Justin D. Radolf, Edmund C. Tramont, Juan C. Salazar Keywords bicillin; cardiovascular syphilis; chancre; congenital syphilis; darkfield microscopy; general paresis; granuloma; Great Pox; gumma; Herxheimer reaction; Jarisch-Herxheimer reaction; latency; leptomeningitis; neurosyphilis; nontreponemal antibodies; otosyphilis; penicillin; RPR; spirochete; tabes dorsalis; treponemal antibodies; treponeme; VDRL
Syphilis (Treponema pallidum)
Syphilis is a complex systemic illness caused by the highly invasive, noncultivable spirochete Treponema pallidum. It holds a special place in the history of Western medicine because of its prevalence in modern times, the many historical personages who had or are presumed to have had the disease, and its protean clinical manifestations, for which it came to be known as “the great imitator” or “the great impostor.”1–4 The first designated and recognized medical specialists were syphilologists. Special clinics were established in Europe and North America to care for the enormous number of persons afflicted with this disorder, and its ubiquity spawned one of the first specialized medical journals, the American Journal of Syphilis, Gonorrhea and Venereal Disease. The identification of the causative agent, initially named Spirochaeta pallida, in 1906 was a milestone in biomedical research.4
Venereal syphilis is usually transmitted by sexual contact and, unlike most other bacterial infections, is never diagnosed in routine clinical practice by isolation of the causative organism. Instead, diagnosis depends upon detection of T. pallidum or T. pallidum DNA in patient specimens or, most commonly, reactivity in serologic assays.5,6 The inability to isolate T. pallidum from patient specimens, with the consequent dependence on serologies, is the root cause of most of the management dilemmas that continue to surround this disorder.7,8 Confusion and controversies also arise from the disease’s protracted natural history, which is classically divided into the following stages: (1) an incubation period lasting up to 90 days (average of 3 weeks); (2) a primary stage characterized by a painless ulcer, the chancre, at the site of inoculation, often associated with regional lymphadenopathy; (3) a florid, disseminated stage (secondary syphilis), typically characterized by generalized skin rash, mucocutaneous lesions, and lymphadenopathy, but capable of involving any organ system, including the central nervous system (CNS); (4) an asymptomatic latent period lasting years, detectable only by reactive serologic tests; and (5) in approximately one third of untreated persons, a recrudescent tertiary stage involving the ascending aorta (cardiovascular syphilis) or the CNS (neurosyphilis) or causing necrotizing granulomatous lesions (gumma) in almost any organ.
History
The historical aspects of syphilis make for fascinating reading and endless speculation.2,4,9 Many contemporary clinicians are unaware of the prevalence and importance of syphilis in Western countries through the middle of the 20th century, the many historical figures who suffered from the ailment, and its pervasive influence on all facets of medical practice. As one example, at the turn of the 20th century, syphilis was the leading cause of neurologic and cardiovascular disease among middle-aged persons.1,10
The likely unresolvable debate about the origins of syphilis centers about whether the disease was imported into the Old World from the New World by shipmates of Christopher Columbus or was an established entity that spread in epidemic form throughout Europe as a consequence of urbanization and social upheaval.11,12,13–15 The controversy over these two mutually exclusive theories is fueled by the imprecision and uncertainties inherent in the historical record, radiocarbon dating, and paleopathologic findings believed to represent the signature of treponemal disease in skeletal remains. Unfortunately, the inability thus far of investigators, despite exhaustive efforts, to polymerase chain reaction (PCR)-amplify ancient treponemal DNA from pre-Columbian archeological specimens precludes definitive resolution of this controversy.16–18 Lacking direct molecular proof, the proponents of the New World or Columbian theory base their argument on the extensive osseous record of presumptive treponemal disease in New World pre-Columbian populations dating back 7000 years as opposed to the sporadic evidence from Old World pre-Columbian excavation sites. In an exhaustive evaluation of all extant data, Harper and colleagues12 concluded that no case of Old World treponemal disease has a radiocarbon date that places it firmly in the pre-Columbian period. These same investigators have used sequence analysis of multiple loci in modern T. pallidum subspecies isolates to buttress their argument that syphilis spirochetes originated in the New World,19 although others have questioned the robustness of their phylogenic scheme.20 From a genetic standpoint, two questions need to be answered: What alterations in the T. pallidum genome enabled treponemes to transition from nonvenereal to venereal transmission and what evolutionary forces prompted the change? Comparisons of genomic sequences of T. pallidum subsp. pallidum and subsp. pertenue strains may provide these answers.21 The recent discovery of yawslike treponemes that cause severe genital ulceration in wild baboons in East Africa22 further deepens the mystery surrounding the geographic and evolutionary origins of sexually transmitted strains of T. pallidum.
Universally agreed upon is that an epidemic known as the Great Pox (as distinguished from smallpox) ravaged Europe shortly after the return of Columbus from his first voyage of discovery.4,23 This plague broke out during the invasion of Italy in 1494 by Charles VIII of France, whose army included numerous mercenaries, some from Spain, and was accompanied by a horde of civilians, many of whom were prostitutes.4,23 Although it cannot be proved with certainty that T. pallidum was the cause of this scourge, the first clear descriptions of this serious illness, including its sexual mode of transmission, were recorded by contemporary physicians and chroniclers who traced the origins of “the French Disease” to the West Indies and members of Colombus’s expedition.4,23 The name “syphilis” derives from an epic poem, Syphilis sive morbus Gallicus (Syphilis or the French Disease), published in 1530 by Girolamo Fracastoro, a Veronese physician, poet, and philosopher. In his treatise, Fracastoro provided a detailed description of the clinical features of syphilis along with recommendations for treatment, ending with the allegory of Syphilus, a shepherd who contracted an illness as punishment for offending the sun god Apollo.4 During the pandemic of the early 16th century, syphilis in adults manifested as a fulminant, often fatal, illness that progressed rapidly from genital ulceration to gummas. Whether the comparatively mild nature of the present-day ailment reflects a change in the virulence of T. pallidum, an adaptation of the human host, or the disappearance of a concomitantly occurring but unknown illness is an enduring mystery.
One of the difficulties in sorting through medical writings from the 17th and 18th centuries is that unequivocal clinical distinctions between syphilis, gonorrhea, and other venereal diseases did not emerge until the late 18th century. John Hunter’s misguided self-inoculation experiment with urethral pus containing both Neisseria gonorrhoeae and T. pallidum only served to prolong the misconception that syphilis and gonorrhea are the same disease.4 By the mid-19th century, however, syphilis had been distinguished from gonorrhea and its principal clinical features defined, although not without some erroneous teachings. One prominent example is the dogma of Ricord, the greatest syphilologist of the 19th century, that material from secondary syphilis lesions is noncontagious.4
In 1903, Metchnikoff launched the era of modern syphilology when he observed that a female chimpanzee inoculated on the clitoris with material from a syphilitic lesion developed a chancre followed by secondary syphilis. Two years later, Schaudinn and Hoffmann discovered Spirochaeta pallida in chancre exudates and adjacent lymph node aspirates. By 1906, Wassermann had developed his complement fixation test using an extract from the liver of a syphilitic stillborn baby. Subsequently, it was shown that extracts from uninfected beef livers and hearts were equally sensitive and that a phospholipid extracted from beef heart (cardiolipin) reproduced the Wassermann reaction when mixed with lecithin and cholesterol. In 1910, Ehrlich introduced his 606th arsenic preparation, arsphenamine or salvarsan, for syphilotherapy, hailed at the time as “the magic bullet.” In 1912, Nichols and Hough24 used rabbit inoculation to isolate the organism from the cerebrospinal fluid (CSF) of a patient who developed neurosyphilis following salvarsan therapy for secondary syphilis. In addition to being the first demonstration of early CNS invasion by T. pallidum, their findings suggested that the CNS may serve as a sanctuary for the spirochete during inadequate therapy of early syphilis, a concern to this day.25 Also noteworthy is that the “Nichols” isolate became the reference laboratory strain of T. pallidum and was the source of DNA for the T. pallidum genome sequencing project.26 In 1927, Dr. Julius Wagner von Jauregg was awarded the Nobel Prize in Medicine for describing malarial fever as treatment for neurosyphilis. In 1937, Thomas Parran, the U.S. Surgeon General from 1936 to 1948, published his landmark book “Shadow on the Land” in which he estimated that 10% of Americans would be infected by syphilis during their lifetimes.10 In 1943, John Mahoney of the U.S. Public Health Service treated the first syphilis patients with penicillin,27 the true magic bullet, work that won him the Lasker Award in 1947. Not surprisingly, syphilis rates plummeted over the next decade. Despite alarming comebacks, most notably the “drugs for sex” cocaine-fueled epidemic of the late 1980s to early 1990s28 and the substantial upswing in cases among men who have sex with men (MSM) beginning in the late 1990s,29 syphilis rates have never approached pre-World War II levels.
The enduring impact of syphilis on the biomedical research community extends beyond its ongoing threat to public health. In an effort to better understand the natural history of syphilis, from 1932 to 1972 the U.S. Public Health Service conducted the “Tuskegee Study of Untreated Syphilis in the Negro Male” in which 412 indigent African-American men with latent syphilis in rural Macon County, Alabama were followed without treatment along with 192 black males without serologic evidence of syphilis.30,31 The revelation 4 decades after the study’s inception that treatment had been withheld for years despite the proven efficacy of penicillin sparked outrage and charges of abuse of authority and racial exploitation on the part of the U.S. government and the medical profession.32 It has also been argued that the continuance of the study was more a reflection of the lack of data and consensus about the efficacy of penicillin therapy for late syphilis than of racial bias.31 Advertent or not, the egregious violations of patient rights brought to light the need for much more stringent regulation of research involving human subjects and led to the 1979 Belmont Report, the establishment of the National Human Investigation Board, and the requirement for Institutional Review Boards. In 2010, the public health and medical communities were stunned by disclosures of bioethical violations even more serious than those of the Tuskegee Study. A Wellesley College historian investigating Dr. John Cutler, one of the physicians involved in the implementation of the Tuskegee Study, inadvertently discovered that from 1946 to 1948 the U.S. Public Health Service conducted studies in Guatemala in which individuals from vulnerable populations were unknowingly inoculated with T. pallidum and allowed to transmit syphilis to their sexual partners as part of a plan to evaluate prophylactic treatment regimens.33 Among the many disturbing revelations was that Parran had approved funding for the studies and appears to have followed their progress.33,34 The National Archives has posted more than 12,000 pages of documents related to this study on its website (www.archives.gov/research/health/cdc-cutler-records). A full report by the Presidential Commission for the study of Bioethical Issues can be found at http://bioethics.gov/cms/sites/default/files/Ethically-Impossible_PCSBI.pdf.
Etiology
The causal agent of venereal syphilis is T. pallidum subsp. pallidum, which belongs to the order Spirochaetales, the family Spirochaetaceae, and the genus Treponema. Other members of the genus Treponema that can infect humans are T. pallidum subsp. pertenue (yaws), T. pallidum subsp. endemicum (bejel or endemic syphilis), and Treponema carateum (pinta). In addition to the pathogenic spirochetes, a number of commensal treponemes have been isolated from humans over the years, particularly from the oral cavity and from the prepuce of uncircumcised men. At one time, investigators believed that these cultivatable treponemes were nonpathogenic variants of T. pallidum.35 Miao and Fieldsteel36,37 disproved this notion by showing using DNA-DNA hybridization that T. pallidum subsp. pallidum and pertenue were indistinguishable, and that there was no discernible homology between the DNAs of pathogenic and cultivatable treponemes. As a result, the agents of venereal syphilis, endemic syphilis, and yaws were reclassified as subspecies of T. pallidum; T. carateum, on the other hand, retained its status as a separate species because no isolates were available for study (as is true today).38 The pathogenic treponemes are believed to have evolved by reductive evolution from a commensal treponeme.39,40 Comparative genomics suggests that the divergence from a common progenitor occurred millions of years ago. In contrast, the divergence of the T. pallidum subspecies is believed to postdate the emergence of modern man, probably occurring within the past 20,000 years.39,40 Isolation of yaws-like treponemes from nonhuman primates22,41,42 suggests that the evolutionary biology of pathogenic treponemes may have involved spillover to humans from a nonhuman primate reservoir.
All four pathogenic treponemes are morphologically indistinguishable and induce antibodies detected by the routine serologic tests used to diagnose venereal syphilis. In clinical situations, distinction between the corresponding infections rests upon geography, clinical manifestations, patient age, and other demographic features. Of the four pathogens, only subsp. pallidum is transmitted routinely by sexual contact.43 Because the other three are transmitted nonsexually, the diseases they cause are collectively referred to as the endemic or nonvenereal treponematoses.44,45 T. pallidum subsp. pallidum is considered the most virulent because it is the only subspecies capable of regularly breaching both the blood-brain and maternal-fetal barriers.43,46 At the opposite end of the virulence spectrum is T. carateum, which disseminates only to cutaneous sites and causes the least severe skin lesions. Subtle differences in the T. pallidum subsp. pallidum and pertenue genomes, which are 99.8% identical, appear to be responsible for profound differences in modes of transmission, tissue tropisms, clinical manifestations, and host range.21,47 No strain or subspecies of T. pallidum can be cultivated continuously in vitro, although limited replication of T. pallidum subsp. pallidum has been achieved by cocultivation with mammalian cells.48 Maintenance of strains requires serial passage by intratesticular inoculation of rabbits.49 Intratesticular inoculation of rabbits, rabbit infectivity testing (RIT), is also the only means of recovering strains from clinical specimens.50,51
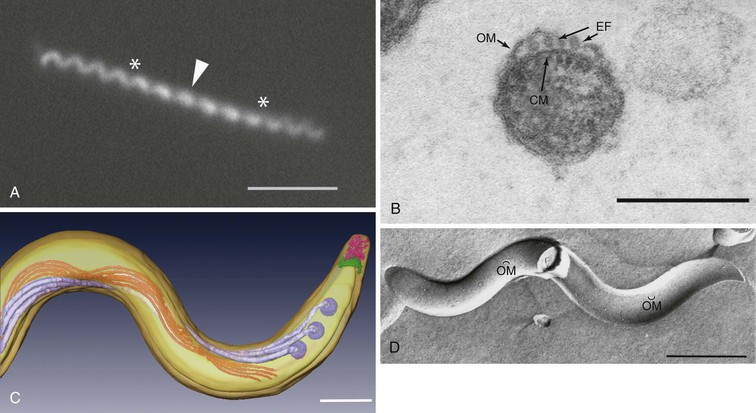
T. pallidum is approximately 0.2 µm in diameter, has tapering ends, and ranges in length from 6 to 20 µm (Fig. 239-1). Because of their small diameter, pathogenic treponemes cannot be visualized by bright field microscopy and are best visualized by darkfield (DF) or phase-contrast microscopy (see Fig. 239-1A). Though often described as spiral or coiled, high-resolution time-lapse microscopy has confirmed earlier reports that, like the Lyme disease spirochete Borrelia burgdorferi, T. pallidum has a flat-wave morphology (see Fig. 239-1A).52 Within the periplasmic space are the flagellar filaments, which extend from motors at each cell pole and overlap in the middle of the cell cylinder (see Fig. 239-1B and C).52,53 The rotation of the filaments, driven by the motors, propagates an undulating wave along the length of the bacterium capable of propelling it through complex fluids, tissue matrices, and intercellular junctions.54,55 T. pallidum is often described as gram negative. In addition to being erroneous from a phylogenetic standpoint,56 the syphilis spirochete lacks lipopolysaccharide (LPS),26 the hallmark glycolipid of gram negatives, and does not take up Gram stain. Other major differences in the molecular architecture and composition of their cell envelopes further underscore the phylogenetic gulf between T. pallidum and gram-negative bacteria.57 The outer membrane of T. pallidum contains an extraordinarily low density of integral membrane proteins (see Fig. 239-1D) that, with one exception (TP0326/TP92/BamA), have no sequence homology with outer membrane proteins (OMPs) of gram-negative bacteria.57–59 In gram-negative bacteria, the peptidoglycan resides directly beneath the outer membrane; in T. pallidum, the murein layer is found midway in the periplasmic space, beneath the flagellar filaments, which rotate against it in order to transfer their motive force to the cell cylinder.52,53 For many years, it was believed that T. pallidum possesses a coat of serum proteins and mucopolysaccharides that shields it from the host’s immune system.60 It is now widely accepted that the paucity of proteins and pathogen-associated molecular patterns (PAMPs) on the spirochetal surface is the basis for the bacterium’s impressive capacity for immune evasion, which has earned for it the name “stealth pathogen.”43,57,58,61
The genome of the Nichols-type strain of T. pallidum consists of a single circular chromosome of approximately 1.14 MB, near the low end of the size range for eubacteria.26 The absence of plasmids, pathogenicity islands, transposable elements, and restriction-modification systems indicates that the organism has little capacity for uptake of exogenous DNA, probably explaining why it has remained exquisitely sensitive to penicillin for more than 7 decades. The syphilis spirochete is the only pathogenic treponeme whose physiology has been examined in detail, although there is no reason to believe that it differs substantively from those of the other subspecies. T. pallidum subsp. pallidum replicates slowly (doubling time of ≈30 hours in rabbits)62 and poorly tolerates desiccation, elevated temperatures, and high oxygen tensions.63 Because optimal replication under in vitro conditions occurs in ambient oxygen concentrations of 3% to 5%, the organism, once considered anaerobic, is now classified as a microaerophile.63 During the course of reductive evolution, T. pallidum has dispensed with a vast amount of the biosynthetic machinery found in other bacterial pathogens. It is unable to synthesize fatty acids, nucleotides, enzyme cofactors, and most amino acids and must, therefore, scavenge all of these nutrients from its obligate human host.26,64 Lacking the components for oxidative phosphorylation, T. pallidum relies entirely on glycolysis for production of ATP, presumably usurping the abundance of glucose in blood and interstitial fluids.
Identification of stable, genetically variable loci within the T. pallidum genome has made possible the development65,66 and further refinement67 of a robust scheme for typing syphilis strains, an essential tool for molecular epidemiologic studies. A recent meta-analysis of global typing studies reported the existence of at least 57 subtypes, with wide geographic variation in subtype distribution, although a small number of subtypes predominate overall.68 As a whole, these results indicate that high prevalence rates in endemic areas can be attributed to concurrent circulation of multiple subtypes.
Epidemiology
Despite the availability of effective and inexpensive antibiotic treatment, venereal syphilis continues to cause significant morbidity and mortality globally. According to the World Health Organization, in 2008, approximately 11 million people acquired the disease and 36 million were estimated to be infected (new cases plus existing cases).69 Although syphilis has recently re-emerged in the United States and Europe,70–72 most individuals (>90%) who acquire syphilis reside in less affluent regions of the world.73
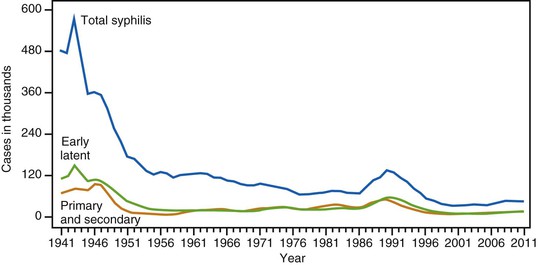
The number of new syphilis cases in the United States has cycled continuously since active reporting began in 1941. The advent of penicillin treatment and the establishment of aggressive syphilis control public health campaigns toward the end of World War II led to notable decreases in the total number of new syphilis cases. As shown in Figure 239-2, the incidence of venereal syphilis steadily decreased over several decades, with periodic outbreaks between 1986 and 1994 and between 2002 and 2011. The upswing in the total number of early syphilis cases in the late 1980s, ascribed principally to heterosexual transmission, led to increases in gestational and congenital syphilis (CS) cases. The reappearance of syphilis during this time period was linked epidemiologically to the exchange of sex for drugs, especially crack cocaine.74 The most recent surge has occurred chiefly in MSM,75 a high-risk group that accounts for nearly three quarters of all primary and secondary syphilis cases reported to the Centers for Disease Control and Prevention (CDC).71 The reasons for this latest increase are likely multifold and include lack of sexual inhibition resulting from the availability of effective treatment for human immunodeficiency virus (HIV), the use of the Internet to meet partners, the practice of HIV serosorting, and the increase in oral sex as a form of “safe sex.”75 Some have suggested that, risk factors notwithstanding, the oscillating pattern of syphilis outbreaks reflects changes in levels of immunity to T. pallidum within susceptible populations.76
In Western Europe and the United States, syphilis is currently characterized by low-level endemicity with concentration among population subgroups with high rates of partner change, poor access to health services, social marginalization, or low socioeconomic status. The highest incidence of syphilis in the United States is evidenced in MSM from poor, underserved minority communities in southern states, from Maryland to Florida to eastern Texas and also in urban centers in California.71 In addition, several other metropolitan areas throughout the country continue to report a high incidence of venereal syphilis in HIV-infected MSM. In Europe, the estimated syphilis rate is 4.4 cases per 100,000 population, with more than half (59%) of all cases diagnosed in four countries (Germany, the United Kingdom, Spain, and Romania).77 The highest incidence was observed in Lithuania (10.4/100,000 population), followed by Romania (8.3/100,000) and Denmark (7.5/100,000).
The social changes and disruption of medical services that followed the breakup of the Soviet Union in the late 1980s led to important increases in syphilis rates in Eastern Europe and Russia.78 More recently, improved prevention strategies have led to a steady decrease in the overall disease burdens in this region of the world. By contrast, in China, a country where syphilis was virtually eradicated in the 1950s, the incidence and prevalence of the disease have more than quadrupled in the past several years.79 By way of comparison, in 2008 there were more syphilis cases in the coastal province of Guangdong than were reported in the entire European Union. Although the causes are not entirely clear, this increase in syphilis rates has been attributed to migration from rural communities to urban environments, leading to earlier sexual debut, later marriage, more lifetime partners, and increased commercial sex, including MSM. These factors are compounded by limited syphilis screening, lack of adequate partner notification, and an overall unwillingness by the general population to access sexually transmitted disease (STD) health care services.80
Regardless of geographic location, the worldwide epidemiology of syphilis has been greatly influenced by developments in the HIV epidemic. A statistical association between the two diseases became apparent at the outset of the acquired immunodeficiency syndrome (AIDS) epidemic in the early 1980s.81 Since that time, numerous studies have documented the high rate of HIV infection among syphilis patients.75,82,83 As already stated earlier, following the steady decrease in the syphilis rates in the 1990s to an all-time low for the United States, by 2008 the rate of primary and secondary syphilis increased to 4.5 cases per 100,000 (www.cdc.gov/std/stats08/syphilis.htm). Not surprisingly, this increase has disproportionately affected large metropolitan areas with well-established populations of HIV-coinfected MSM, including San Francisco, where the incidence of primary and secondary syphilis increased more than 600% (26 cases per 100,000).84 This increase has been attributed to increased reports of unprotected sex among HIV-positive MSM and increased HIV prevalence (www.cdc.gov/std/stats08/syphilis.htm). Simultaneously, there also has been a resurgence of traditional sexual marketplaces such as saunas and cruising grounds, together with a new and rapid growth of Internet chat rooms, increasing the opportunity for rapid and easy access to new sexual partners. The overall effect has been to join previously isolated sexual networks, increasing the size of the sexual networks and thereby reducing the time taken for the spread of syphilis to evolve. In addition, the idea that oral sex is “safer” sex and rarely associated with HIV transmission may explain the role of oral sex in syphilis transmission.85
Although the association between syphilis and HIV infection initially was thought to simply reflect similar behavioral risk factors for acquisition and transmission, it has become apparent over the years that complex biologic relationships exist between these two diseases.75,86,87 The presence of a syphilitic chancre can theoretically facilitate HIV transmission by either increasing the host’s susceptibility to infection with the virus or the HIV-infected host’s infectiousness to discordant sexual partners. The former is associated with the disruption of the protective epithelial and mucosal barriers present in genital chancres and the known enrichment of the lesion with activated lymphocytes, macrophages, and dendritic cells, all of which are potential targets and donors for HIV88–91 and which differentially increase expression of key HIV co-receptors (i.e., CCR5 and DC-SIGN) in untreated patients.91,92 With respect to increased infectiousness, the inflammatory response elicited by T. pallidum is capable of inducing HIV gene expression, thereby promoting viral replication.93 Not surprisingly, in HIV-infected patients, CD4 counts decrease and HIV viral loads increase in subjects with untreated syphilitic coinfection.94
Nearly 1.5 million pregnant women are estimated to be newly infected with syphilis every year, and approximately half of pregnant women who are left untreated will experience adverse outcomes in their pregnancies. These include early fetal loss (20 to 28 weeks’ gestation) and stillbirth (>28 weeks), neonatal death, low-birth-weight infants, and infants with clinical evidence of infection.95 In 2008, syphilis in pregnancy contributed to 305,000 stillbirths and fetal and neonatal deaths, and an additional 215,000 infants were at increased risk of dying from low birth weight, prematurity, or complications of infection related to syphilis.95 Of particular concern, in sub-Saharan Africa, syphilis contributes to approximately 20% of all perinatal deaths.
Pathogenesis
Studying syphilis pathogenesis poses a formidable and unique set of challenges. First and foremost is the fact that T. pallidum has defied all attempts at continuous cultivation in artificial medium48 and, therefore, cannot be genetically manipulated. A second serious limitation is the lack of a murine model for dissecting the complex host response to this pathogen.96 Although the outbred rabbit model recapitulates many facets of human disease, rabbits do not develop typical secondary lesions following intradermal inoculation, nor do they develop neurologic complications or neuropathology comparable to those of humans, even when inoculated intravenously.49,97 Investigators who wish to circumvent these deficiencies by studying infection in humans must cope with an enormous spectrum of disease manifestations, person-to-person variability in immune responses, high rates of HIV coinfection, and the fact that results from blood and skin (the two most accessible sites) represent snapshots from just two compartments of a dynamic, systemic process.98 A third obstacle is the fragility of T. pallidum, particularly its outer membrane, a physical property that has greatly hampered efforts to identify and examine molecules that function at the host-pathogen interface.57,99 Lastly, transcriptional and proteomic analysis of syphilis spirochetes has, thus far, been confined to organisms extracted from inflamed rabbit testes,100 a circumstance unlikely to represent the expression profiles in other tissues, especially those of humans, and stages of disease. Given all of these impediments, it is not surprising that our current understanding of the microbiologic and immunologic factors that determine the outcome of human infection lags far behind most other common bacterial infections.
Person-to-person transmission of spirochetes initiates the pathogenic sequence. With venereal syphilis, this typically occurs when treponemes are transferred during intimate contact, usually sexual (oral-genital as well as genital-genital, rarely kissing), with an actively infected partner. In their human inoculation studies conducted at Sing Sing Prison in the 1950s, Magnuson and colleagues101 (please see ethics statement later) found that as few as 10 organisms of the Nichols strain could cause lesions. Thus, only miniscule amounts of infectious exudate need be exchanged when micro-environmental conditions are conducive to survival of this fastidious pathogen. Individuals with venereal syphilis are most infectious during the primary and secondary stages of disease (including secondary relapses of early latency) when moist, mucocutaneous lesions are present. Persons with early syphilis may be infectious, however, even if they lack open lesions. Micrographs showing that spirochetes are abundant within the epidermis and superficial layers of the dermis in secondary syphilitic lesions102 suggest how minute abrasions created during sexual activity might result in infection. Transfusion syphilis, a well-recognized nonsexual mode of transmission before World War II, no longer occurs in the United States103 but remains a significant concern in underdeveloped countries.104 The agents of yaws, pinta, and endemic syphilis are transmitted by nonsexual contact with open lesions, usually during childhood.44
Syphilis spirochetes rapidly gain entry into their new host either by directly penetrating mucous membranes or via abrasions and cracks in skin. Remarkably, treponemes applied to the preputial mucosa of rabbits migrate into the subepithelium within several hours.105 Once below the epithelial surface, they then begin to multiply locally and disseminate through lymphatics and blood vessels. Why some members of the bacterial population remain localized, whereas others respond to the same environmental signals by migrating away from the inoculation site, is a fascinating question. The experimental and clinical evidence that T. pallidum disseminates systemically early during the course of infection is overwhelming.43 Brown and Pearce106 recovered T. pallidum from inguinal lymph nodes and blood of rabbits within 48 hours of intratesticular inoculation, well before the onset of orchitis; these findings have been replicated using quantitative PCR.106a Numerous reports in the early 20th century documented transmission following the transfusion of blood from seronegative donors (i.e., during the incubation period).107 Almost any organ in the body can be invaded during the spirochetemia of early syphilis, including the CNS.1,51,108 Using both RIT and PCR, investigators in the molecular era have confirmed that substantial percentages of early syphilis patients without neurologic signs or symptoms harbor T. pallidum in their CNS.109,110
It is widely believed that T. pallidum must adhere to epithelial cells and extracellular matrix components in order to gain the “foothold” needed to establish infection; spirochetes subsequently disseminate within tissues by coordinating adherence with motility in a series of “stop and go” movements.43,54 Binding studies suggest that fibronectin and laminin are important substrates for this attachment-mediated form of motility.111–113 Light micrographs showing T. pallidum attached end-on to cell surfaces suggested years ago that the tip of the bacterium functions as an attachment organelle114; this notion has been buttressed by recent cryoelectron tomographic analyses (see Fig. 239-1C).52,53 Chemoreceptor arrays near the cell ends translate environmental cues (chemotactic signals) into a chain of phosphorylation events that determine whether the flagellar motors turn clockwise or counterclockwise.53,54 Directed motility does not occur unless the motors at each end of the cell turn in opposite directions. How a spirochete coordinates the direction of rotation of the motors at opposite ends of the cell is a mystery.54 Circulating treponemes bind to vascular endothelium within target organs, reaching the parenchyma by negotiating their way through the tight junctions separating endothelial cells, a process called interjunctional penetration.55
Although rare examples of intracellular treponemes have been reported, the syphilis spirochete is believed to have little capacity for establishing long-term residence in phagocytic or nonphagocytic cells.43,115 There is also now a consensus among syphilologists that clinical manifestations result from the inflammatory processes driven by the presence of treponemes and treponemal constituents within infected tissues.115,116 How does the spirochete flourish within the extracellular space in the face of the increasingly vigorous humoral and cellular responses it elicits as the disease progresses? Current thinking is based on an infection model that pits innate and adaptive immunity against a motile invader structurally equipped to “fly” beneath the host’s “immunologic radar,” perhaps even manipulating the host response to facilitate dissemination and immune evasion.61,98
Though lacking LPS, T. pallidum contains abundant lipoproteins, which are capable of activating macrophages and dendritic cells (DCs) via CD14- and Toll-like receptor 1/2 (TLR1/2)-dependent signaling pathways.61,116 However, because of the bacterium’s unique outer membrane ultrastructure,57,58 these PAMPs are not readily accessible to TLRs or other pattern recognition receptors (PRRs) expressed on the surface of innate immune cells. As a result, spirochetes can replicate at the site of inoculation, disseminate hematogenously, and then replicate at metastatic sites unchecked by innate surveillance systems. At some point, and through poorly understood mechanisms, pathogen sensing is triggered and organisms are taken up by tissue-based DCs, which then traffic to draining lymph nodes to present cognate treponemal antigens to naïve B and T cells. The production of opsonic antibodies, presumably directed against surface-exposed epitopes on rare outer membrane proteins, enhances the uptake and degradation of spirochetes by phagocytes, allowing spirochetal PAMPs to gain access to PRRs lining the phagosomal vacuole, while production of interferon-γ (IFN-γ) by recruited, locally activated T cells bolsters macrophage-mediated clearance and inflammation.98,117,118 Although these adaptive responses collectively help to shift the balance in favor of the host, the spirochete is not without countermeasures. The paucity of antigenic targets on the spirochetal surface, perhaps together with the emergence of antigenically variant subpopulations,119,120 enables some bacteria to avoid opsonophagocytosis and other forms of antibody-mediated killing, prolonging disease manifestations and fueling the relapses of early latency. How, then, is the pathogen contained, bringing about latency? Possibly, as infection proceeds, the antibody repertoire broadens and intensifies sufficiently to the point where the spirochete’s antigen-poor surface is overwhelmed or its capacity for antigenic variation is exhausted, or both.119,120 Nevertheless, though no longer capable of disseminating, organisms survive for years in a substantial proportion of untreated individuals, establishing niduses of inflammation that set the stage for recrudescent (i.e., tertiary) disease when, for reasons unknown, the balance shifts back in favor of the pathogen (see “Natural Course of Untreated Syphilis,” later).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree