani carries the plasmid required for toxin production.
The spores germinate when introduced into a wound and proliferate if the redox potential of the tissue is low. During growth, C. tetani produces two exotoxins: tetanospasmin (TS) and tetanolysin. The potential role of tetanolysin in human tetanus is unclear; at worst, it may damage otherwise viable tissue in the vicinity of an infected wound, lowering the redox potential and promoting the growth of anaerobic organisms. Tetanolysin can disrupt cell membranes, apparently by more than one mechanism. Although systemic administration of tetanolysin in animals produces electrocardiographic abnormalities and disseminated intravascular coagulation, the relevance of these findings to clinical tetanus in humans is uncertain.
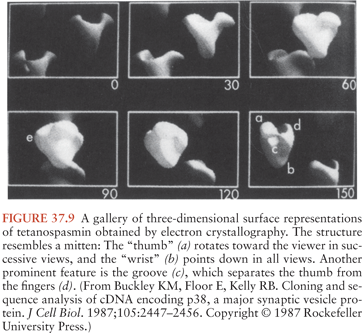
TS, the substance commonly called tetanus toxin, is synthesized as a single 151-kd, 1,315–amino-acid polypeptide chain (36) (Fig. 37.7). The genetic information for this molecule resides on a single large plasmid (37) (Fig. 37.8). Strains of C. tetani lacking this plasmid are not toxigenic and do not cause clinical tetanus. The native molecule has little or no activity but becomes potent when nicked at serine-458 by a bacterial protease (38). This produces one heavy (100-kd) chain and one light (50-kd) chain, connected by a disulfide bridge. This bridge, as well as another one on the heavy chain, is required for the activity of the toxin. These chains or their fragments affect different phases of toxin binding, cell entry, and toxicity. T cells commonly produce an immune response against two particular amino-acid sequences from the amphipathic alpha-helical portion of the molecule. The three-dimensional structure of TS has been determined (39) (Fig. 37.9). The genomic sequence of the bacterial chromosome and the plasmid is now available (40).
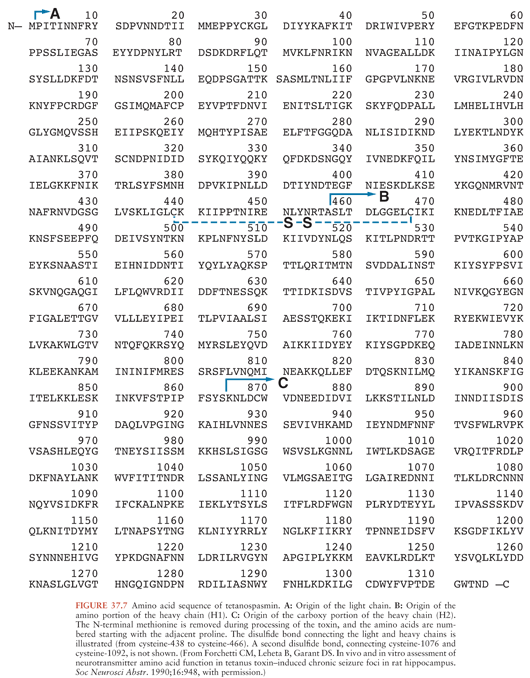
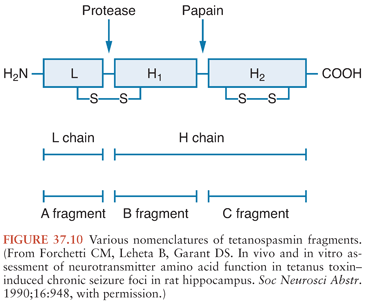
Investigators have described several enzymatic digestion products of TS (Fig. 37.10), but their clinical relevance is uncertain. Now that the toxin molecule has been sequenced, reference to the specific amino acids of a fragment is preferred (41). The more commonly investigated fragments are those derived from papain treatment, which cleaves the heavy chain at lysine-865, about 50 kd from the carboxy-terminal end (the C fragment) (42). The light chain and the amino-terminal end, still linked by the disulfide bridge, are variously called the B fragment or the A-B fragment. Attachment and internalization of TS into its target neurons is mediated by the heavy chain (43) or its C fragment (44), whereas the light chain is responsible for inhibiting transmitter release (45,46). The C fragment may also be responsible for the transsynaptic transport of the toxin (46). However, some disagreement remains about these distinctions.
The manifestations of tetanus result from the propensity of TS to inhibit neurotransmitter release by a presynaptic terminal (47). This is a three-step process (Fig. 37.11): binding to the presynaptic membrane, translation of the toxin to the active site, and induction of paralysis. Because TS avidly binds to gangliosides, these molecules were proposed as being the “receptors,” but several lines of evidence suggest that this binding is nonspecific (48). Other evidence implicates a nonganglioside receptor (49), but it has not been characterized. Similarly, the process by which the toxin breaches the cell membrane is not well understood. TS enters the neuron from the extracellular space via noncoated vesicles (50); Pelkmans and Helenius (51) stress the endocytosis of TS via caveolae, as is also the case for cholera toxin and some nonenveloped viruses. The discovery that bafilomycin A1 prevents the action of TS on cultured spinal cord neurons (52) implies that the toxin enters the cytoplasm in an acidic vacuole, analogous to that described for diphtheria toxin (53). Other clostridial neurotoxins appear to share this entry system. The internalization of TS is protease sensitive and appears to require glycosyl-phosphatidylinositol–anchored proteins, which are present in the vicinity of synaptic release site (54). The binding sites may differ biochemically in the central nervous system (CNS) and the peripheral nervous system (55).
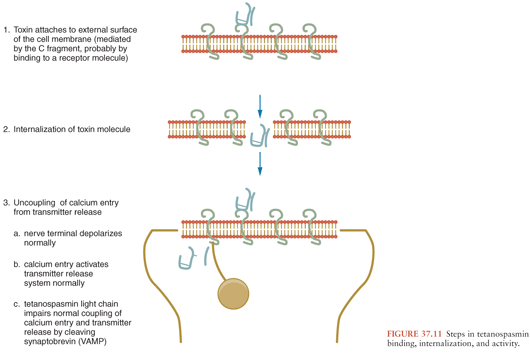
Once inside the presynaptic terminal, TS exerts a local effect to inhibit transmitter release for several weeks. Although the synaptosomal content of γ-aminobutyric acid (GABA) is not altered in epileptogenic cortical foci induced by TS, the GABA release evoked by depolarization remains decreased for at least 3 weeks (56). Thus, inhibitory failure is due not to a decrease in available GABA, but to the inability of the presynaptic neuron to release the transmitter it contains.
In contrast to the lack of understanding of binding mechanisms, research in presynaptic function (much of it dependent on TS and other clostridial neurotoxins as tools for dissecting the molecular steps involved) paints an elegant and coherent picture of the mechanism by which these toxins prevent transmitter release (57).
The release of synaptic vesicles by an action potential is initiated by an abrupt rise in the intracellular free Ca2+ concentration, mediated by voltage-dependent calcium channels (Fig. 37.12). This increase in free Ca2+ triggers an interaction between synaptotagmin (in the vesicle membrane) and syntaxin (on the presynaptic cell membrane), clamping the vesicle to the presynaptic membrane. Synaptobrevin (also referred to as vesicle-associated membrane protein [VAMP] [58]) also binds to syntaxin and appears to dock the vesicle to the membrane at the proper location for fusion. There are different isoforms of synaptobrevin within neurons; a protein termed cellubrevin performs a similar function in nonneuronal secretory cells (59). Synaptophysin, the third major component of this mechanism, probably forms the fusion pore that allows release of the vesicle contents into the synaptic cleft (60).

Clostridial neurotoxins inhibit vesicle release by cleaving peptide bonds in these proteins (59). Each toxin has a specific locus of activity (61). TS, along with botulinum neurotoxins B, D, F, and G, cleaves synaptobrevin (62). TS and botulinum neurotoxin B may share the same cleavage site on synaptobrevin. The toxins affect only the free proteins; once they have complexed to cause transmitter release, they are not subject to attack (63). Synaptobrevin and synaptotagmin cleavage also occurs normally, as an effect of an endogenous protease, and they are probably involved in organelle recycling (64). The endogenous protease does not appear homologous to the clostridial toxins.
From the medical standpoint, one of the most important properties of intraneuronal TS is its propensity to travel via the retrograde transport system back to the cell body, which allows access to various other neurons (65) (Fig. 37.13). This process extends from the periphery into the spinal cord as well across several orders of synaptically connected neurons in the brain. The particular clinical manifestations of tetanus depend on the classes and locations of the affected cells, as discussed in the section “Pathogenesis and Pathophysiology,” later in this chapter.

CLINICAL MANIFESTATIONS
Tetanus is traditionally classified into four clinical types: generalized, local, cephalic, and neonatal. These distinctions are useful diagnostically but do not reflect toxicologic differences. Rather, they reflect variations in the site of toxin action.
Although a portal of entry can usually be determined, the lack of a defined wound does not exclude tetanus. Similarly, bacterial stains and cultures of wounds are of no consequence in its diagnosis or management. A “protective” titer of antitetanus antibody may help exclude the diagnosis, but only in retrospect. This is an area of controversy.
The temporal development of symptoms in each form of tetanus is of great prognostic significance. The incubation period extends from the time of spore inoculation to the first symptom, and the period of onset marks the time from that first symptom to the first reflex spasm (i.e., a spasm produced in response to sensory stimulation, as opposed to an apparently spontaneous spasm). In a study of 176 patients from Brazil, the incubation period for patients with severe disease (N = 116) was 8.3 ± 4.7 days, whereas that for patients with moderate and mild disease severity (N = 60) was 11.0 ± 6.7 days. The corresponding periods of onset were 1.9 ± 1.5 days and 3.2 ± 1.9 days, respectively (66). Regardless of the clinical type of tetanus, shorter incubation and onset periods indicate a poorer prognosis.
A general assessment of the disease severity can be predictor of the outcome and can assist in determining the timing and need for airway protection. The portal of entry is another important prognostic factor, with burns, head and neck infections, umbilical stumps, surgical procedures, compound fractures, septic abortions, and intramuscular injections all associated with lesser chances for recovery. Injection drug users, especially those injecting narcotics and heroin, appear to develop particularly severe tetanus (67). Tetanus following the intramuscular injection of quinine, used in some third-world countries for malaria treatment, has an unusually high mortality rate and develops very quickly (68). This probably reflects the very acidic milieu produced by quinine injection. Fever and tachycardia, if reflecting autonomic dysfunction rather than wound infection, are similarly poor prognostic signs (69). The outcome is also influenced by age and comorbidity.
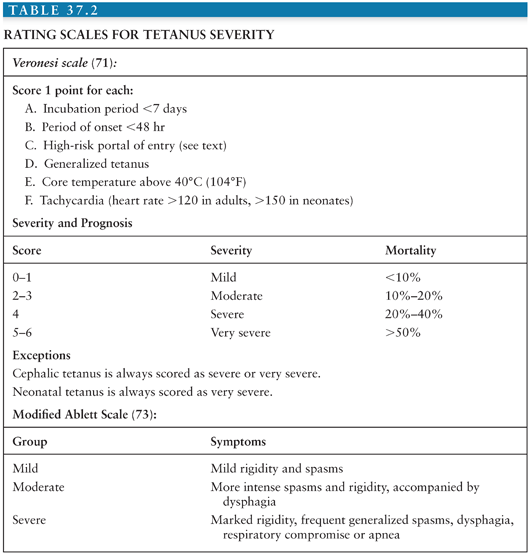
Several authors have developed rating systems for severity and prognosis (70–72), summarized in Table 37.2. The modified Ablett scale is frequently used for making decisions regarding tracheostomy, which is performed on moderate or severely affected patients (73).
Generalized Tetanus
This is the most common form of clinical tetanus. It may occur after relatively minor injuries. Patients generally have tonic contraction of their skeletal muscles and intermittent intense muscular spasms. Tonic and periodic spasmic muscular contractions account for the classic clinical findings: opisthotonus, stiff neck, risus sardonicus (sardonic smile) (Fig. 37.14), a boardlike rigid abdomen, periods of apnea caused by viselike contraction of the thoracic muscles and/or glottal or pharyngeal muscle, and dysphagia.
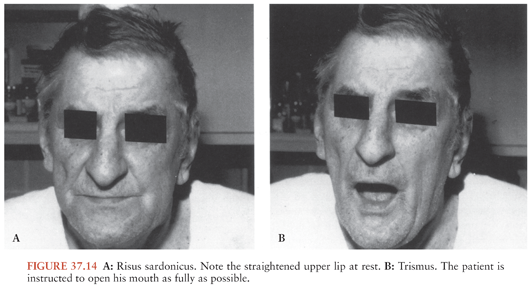
The onset can be insidious; however, the typical initial findings of trismus, or lockjaw, due to parapharyngeal and masseter muscles spasms is observed in about half of cases (32,33). Patients manifest tonic contraction of their skeletal muscles and intermittent intense muscular spasms. These may be triggered by sensory stimuli such as loud noises, contact, or light. Tonic and periodic spasms are responsible for most of the clinical findings of tetanus. Trismus is caused by rigidity of the masseter muscles, producing inability to open the mouth (see Fig. 37.14); its course can be followed by measuring the distance between the upper and the lower teeth with the mouth maximally open. Trismus is the most common presenting sign, although back or shoulder stiffness may have been present for hours. The most common complaints are pain, swallowing difficulty, and unilateral or bilateral stiffness of the neck and other muscle groups, such as those of the thorax or abdomen (74). Persistent trismus accounts for “risus sardonicus,” which is the classic finding of tetanus (see Fig. 37.14). Risus sardonicus is often a subtle finding that may best be diagnosed by the family or friends of the patient. Physicians can diagnose risus sardonicus by comparison with photographs or in retrospect.
As the illness progresses, more muscle groups get involved. One of the most significant findings occur with spasm of the paraspinal musculature, resulting in severe opisthotonos (see Fig. 37.1), where in young infants, the soles of the feet may touch their head. The typical generalized spasm resembles decorticate posturing, consisting of “a sudden burst of tonic contraction of muscle groups causing opisthotonos, flexion and adduction of the arms, clenching of the fists on the thorax, and extension of the lower extremities” (75). Although the spasms may be confused with posturing or epileptic seizures, they do not produce loss of consciousness and are extremely painful. The tetanic contractions progress further for several more days, with recruitment of additional muscle groups and significant aggravation of symptoms. Painful spasms and contraction can also contort and distort the patient’s posture. All voluntary muscles may be affected, and the disease can involve the larynx, which can be fatal. Fractures of vertebrae or other bones and hemorrhage into muscles can also take place. Even minor stimuli including light, drafts, noises or voices, and light touch may trigger reflex spasms. Because patients remain conscious throughout these spasms, anxiety and pain further complicate management and can contribute to the severity of disease.
Symptoms of autonomic overactivity are generally manifested in the early phases as irritability, restlessness, sweating, and tachycardia. In later phases of illness, profuse sweating, cardiac arrhythmias, labile hypertension or hypotension, and fever are commonly present (76–78). Episodes of bradycardia and hypotension can lead to cardiac arrest. Cardiac arrest has also been attributed to myocardial damage caused by the high catecholamine level (79) and toxic damage to the brainstem (80). Fever can be due to the sympathetic overactivity or superinfections, such as pneumonia (81). Cardiovascular instability due to spasms and inadequate sedation need to be excluded. Cardiovascular complications are managed in the intensive care setting, where ventilatory support and therapeutic paralysis are available. Spasms and cardiovascular complications occur most commonly during the first week and resolve slowly during the ensuing 2 to 4 weeks.
Respiratory compromise is the most serious early problem in generalized tetanus. Upper airway obstruction is common during spasms. The diaphragm and abdominal musculature are often involved, and they can produce apnea in inadequately treated patients in spite of mechanical ventilation. The neuromuscular junction (NMJ) effects of the toxin may produce diaphragmatic paralysis or bilateral vocal cord paralysis (82). Severity may continue to increase for 10 to 14 days after diagnosis, reflecting the transport time of intraneuronal toxin into the CNS. Recovery then begins, usually requiring about 4 weeks. This period probably reflects the time needed for synthesis and transport of presynaptic constituents. In the absence of antitoxin, disease persists as long as TS is produced. The total amount of toxin produced is so small that it is inadequate to prompt an immune response; therefore, patients with newly diagnosed tetanus must be actively immunized to prevent recurrence. Recurrent tetanus is well documented if this is not done (83).
Local Tetanus
This is an unusual presentation of tetanus that occurs when circulating antitoxin prevents general spread of the toxin but is insufficient to stop local uptake at a wound site (75,84). This causes mild, prolonged, steady without progression, and painful muscle contraction in the wounds’ region, which can last for several weeks to months, with subsequent complete spontaneous resolution. Localized tetanus may be unrecognized or mistaken for pain-induced muscle spasms. Neuromuscular transmission may be affected locally, producing weakness in addition to rigidity. Partial immunity to TS may contribute to the development of local tetanus by decreasing the hematogenous spread of toxin (85). More commonly, however, local tetanus is the harbinger of the generalized form, unless treated.
Cephalic Tetanus
This condition is a rare manifestation of tetanus that involves only the cranial nerves after C. tetani enters wound(s) or chronic infection(s) in the head and neck. Although any of the cranial nerves may be affected, singly or in combination, cranial nerve VII is most frequently involved, but involvement of cranial nerves VI, III, IV, and XII may also occur. Patients may present with confusing clinical findings including facial paresis, dysphagia, trismus, and other focal cranial neuropathies. Cephalic tetanus may precede generalized disease, and isolated cephalic tetanus can occur and follows a chronology similar to generalized disease.
Although most series suggest a poor prognosis, a large report from India described mild cases associated with chronic otitis media (86). This may represent colonization of the infected tissue with C. tetani and subsequently the production of toxin. Of 22 patients in one series with otogenic tetanus, 17 had acute otitis media (87). A coexisting aerobic infection was diagnosed in 85%, most commonly with Staphylococcus aureus. Rarely, extraocular movements are affected in patients with tetanus, causing “ophthalmoplegic tetanus” (88) or supranuclear oculomotor palsies (89). Horner syndrome has been reported as a presenting feature (90).
Neonatal Tetanus
This is a generalized form of the disease that often develops in infants delivered to mothers who have not been immunized. This is because passively transferred maternal antibody is protective. The lack of immunization of mothers and the birth practices in developing countries that include lack of aseptic techniques in managing the umbilical stump and application of mud, clarified butter, or feces to the umbilical stump increase the risk of acquiring this illness and are responsible for a large proportion of cases (91). Five factors are involved with NT: (a) the length of the stump (longer seems safer), (b) the care with which the cord is ligated, (c) the cleanliness of the instruments and dressings, (d) the cleanliness of the environment and of the patient’s and mother’s clothing, and (e) the application of mud, feces, or unclean material on the stump (92).
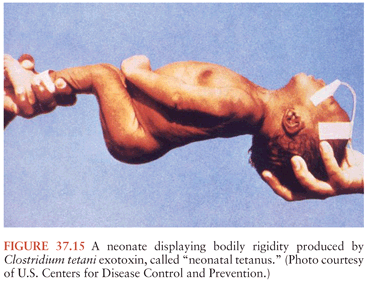
This form of tetanus usually occurs in infants within 14 days of birth and generally manifests initially by inability to suck, and then by tetanic spasms and rigidity producing the typical opisthotonic posture, and trismus (Fig. 37.15). The hypersympathetic state occurs commonly in these infants, and the mortality rate is high, with infants dying of complications such as CNS hemorrhage, pneumonia, pulmonary hemorrhage, and laryngeal spasms with asphyxia. One study found that 33% of infants with tetanus were also bacteremic, mostly with coliforms or S. aureus, most commonly from an infected umbilical cord stump (93). Developmental delay is common in survivors (94,95).
A Turkish study implicated unhygienic home deliveries in more than 90% of NT cases (94). A recent study in Pakistan showed that delivery onto soil compared to a clean surface resulted in a 3.2-fold increase in the odds ratio (OR) of death from NT (95). Other statistically significant risks included paternal illiteracy (OR = 3.2) and having sheep at home (OR = 2.0). Soil as a delivery surface accounted for 64% of the neonatal deaths. Although these concerns are important, active maternal immunization—or passive immunization of the mother before delivery and of the child at birth—would eliminate the disease.
PATHOGENESIS AND PATHOPHYSIOLOGY
After introduction into tissues, C. tetani spores convert to vegetative forms, multiply, and produce TS. In numerous instances, there is no associated inflammation or apparent local infection. At the site of entry, the toxin enters the peripheral nerve and reaches the CNS through the nerves (25,84,96).
The actions of TS predominantly involve three components of the nervous system: central motor control, autonomic function, and the NMJ (76). The central effects have been studied most intensively, and they provide paradigms for understanding the toxin’s effects on other nervous system components.
Central Motor Control Effects
To express its toxic potential, TS must gain access to its target neurons. The toxin appears to enter the nervous system predominantly through the NMJ of alpha motor neurons. Some toxin enters sensory and autonomic neurons, but the amount appears small and its contribution to symptoms is uncertain. It then moves, via the retrograde transport system, to the cell body (57). This system, consisting of microtubules and transport proteins, is normally used to bring signal molecules and exhausted presynaptic components back to the cell for processing. The heavy chain or the C fragment of the toxin is necessary for retrograde transport. TS also spreads hematogenously from its site of production, but it still must enter via neurons (97). Toxin already in transit within the neuron is inaccessible to antitoxin, which partly explains the progression of the disease for several days after treatment. The intrathecal administration of human tetanus immune globulin (HTIG) is an attempt to circumvent this by allowing access to TS moving across synapses in the CNS (see the section “Immunotherapy,” later in this chapter).
Once transported to the spinal cord or the brainstem, the toxin then migrates transsynaptically into presynaptic inhibitory cells (98), which use either glycine or GABA as transmitters (Fig. 37.16). The glycinergic cells are most important in the spinal cord, whereas the GABAergic cells are responsible for decreasing inhibition from the brainstem (99). By preventing glycine or GABA release, TS denies the alpha motor neuron its most essential inhibitory transmitters. This raises its resting firing rate, causing muscle rigidity. Moreover, the normal inhibition of other motor neurons during movements of a particular motor group depends on these inhibitory transmitters, as does the termination of reflexive contractions. Deprived of this inhibition, the motor system responds to an afferent stimulus with the intense, sustained contraction of a wide range of muscles that characterizes the tetanic spasm.
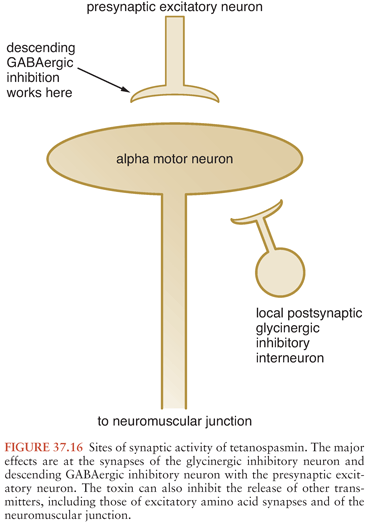
There may be some differences in the predominant effects of local and generalized tetanus regarding the loci of inhibition. Following intravenous injection, TS appears to affect supraspinal (i.e., GABAergic) inhibitory mechanisms exclusively, whereas local tetanus likely has more effect on the spinal glycinergic systems. Because the human disease is caused by spore inoculation rather than toxin administration, clinical generalized tetanus represents a variable combination of these mechanisms. In addition to the loss of inhibitory systems, excitatory transmission is also disrupted (100). This may partially explain the weakness associated with local tetanus.
Histologic abnormalities have been reported in the brainstem nuclei in fatal cases (101), but the significance of these changes is unknown. They most likely reflect terminal hypoxia and autonomic dysfunction. Reversible chromatolysis of motor neurons occurs in experimental tetanus when markedly suprathreshold doses of toxin are employed. Whether this is simply a manifestation of hypermetabolism or reflects another pathophysiologic process is unresolved.
Once transported into the CNS, the toxin continues its rostral movement by retrograde transport (97,102). This implies that structures above the brainstem could be affected by TS; however, there is no evidence that this occurs in the natural history of tetanus. It is likely that generalized tetanus does involve some vertical movement of toxin within the neuraxis, but the widespread manifestations in this form are mostly due to the hematogenous dissemination of toxin to NMJs throughout the body. TS does inhibit the evoked release of norepinephrine (103), acetylcholine (104), serotonin (105), and enkephalin (106) from brain tissue in vitro. TS is also epileptogenic, producing depolarizing shifts when added to CNS tissue in culture and partial seizures when injected intracerebrally. These acute seizures are associated with diminished extracellular GABA levels (107). However, the abnormal electrical activity outlasts the period of GABAA inhibition, indicating long-lasting reorganization of synaptic function (108).
Autonomic Nervous System Effects
Before modern intensive care, most patients with severe tetanus died quickly from ventilatory failure (109). Although autonomic involvement was mentioned in 1954, the first major report of this aspect of tetanus appeared in 1968 (74). The latter group described “a characteristic syndrome whose features include sustained but labile hypertension and tachycardia, irregularities of cardiac rhythm, peripheral vascular constriction, profuse sweating, pyrexia, increased carbon dioxide output, increased urinary catecholamine excretion, and, in some cases, the development of hypotension.” Although these signs are usually present toward the end of the first week, they may develop during the second (110). Most are associated with elevated catecholamine levels.
An elevated plasma epinephrine and norepinephrine concentration (74,111) and 24-hour urine catecholamine excretions (112) were reported in patients with tetanus. These manifestations resemble the effects of a pheochromocytoma; a similar cardiomyopathy is seen in both conditions (78).
Udwadia et al. (113) performed invasive hemodynamic monitoring of 27 patients with severe tetanus. The major finding was a hypersympathetic state, with tachycardia (mean, 131 beats per minute) and increased cardiac (5.48 liters per minute) and stroke volume (43.1 mL/m2) indexes. A total of 19 patients experienced episodes of sustained tachycardia (>150 beats per minute); 17 had hypertension of varying degree, whereas 8 experienced alternating hypertension and hypotension, and 5 had episodic hypotension only. Seven patients had paroxysmal supraventricular tachycardia, and three had brief runs of ventricular tachycardia. Despite the difficulties experienced by their patients, the mortality rate was 6.25%.
TS disinhibits sympathetic reflexes at the spinal level, implying that the hyperadrenergic findings are not dependent on hypothalamic or brainstem dysfunction (114). Parr et al. (115) later showed that cutaneous stimulation of animals with local tetanus increased firing rates in renal sympathetic nerves. Conversely, the development of inappropriate antidiuretic hormone secretion supports hypothalamic involvement (116).
The identification of TS as a Zn2+-dependent peptidase (117) leads to a new hypothesis to explain the hypertension seen in tetanus patients. Captopril, an inhibitor of Zn2+-dependent proteases, antagonizes the effect of TS on synaptobrevin and allows exocytosis to proceed normally (118). Antibodies directed against the Zn2+-binding domain of TS restore exocytosis in chromaffin cell cultures over 4 days (119); however, this model differs substantially from the synapse, so such rapid restoration of function would not be anticipated in clinical disease; chelation of Zn2+ by ethylenediaminetetraacetate (EDTA) and other chelators is also effective in experimental systems (120). Nonetheless, these results suggest that TS might, in addition to its other effects, have an angiotensin-converting enzyme (ACE)–like effect, and a component of the hypertension in patients with tetanus may be related to excess angiotensin-II effects rather than catecholamine effects.
Clinical evidence suggests some disruption of parasympathetic function as well. Bradycardia is occasionally noted as is hypotension (121) without previous evidence of increased sympathetic tone (122). Although both of these findings can be reproduced by injection of TS into a rat’s third ventricle (123), a more peripheral mechanism appears likely in human tetanus.
Neuromuscular Junction Effects
Because the central effects of TS are so dramatic, the peripheral disorder it produces received scant clinical attention until 1977. Investigators showed denervation changes in the muscle closest to the site of the injury that introduced C. tetani, which reflected failure of neuromuscular transmission (124). Experimental studies by Harvey (125) established the presence of neuromuscular blockade 40 years earlier. Single-fiber electromyography (EMG) confirmed a presynaptic defect of acetylcholine release qualitatively similar to that of botulism (126). The NMJ may be permanently disabled by TS; return of function requires sprouting of the terminal end to produce new synapses (124).
APPROACH TO DIAGNOSIS
Tetanus can be diagnosed only clinically. Generalized and cephalic tetanus are easily recognized if one thinks of the disease. Localized tetanus is more difficult to detect early. The neonatal form is less easily recognized on initial presentation, but it rapidly becomes obvious. Laboratory assistance is confined to the exclusion of other diagnoses and to the determination of immunity. Tetanus can follow an incubation period from 3 to 14 days after an injury.
EMG studies may be useful by showing evidence of denervation, reinnervation, and increased excitability of the motor neuron pool (127). The EMG differential diagnosis of tetanus has been reviewed (128). Blood counts are normal or slightly elevated; CSF measures are normal; and electroencephalogram (EEG) and EMG are normal and nonspecifically abnormal, respectively. Gram stains of wounds may reveal the characteristic gram-positive bacilli with terminal spores in as many as one third of tetanus patients. Even though a positive wound culture can support the clinical diagnosis, a positive culture in the absence of symptoms does not indicate that clinical tetanus will develop.
The spatula test is a simple test that can be diagnostic (129). The posterior pharyngeal wall is touched by a spatula, which induces a reflex contraction of the masseters in the presence of tetanus.
Gowers (4), again, provided an unequaled clinical description in 1888:
The first symptom is usually a sense of stiffness in the neck or jaw, sometimes difficulty in swallowing, or stiffness of the tongue. . . . In the course of a few hours, or at most a day or two, the difficulty in separating the jaws becomes greater, and is clearly due to increasing rigidity of the masseters. With this there is also more stiffness in the neck, and the head is slightly bent backward from the preponderance of spasm in the extensor muscles. As the rigidity in the neck increases, it passes down the spinal muscles. . . . The legs may also become extended and rigid, but the arms are little affected.
A few patients have atypical features on presentation. The lack of an easily identified portal of entry, discussed earlier, does not exclude the diagnosis but makes prognostication more difficult because the incubation period cannot be determined. These patients should be carefully examined for signs of parenteral drug abuse, otitis, or rectal or vaginal instrumentation. Recent injections or minor surgical procedures may be uncovered by questioning. Lesions of the gastrointestinal tract are occasionally implicated, especially among inhabitants of rural areas among whom the carriage rate of C. tetani may be 20-fold higher than that of urban dwellers (130).
Differential Diagnosis
Although many disorders have small areas of overlap with tetanus, strychnine intoxication is the only true mimic (131). Strychnine is a component of some rodenticides. This drug is a direct antagonist at the glycine receptor. Lack of abdominal rigidity between spasms may be more common with strychnine than with tetanus, and trismus may be absent in the former; otherwise, the clinical presentations are similar. For this reason, biochemical analyses of serum and urine for strychnine should be performed in suspected tetanus cases, and tetanus should be considered even when strychnine poisoning is likely. The initial symptomatic management of both conditions relies on benzodiazepines, but their subsequent treatments and complications differ substantially. Many other glycine and GABA antagonists have been exploited as experimental convulsant agents; although human poisoning has not been reported, conditions resembling tetanus may be expected.
Dystonic reactions to dopamine blockade usually involve torticollis, and oculogyric crises are common. Neither of these phenomena is part of tetanus, and reflex spasms are not seen. A rapid response to anticholinergic agents (benztropine, 1 to 2 mg; or diphenhydramine, 50 mg) and subsequent toxicologic studies help confirm the diagnosis of dystonic reaction. A trial of anticholinergic agents is reasonable in suspected tetanus if the diagnosis is in doubt. There are anecdotal reports of transient improvement in the symptoms of tetanus after anticholinergic drug administration, perhaps reflecting a sedative effect (132).
A report of opisthotonic posturing and risus sardonicus in a patient suffering from an amphetamine overdose is difficult to reconcile with the expected effects of amphetamines but reminds us of the need to keep an open mind in differential diagnosis (133).
The nuchal rigidity of meningitis may resemble the neck stiffness of tetanus, but the other manifestations of each disorder should resolve any confusion. The cerebrospinal fluid is normal in tetanus. The other infection that may raise a question of tetanus is an alveolar ridge abscess producing trismus. Oral pain and tenderness is not characteristic of tetanus; the patient with a dental disorder will not display spasms or rigidity. However, various dental infections may produce tetanus (134), and temporomandibular joint dislocation may be a symptom of the disease (135). Verma et al. (136) described a single case of unilateral trismus resulting from a tuberculoma of the brainstem.
Patients experiencing generalized convulsive status epilepticus may at first appear to have tetanic spasms, but the loss of consciousness and evolutionary movements of the former should quickly resolve any question.
Tetany precipitated by hypocalcemia or alkalosis will be accompanied by Chvostek and Trousseau signs. In contrast to tetanus, tetany involves the extremities more than the axial musculature.
Progressive fluctuating muscular rigidity (the “stiff person” syndrome) has been likened to a chronic form of tetanus (137). This idiopathic syndrome has an insidious onset, usually has minimal cranial nerve involvement, lacks trismus, and is relieved during sleep. Current evidence links this disorder to autoantibodies against GABAergic neurons (138).
Local tetanus is rarely confused with other disorders; the major problem is failure to consider the diagnosis. It has been confused with transverse myelitis (139).
“Pseudotetanus” has been used in the past to describe a broad variety of disorders (140) and may be due to malingering or a conversion disorder (141). In the former, the patient’s posture may be complex or inconsistent; rigidity is lacking or feigned, the patient is distractible, and some secondary gain is identified. Preexisting psychologic problems may also complicate the diagnosis and management of tetanus (142).
Determination of Immunity
Tetanus Toxoid Adsorbed, for intramuscular use, is a sterile suspension of alum-precipitated (aluminum potassium sulfate) toxoid in an isotonic sodium chloride solution containing sodium phosphate buffer to control pH. When properly administered and maintained, immunization with tetanus toxoid is highly effective, with an estimated failure rate of less than 4 per 100 million persons in those who are immunocompetent (143). Therefore, a confirmed history of active immunization almost eliminates tetanus as a diagnosis. This includes both an acceptable initial series and a booster within 10 years.
Human antibody against tetanus toxoid is predominantly in subclass immunoglobulin G1 (IgG1); there is a moderate amount of IgG3 and IgG4, and minimal IgG2 (144). The serum concentration of antitetanus antibodies can be measured by immunoassay or hemagglutination, but the results are seldom available in time to influence initial diagnosis and management. The results are quantitated in international units (IU) of antitoxin by reference to an arbitrary international standard, which has achieved international agreement (145). The development of a rapid test holds promise not only for the exclusion of tetanus but also for decisions regarding antitoxin administration (see the section “Therapy,” later in this chapter) (146).
In most epidemiologic studies, a level of 0.01 IU/mL is accepted as protective, based on one study in guinea pigs reported in 1937 (147). This level prevented death in the animals, but a few developed tetanus with concentrations between 0.1 and 0.5 IU/mL, from which they recovered. Nonetheless, cases of human tetanus have been reported with titers as high as 0.16 IU/mL (148–150). In one study of 64 tetanus patients, 24 had detectable antitoxin levels, and 10 had levels greater than 0.01 IU/mL (151). The severity of tetanus tended to be less in the patients with higher antibody levels, but six of the ten required tracheostomy and mechanical ventilation. In a study of 20 cases of NT in Nigeria, the disease developed in six infants whose mothers had been immunized; the mean antitetanus IgG concentration in these babies was 0.70 IU/mL, with a range of 0.16 to 2.83 (152). The authors speculated that the toxin load in neonatal tetanus may be greater than that in other forms of tetanus, suggesting that the antibody concentration needs to be higher to be protective.
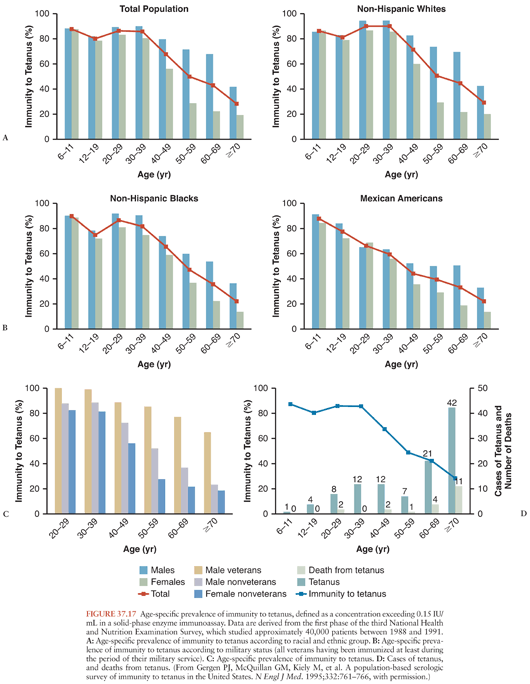
A population-based study, employing 0.15 IU/mL as the threshold for immunity, confirmed that antibody levels wane with aging (153). Individuals with histories of military service, higher educational attainment, and incomes exceeding the poverty level were more likely to have protective antibody titers. Women and members of several minority groups were less likely to have protective titers (Fig. 37.17), presumably because of failure to receive initial or booster vaccinations. An accompanying editorial reinforced the responsibility of physicians to maintain their patients’ immunity to tetanus (154). A more recent analysis confirms that failure to maintain tetanus immunity is an ongoing problem (155).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree