15 Systemic Therapy for Colon Cancer
Introduction
The fluoropyrimidine analog fluorouracil (5-FU) first developed by Heidelberger and coworkers1 in 1957 was the only first-line chemotherapeutic option for patients with advanced colorectal cancer until the late 1990s. As such, 5-FU underwent extensive dose and schedule optimizations and combinations with different modulators to improve response and survival rates. Median survival beyond 12 months, however, was rarely attainable.2–6
Metastatic Colorectal Cancer
5-Fluorouracil and Leucovorin
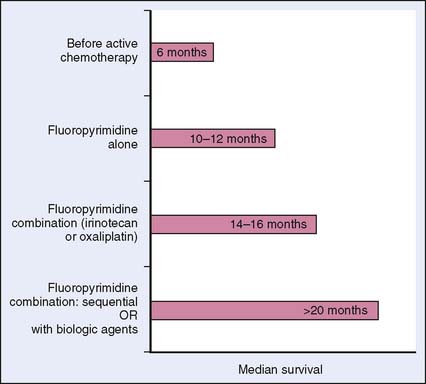
The benefit of systemic therapy in the management of metastatic colorectal cancer was initially established in the 1980s and 1990s. Several phase III randomized trials showed improvement in the quality of life and overall responses despite low objective response rates (RR 10%–20%) with the use of 5-FU and leucovorin (LV).7 According to a meta-analysis of 13 such trials, systemic chemotherapy significantly improved overall survival compared with best supportive care.8 Fluorouracil forms the backbone of systemic therapy for advanced colorectal cancer, and the survival of these patients nearly doubled with the advent of new chemotherapeutic and targeted agents (Fig. 15-1).
Fluorouracil and leucovorin are administered intravenously in a variety of dosing schedules. In the loading bolus schedules, 5-FU and leucovorin are administered daily in bolus for 5 consecutive days and repeated every 28 days (Mayo Clinic Protocol)9; alternatively, in the weekly bolus schedule, 5-FU and leucovorin are given weekly for 6 of every 8 weeks (Roswell Park regimen).10 For the infusional regimen, leucovorin and 5-FU are administered in bolus followed by 22 hours of 5-FU infusion through a central venous catheter on days 1 and 2 and repeated every 2 weeks (5-FU/LV2).11
These regimens differ in their toxicities, which may partly guide their use clinically. The bolus regimens tend to be associated with bone marrow suppression, mucostomatitis, and diarrhea; whereas palmar-plantar erythrodysesthesia (hand-foot syndrome) is more common in the infusional regimen. Central venous access is required for the infusional regimen. The difference in quality of life and cost between the infusional and bolus regimens is marginal, and the infusional method seems to be marginally more effective than the bolus approach.12–16
Irinotecan
The advent of a number of active agents allowed the use of combination chemotherapy (Box 15-1). The earliest combination regimens used irinotecan with various schedules of 5-FU/LV in first-line therapy of metastatic colorectal cancer.17–19 Irinotecan (also known as CPT-11) is a semisynthetic derivative of the natural alkaloid camptotecin and exerts its cytotoxic effects by inhibiting topoisomerase I, which is necessary for the proper uncoiling of DNA for replication and transcription. The drug is hydrolyzed to its more potent active metabolite, SN38, by carboxylesterase.20 Irinotecan and its metabolites interact with DNA replication forks, leading to DNA fragmentation by stabilizing single-chain DNA breaks and subsequent cell death. When compared with infusional 5-FU or best supportive care, irinotecan showed single-agent activity in patients with advanced colorectal cancer who had previously received bolus 5-FU with improvement in median survival and quality of life.21,22
Box 15-1 Selected Agents with Proven Efficacy in Metastatic Colorectal Cancer
In first-line advanced colorectal cancer therapy, irinotecan improved median survival by about 2 months and almost doubled the response rate when combined with 5-FU and leucovorin in two randomized trials16,19 (Fig. 15-2). In the North American trial, irinotecan added to weekly bolus schedule 5-FU (IFL) was compared with bolus 5-FU (Mayo regimen) and patients who received IFL had a longer median overall survival (14.8 versus 12.6 months; P = .04) and a higher response rate (39% versus 21%; P < .001). In the European trial, irinotecan combined with infusional 5-FU (FOLFIRI) was compared with infusional 5-FU regimens. Similar efficacy was found in patients who received the FOLFIRI with improvement of median overall survival from 14.1 to 17.4 months (P < .001) and response rate from 22% to 35% (P < .01).
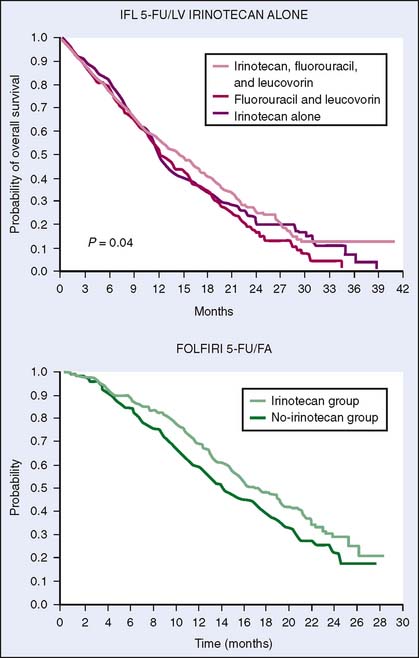
Figure 15-2 Kaplan-Meier estimates of overall survival for irinotecan-based regimens. FA, folinic acid.
(A, Reprinted from Saltz LB, Cox JV, Blanke C, et al: Leucovorin and fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 343:905–914, 2000. B, Reprinted from Douillard JY, Cunningham D, Roth AD, et al: Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomized trial. Lancet 355:1041–1047, 2000.)
The toxicity of irinotecan includes diarrhea, bone marrow suppression, nausea and vomiting, and alopecia. Certain toxicities, especially diarrhea and neutropenia, correlate with a polymorphism of uridine diphosphate glucuronosyltransferase isoform 1A1 (UGT1A1) in retrospective studies.23,24 SN38, the active metabolite, is glucuronidated by UGT1A1 before elimination, so reduced UGT1A1 activity increases SN38. An (FDA)-approved clinical test for UGT1A1 polymorphism is available, and individualizing irinotecan dosage based on patients’ pharmacogenomic profile may eventually be clinically feasible.25 With no data from a prospective clinical trial, the usefulness of testing UGT1A1, and how to adjust dosages depending on the test results, are debatable.
Oxaliplatin
Oxaliplatin is a third-generation diaminocyclohexane-containing platinum compound that forms bulky DNA adducts and induces cellular apoptosis.26 Unlike previous generations of platinum compounds, oxaliplatin showed promising activity against human colorectal cell lines in preclinical studies. In clinical studies, oxaliplatin had limited single-agent efficacy but was highly synergistic with fluoropyrimidines in first- and second-line therapy for metastatic colorectal cancers.27–29 A possible mechanism was downregulation of thymidylate synthase by oxaliplatin.30–32
In April 2002, an interim analysis of a landmark trial ushered in oxaliplatin-based therapy to the first-line treatment of advanced colorectal cancer. The North Central Cancer Treatment Group/Intergroup Trial N9741 compared IFL with oxaliplatin/folinic acid/5-FU (FOLFOX4 regimen with oxaliplatin 85 mg/m2 on day 1; LV 200 mg/m2 for 2 hours; and bolus 5-FU, 400 mg/m2 followed by 600 mg/m2 continuous infusion for 22 hours on days 1 and 2) and a combination of irinotecan and oxaliplatin (IROX).33 FOLFOX4 compared favorably with IFL, which was the standard of care at the time, in all efficacy parameters including response rate (45% versus 31%), time to progression (TTP, 8.7 months versus 6.9 months), and median overall survival (OS 19.5 months versus 15 months). In addition, toxicity analysis favored FOLFOX4. This trial eventually set the new standard of care in first-line therapy of colorectal cancer. Based on its results, FOLFOX has become the most widely used combination regimen in newly diagnosed advanced or metastatic colorectal cancer, particularly in the United States.
In the second-line setting, patients with metastatic colorectal cancer who received FOLFOX after IFL failure had a better outcome in median time to progression and response rate compared with those who received 5-FU and leucovorin.34 In three first-line randomized trials, the oxaliplatin-containing regimens showed consistently better time to progression, response rate, and overall survival than did 5-FU and leucovorin alone.35,36
When combined with 5-FU and leucovorin, oxaliplatin has side effects that are different from those of the other platinum compounds, namely, cisplatin and carboplatin. Nephrotoxicity, ototoxicity, and alopecia have been less common.37–39 Approximately 15% of patients who received oxaliplatin-based regimens, however, have developed a unique neuropathy: an acute, reversible, transient, and cold-induced paresthesia of hands, feet, oral, or pharyngeal regions during or for a short period of time after infusion and a cumulative, dose-dependent, longer-lasting peripheral dysesthesia after months of treatment. The neuropathy is usually reversible when oxaliplatin is stopped and fortunately reverses in more than 99% of patients to a level not interfering with activities of daily living within 18 months after discontinuation.40
FOLFOX versus FOLFIRI
Despite the fact that FOLFOX showed significantly higher efficacy compared with irinotecan and bolus 5-FU (IFL), when administered in combination with infusional 5-FU and leucovorin, irinotecan (FOLFIRI), and oxaliplatin (FOLFOX) appear to be equally efficacious in patients with previously untreated metastatic colorectal cancer. The choice for first-line therapy is then often guided by the regimens’ adverse effects and institutional practice. A phase III trial by Tournigand and associates41 compared FOLFOX with FOLFIRI with crossover at progression. Response rates (56% versus 54%), progression-free survival ([PFS] 8.5 months versus 8.0 months), and median overall survival (21.5 versus 20.6 months) were almost identical. Comparable results were also reported by an Italian group.42
It is clear that the maximum impact on overall survival is related to exposure to all active drugs, with the actual sequence of this exposure playing a minor role or no role in changing the overall survival outcome. This observation was emphasized in a meta-analysis of seven phase III studies showing that the median overall survival correlates with the percentage of patients receiving all three active agents (5-FU, irinotecan, and oxaliplatin).43 Regimens with all three drugs simultaneously (FOLFOXIRI) have also been tested with promising results.44
Oral Fluoropyrimidines
Capecitabine, a fluoropyrimidine carbamate, is an oral prodrug that undergoes enzymatic conversion in the liver to 5-FU.45 The adverse-effect profile is similar to that of infusional 5-FU regimen, with hand-foot syndrome as the predominant toxicity. However, stomatitis, nausea, vomiting, bone marrow suppression, and diarrhea are observed as well. Capecitabine is superior to daily bolus Mayo regimen in terms of objective response rate (about 25% versus 16%, respectively) in two randomized controlled trials, but it did not confer significant survival advantage (median survival about 12.9 versus 12.8 months, respectively).46–48
Another oral fluoropyrimidine with similar response and survival rates compared with intravenous 5-FU was tegafur, a prodrug of 5-FU, plus uracil, an inhibitor of dihydropyrimidine dehydrogenase.49,50 The combination is usually administered together with oral leucovorin and is approved by regulatory agencies outside of the United States. S-1 is another 5-FU-based oral combination drug, which has been developed mainly in Asia.51–56
The oral fluoropyrimidine that has been studied the most is capecitabine. Having been designed to recapitulate the pharmacokinetics of continuous infusion 5-FU via oral administration, capecitabine was compared with the Mayo Clinic regimen of bolus 5-FU/LV in a phase III trial. It was found to have superior response rates, equivalent PFS and overall survival, and a favorable toxicity profile.48 These observations led to studies exploring capecitabine as an alternative to infusional 5-FU in combination with oxaliplatin or irinotecan. Two studies, TREE-1 and TREE-2, explored the best fluoropyrimidine in combination with oxaliplatin. Patients were randomized to FOLFOX6, bolus 5-FU/LV/oxaliplatin, or CAPOX (capecitabine + oxaliplatin).57 Since the FDA approval of bevacizumab took place while the trials were ongoing, an amendment was made to add bevacizumab to various arms of the studies. Although bevacizumab appeared to enhance the efficacy of each individual regimen, the CAPOX regimen was comparable to its infusional counterpart FOLFOX6 as far as response rates, TTP (time to progression), and overall survival are concerned, with the regimen containing the bolus 5-FU/LV being the least effective.
Moreover, a large, randomized, international, phase III trial of 2035 advanced colorectal cancer patients, NO16966, compared first-line XELOX (capecitabine [Xeloda] + oxaliplatin) with FOLFOX4 (intravenous bolus and infusional 5-FU + oxaliplatin). After release of bevacizumab data in colorectal cancer in 2003, the protocol was amended to investigate, using a 2 by 2 factorial design, XELOX + placebo versus XELOX + bevacizumab (7.5 mg/kg every 3 weeks) versus FOLFOX4 + placebo versus FOLFOX4 + bevacizumab (5.0 mg/kg every 2 weeks). The primary objective of the study was to answer two questions: (1) whether the XELOX regimen is noninferior to FOLFOX and (2) whether the addition of bevacizumab to chemotherapy improved results compared with chemotherapy alone. The secondary endpoints included overall survival, overall response rates, time to and duration of response, and safety profile. The study showed that XELOX (capecitabine + oxaliplatin) is as effective as FOLFOX4 (infused 5-FU + oxaliplatin) in terms of PFS (hazard ratio [HR] 1.05; upper limit of the 95% confidence interval was below the noninferiority margin of 1.23). Adding bevacizumab to chemotherapy (FOLFOX4 and XELOX) significantly improved PFS compared with that with chemotherapy alone (HR 0.83), which meant that adding bevacizumab to chemotherapy combination with either 5-FU or capecitabine improves the chances of delaying progression of the disease by 20%.58 In contrast, the combination of capecitabine and irinotecan (CAPIRI) is more controversial. CAPIRI was found to be inferior to its infusional counterpart (FOLFIRI) both in terms of PFS and tolerability in the phase III trial BICC-C,59 but the difference in overall survival was not significant. Diarrhea appears to be a major hurdle in combining these agents, and care must be taken to prevent dehydration.
Epidermal Growth Factor Receptor (EGFR) Antibodies
The epidermal growth factor receptor (EGFR, or HER1) is a transmembrane receptor tyrosine kinase that belongs to the erbB, or HER, receptor family. Upon binding with a ligand such as epidermal growth factor (EGF) or transforming growth factor (TGF-α), EGFR pairs with another EGFR receptor (homodimerization) or another member of the erbB family (heterodimerization). Dimerization leads to receptor autophosphorylation and activation of intracellular signaling pathways that regulate cellular growth, survival, migration, adhesion, and differentiation (Fig. 15-3). The receptor was found to be abnormally activated in many epithelial malignancies including colorectal cancer.60,61
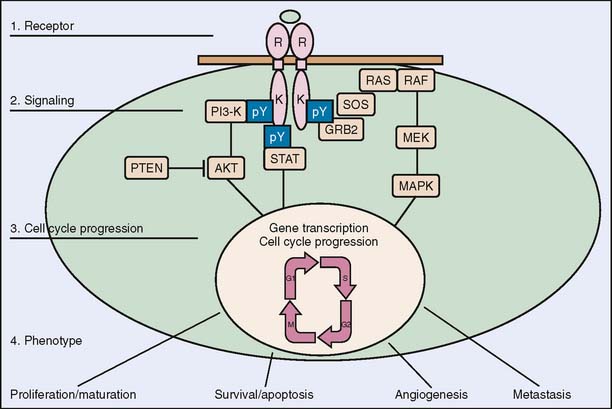
Figure 15-3 Epidermal growth factor signaling pathway.
(Reprinted from Baselga J: Targeting the epidermal growth factor receptor: a clinical reality. J Clin Oncol 19:41S–44S, 2001.)
EGFR became a target of interest in colorectal cancer therapy when expression or upregulation of the EGFR gene was found in 60% to 80% of cases.62–65 Overexpression of EGFR in colorectal cancer was also associated with poorer prognosis.66,67 Inhibition of EGFR can be achieved by targeting the extracellular domain with monoclonal antibodies or the intracellular tyrosine kinase with small molecule inhibitors.68
Cetuximab is a chimeric murine/human IgG1 monoclonal antibody highly specific for EGFR and has a higher affinity than EGF and TGF-α, thereby blocking the ligand-dependent autophosphorylation of the receptor. Although preclinical studies showed primarily cytostatic single-agent activity, cetuximab plus irinotecan was highly synergistic in irinotecan-refractory colorectal cancer xenografts when tumor growth would have continued with respective agents alone.69–71 As an IgG1 subtype antibody, cetuximab can theoretically mediate immune functions and thus may have immunologic mechanisms as well.
Cetuximab was first of its class to be approved by the FDA in the United States for treatment of colorectal cancer in patients who had previous unsuccessful chemotherapy. The response rates for cetuximab alone and cetuximab plus irinotecan were 9% and 17%, respectively, in two nonrandomized trials involving colorectal cancer patients previously treated with an irinotecan-based regimen.72,73 In a multi-institutional randomized study, 329 patients with metastatic colorectal cancer who progressed on irinotecan-based regimen were randomized to receive either cetuximab plus irinotecan or cetuximab monotherapy.74 The patients receiving cetuximab plus irinotecan had a better response rate (22.9% versus 10.8%; P = .007) and median TTP (4.1 versus 1.5 months; P < .001) than cetuximab monotherapy, although median survival was not significantly different (8.6 versus 6.9 months; P = .48).
Cetuximab as monotherapy versus best supportive care (BSC) was investigated in the NCIC CO.17 trial.75 In this trial, 572 metastatic colorectal cancer patients who progressed after treatment with fluoropyrimidines, oxaliplatin, and irinotecan were randomized to cetuximab with BSC versus BSC alone without crossover. Cetuximab improved overall survival (HR for death 0.77; P = .005) from 4.6 months in the BSC-alone arm to 6.1 months in the cetuximab/BSC arm in unselected patients. The hazard ratio for PFS was 0.68; P < .001.
A more recent trial, CRYSTAL, evaluated cetuximab combined with the irinotecanbased chemotherapy regimen FOLFIRI versus FOLFIRI alone. Median PFS was slightly improved from 8.0 to 8.9 months (P = .036), but there was no significant difference in overall survival. The response rate was also improved by the addition of cetuximab (46.9% versus 38.7% in the FOLFIRI-only arm; P = .0038).76 Furthermore, in a subgroup analysis of patients who had liver-limited disease, PFS was 11.4 months with cetuximab versus 9.2 months in the control arm. The number of complete resections of the metastases in the subgroup who had only liver metastases was more than double with cetuximab plus FOLFIRI compared with the control arm (9.8% versus 4.5%).
In addition, the combination of cetuximab plus the oxaliplatin-based regimen (FOLFOX) in irinotecan-refractory metastatic colorectal cancer appeared to be safe when examined in a multi-institutional randomized trial, the EXPLORE trial.77 The OPUS trial, a phase II randomized study, further confirmed this result. In this trial, 337 patients were randomized to receive FOLFOX4 + cetuximab versus FOLFOX4. The best overall response rate was 45.6% in the FOLFOX/cetuximab arm versus 35.7% in the FOLFOX arm.78
The side effects of cetuximab are fairly well tolerated. About 75% of patients developed a mild acneiform rash. About 3% of patients developed hypersensitivity infusional reactions when receiving therapy. The development of rash to anti-EGFR therapy seemed to correlate with objective response, but this relationship needs to be better defined in a properly designed clinical study.79
Panitumumab is a fully human monoclonal antibody of the IgG2 type against EGFR with single-agent activity in chemotherapy-refractory colorectal cancer comparable to that of cetuximab.80,81 Different dosing schedules have been proposed (weekly, every 2 weeks, or every 3 weeks).82 Although more than 90% of patients experienced some degree of acneiform rash, only 3% were severe (grade 3 or 4). Based on the results of a phase III trial showing improved PFS compared with BSC in metastatic colon cancer, biweekly panitumumab has received FDA approval for use in chemotherapy-refractory advanced colorectal carcinoma.83
In previous cetuximab trials, only patients with EGFR expression by immunohistochemical stain were eligible, owing to preclinical evidence, which suggests the predictive value of EGFR expression for cetuximab efficacy. This led to the initial approval of cetuximab therapy for patients with EGFR-expressing colorectal cancer by FDA in the United States. Data from multiple EGFR antibody trials show that the degree of EGFR expression seems to be unrelated to response, and patients with EGFR-negative colorectal cancers have responded to cetuximab as well.84,85 Hence, EGFR expression by the contemporary immunohistochemical analysis techniques did not seem to predict cetuximab response.
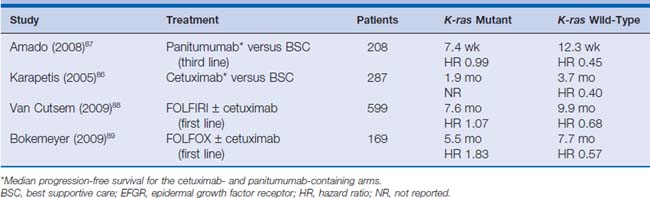
The predictive role of K-ras mutational status was retrospectively analyzed in the two trials in which cetuximab or panitumumab were compared with BSC in third-line setting.86,87 In the NCIC CO.17 trial, in patients with wild-type K-ras, treatment with cetuximab compared with supportive care alone significantly improved overall survival (median 9.5 versus 4.8 months; HR for death, 0.55; P < .001) and PFS (median 3.7 months versus 1.9 months; HR for progression or death 0.40; P < .001).86 Among patients with mutated K-ras tumors, there was no significant difference between those who were treated with cetuximab and those who received supportive care alone with respect to overall survival (HR 0.98; P = .89) or PFS (HR 0.99; P = .96).
The impact of K-ras status on response rate and PFS in the first-line treatment of patients with FOLFIRI or FOLFOX with or without cetuximab was also retrospectively investigated in the CRYSTAL, EVEREST, and OPUS trials. Data from all these trials suggest that the benefit from addition of cetuximab to standard treatment is higher in patients with wild-type K-ras. For patients with K-ras mutations, no benefit could be shown for adding cetuximab to FOLFOX or FOLFIRI. This led the FDA and the European Medicines Agency (EMEA) to change the labeling for cetuximab and panitumumab for patients with only wild-type K-ras colorectal cancer.
Other potential predictive biomarkers have been explored but are not ready for clinical implementation. Since colorectal cancer also has BRAF mutations, and K-ras and BRAF are mutually exclusive, the role of BRAF mutations as prognostic or predictive factors for response to cetuximab or panitumumab was analyzed in a retrospective analysis of 113 patients treated with either EGFR-targeting antibody. In this study, researchers confirmed that K-ras and BRAF mutations are mutually exclusive and that patients with BRAF mutations also did not respond to either cetuximab or panitumumab88–90 (Table 15-1). Patients with BRAF mutations also had shorter PFS and overall survival, suggesting a role of BRAF as prognostic marker, but this result needs to be validated in large trials. Other predictive biomarkers such as high mRNA levels of the EGFR ligands epiregulin and amphiregulin as well as PTEN status are being investigated, with the hope of leading to better patient selection for these targeted therapies.91,92
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree