17 Surveillance and Follow-up
Introduction
In the United States, approximately 146,970 new cases of colorectal cancer are estimated to occur in 2009, which will account for more than 49,900 (49,920 to be exact) cancer-related deaths.1 Up to 30% to 50% of patients with stage II or III colon cancer are estimated to develop locoregional recurrence, distant metastasis, and/or metachronous colon cancers after 5 years of follow-up.2–5 Most of these recurrences occur in the first 2 (60%) to 3 (80%) years.6 Given the high percentage of patients who have a recurrence within 2 to 3 years after curative intent therapy, surveillance performed after treatment for colon cancer is aimed at improving overall survival and the chances for long-term cure. Surveillance improves outcomes by maximizing the efficacy of and potential for curative intent re-resection by (1) identifying locoregional or distant metastasis (liver, lung) recurrence as early as possible and (2) detecting metachronous primary tumors at the earliest stage.
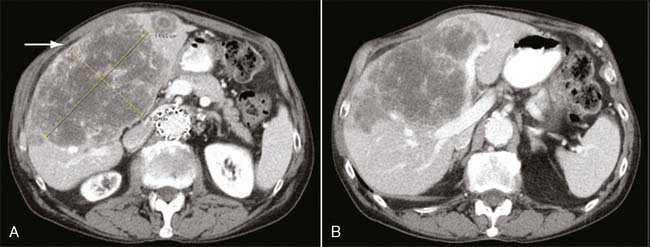
The most common site of recurrence after curative resection of colon cancer is the liver; in fact, at least 50% of patients with colon cancer develop liver metastases during the course of their disease.7 In up to 30% of patients with liver metastases, the liver is the only site of metastatic disease.8 Although historically an estimated 8% to 10% of patients who have undergone curative intent therapy for colorectal cancer develop isolated liver metastases,3,9,10 improvements in imaging, notably helical spiral computed tomography (CT), have increased the ability to detect such recurrence, presumably before additional evidence of spread. Patients with isolated liver metastases are potential candidates for curative intent surgical resection, with the possibility of long-term survival. Several series report overall 5-year survival rates of 35% to 58% after curative intent hepatic resection.11–13 Surveillance regimens that aim to detect metachronous metastatic colon cancer liver metastases with such methods as measurement of serum carcinoembryonic antigen (CEA) and liver imaging (CT, ultrasound) may therefore be the most successful strategy for achieving the ultimate goal of improving overall survival (Fig. 17-1).
Although the rate of metachronous, or second, neoplasms is relatively low (7%–8%),14 the early detection of presumably early-stage new primary lesions—and subsequent adequate surgical treatment—may provide the most efficacious target for surveillance. Although the rate of second neoplasms is relatively low compared with the rates of true recurrence, patients with diagnosed colon cancer are clearly at much higher risk and might benefit from the identification of lesions more likely to be amenable to cure than true recurrences. The population of patients with the best prognosis with respect to their colon cancer is most likely to benefit from such a strategy. The identification of metachronous new primary colonic neoplasms is clearly best accomplished through endoscopy, although fecal occult blood testing has also been promoted for detecting such new tumors.
The rate of local recurrence in colon cancer is decidedly low and is at least half the rate of distant recurrence. For colon cancer, about 15% of patients are estimated to develop locoregional recurrence.15,16 In a recent prospective randomized controlled trial of patients with stage II and III resected colorectal cancer, the rate of local recurrence was surprisingly high (approximately 30%) but still about half the rate of distant recurrences (almost 60%). These recurrences, including metachronous tumors, occurred in a background of a total recurrence rate of about 27%.17 The authors themselves were surprised at the high rate of local recurrences seen in their study. They concluded that it was possible, even likely, that some intramural relapses were misdiagnosed and were in fact metachronous new primaries (which would also explain the higher than expected resectability rate).17
Although the endoscopic identification of both local recurrences and metachronous tumors may offer the greatest chance for discovering recurrences that can be both resected and cured, several studies have shown that the rate of intraluminal recurrences and metachronous tumors is very low.15,18,19 This low rate has caused some investigators to state that the intensification of efforts to identify these lesions is of little benefit.20 Therefore, the contribution of the identification of these lesions, primarily through endoscopy, to the overall efficacy of the strategy of surveillance remains controversial.21
Ultimately, the goal of post-treatment surveillance of patients who have undergone curative resection of colon cancer is to improve overall survival and chances for long-term cure. By identifying recurrences as early as possible, the oncology community aims to maximize the efficacy of and potential for curative intent resection. Several overlapping, yet distinct, goals of surveillance regimens include the following:
All these goals are to improve the ability to provide curative intent, margin-negative (R0) resection. An often unstated and less optimal but important goal in post–curative intent resection of colorectal cancer is to identify incurable disease while it can still be reasonably palliated and before it becomes so advanced as to preclude even effective palliation. An additional goal is to provide a proven survival advantage—though not a cure—for patients with unresectable metastatic disease by initiating earlier systemic therapy.22
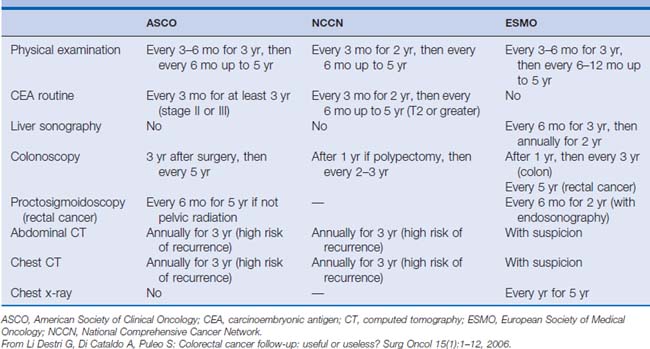
The optimal frequency and specific strategies of surveillance have not been defined; however, the overall interpretation of several prospective randomized controlled trials and meta-analyses indicates that rates of resectable tumor recurrence and overall survival are increased in intermediate-risk patients who undergo high-intensity rather than low-intensity programs of surveillance.15,19,20,23,24 Controversy remains over the right combination of tests and the optimal frequency of evaluations, as evidenced by the different (although similar) surveillance guidelines from three major oncology organizations (Table 17-1). Although there may be disagreements about the optimal and most cost-effective strategy, general consensus exists that surveillance of patients with colon cancer who have undergone curative intent therapy improves overall survival.
Overview of Prospective Evidence
To date, eight prospective randomized controlled trials have been performed studying the possible benefit of colon cancer surveillance after apparent cure17,21,25–30 (Table 17-2); only half of these trials17,21,25,29,30 have been categorized as being of good quality.20 Most of these prospective studies have compared lowintensity with high-intensity surveillance programs. The low-intensity surveillance was truly minimal in two studies,25,29 whereas only one trial compared a schedule of surveillance with that with essentially no follow-up.27 Only three trials—one overall28 and two in subset analysis only17,29—were able to demonstrate a survival benefit of more intensive surveillance. Pietra and associates28 found that the overall survival advantage was attributed to earlier detection of asymptomatic local recurrence (primarily by CEA). However, these results were confounded by the fact that 30% of the patients had rectal cancer and more than half of the local recurrences in both the intensive and conventional follow-up groups were in patients with a history of rectal cancer.28 To date, no single prospective randomized controlled trial provides definitive evidence of improved survival compared with surveillance, or with intensifying surveillance.
Table 17-2 Comparison of All Randomized Controlled Trials Performed to Evaluate the Efficacy of More Intensive versus Less Intensive Surveillance After Curative Resection of Colorectal Cancer
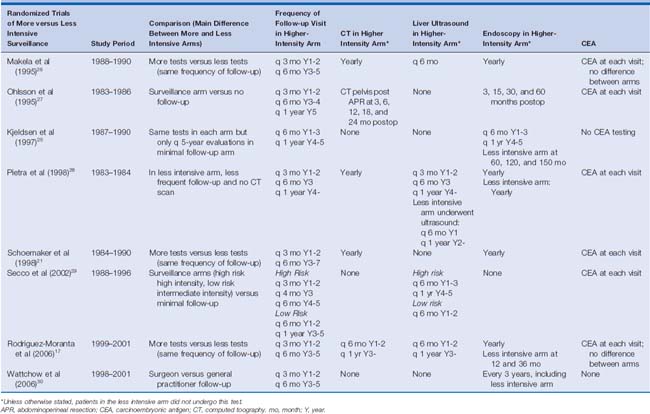
Without a gold standard multi-institutional study to provide guidance, at least five meta-analyses have coalesced the results of these prospective single-institution reports. Each meta-analysis independently revealed a survival benefit to high- versus low-intensity surveillance.15,19,20,23,24 It is interesting and perhaps obvious to note in hindsight that the intensification of surveillance did not result in an increase or decrease in the rate of recurrence, presumably because it is inevitable. It is hoped that such an effort at least changes the type, if not the pattern, of recurrence, perhaps identifying it earlier before the progression or spread of locally recurrent lesions or before the local progression of distant hepatic or pulmonary metastases. Contrary to expectations, in a study by Secco and associates,29 high-risk patients randomized to the minimal follow-up arm were found to have a shorter disease-free survival compared with the intensive surveillance arm. More intensive surveillance should result in finding recurrences sooner (and thus the patients will artificially have “shorter” disease-free intervals). Therefore, the expectation is that high-risk patients in the minimal follow-up arm would have longer disease-free intervals because the recurrence would be found later. The paradoxical finding in Secco and associates29 that high-risk patients in the minimal follow-up arm had a shorter disease-free interval implies that more intensive surveillance does not identify recurrence earlier.
Despite this anomaly in a single study,29 the meta-analyses provide support that intensifying follow-up significantly reduces the time to diagnosis of such inevitable recurrence. In doing so, intensive surveillance presumably aids in identifying a greater proportion of recurrences amenable to curative intent therapy. This reduction in time has been estimated to be as long as 8.5 months (95% confidence interval [CI], 7.6–9.4).19 Many studies corroborate that under intensive surveillance, recurrences are more often discovered while asymptomatic, are uncovered at routine visits, and/or are amenable to curative intent therapy.17,25,26,29 Perhaps because there are fewer patients in some of these reports, this benefit does not necessarily result in a measurable survival benefit,17,25,29 or only in subset analysis.29 Even in several of the meta-analyses, the overall survival benefit of more intensive surveillance (estimated to be about 10%) is disproportionately greater than the survival advantage obtained from detecting more recurrences amenable to curative intent therapy (estimated between 2% and 5%).24 This conclusion has led some to speculate that the increased intensity of surveillance has other, less tangible, benefits such as improved sense of well-being, decreased anxiety, or enhanced global care of unrelated but coincident medical disease, which contribute to the reduction in mortality rate related to the intensive surveillance of colon cancer.24
The interpretation of these studies is hampered by the broad span of time in which they were performed (1983–2002), by the inclusion of both colon and rectal cancers, by the wide variation in surveillance strategies, and by the single-institutional nature of and small numbers of patients included in many of these reports. The inclusion of rectal cancers in the surveillance regimens complicates the interpretation of the results because of the much higher rate of locoregional recurrence in rectal cancer, as well as the relative ease of identifying endoscopic evidence of recurrence in left-sided or rectal cancers compared with right-sided lesions. Because of the broad span of time of these reports, many of these studies were performed before the more efficacious and better-tolerated chemotherapy of today, before the much higher-quality and sensitive CT scans of the current era, and before the much more aggressive, efficacious, and safer major liver resections seen in the last decade. Perhaps most significantly, the combination of improved CT imaging and of more aggressive and simultaneously safer major liver resection expands the ability to provide curative intent therapy to patients with metachronous colon cancer liver metastases. These relatively recent changes thus increase the chances that the earlier discovery of asymptomatic colon cancer liver metastases may provide a survival benefit.
In the background of these limitations and uncertainties, the heterogeneity of surveillance strategies studied to date prevents definitive determinations of the frequency or type of tests and prevents an estimation of the harms or costs of such tests. Given these lingering uncertainties, we eagerly await the final or preliminary results of three large-scale prospective randomized controlled trials: the GILDA study in Italy,31 the Follow-up After Colorectal Surgery (FACS) study in the United Kingdom,32 and the multicenter COLOFOL study in Denmark, Sweden, Poland, Ireland, and Uruguay.33 All three large-scale studies are said to be adequately powered to independently support different aspects of the question of whether more intensive surveillance improves outcomes, including whether increased frequency of colonoscopy and liver ultrasound (GILDA), hospital-/imaging-based follow-up over primary care/CEA (FACS), or more versus fewer clinic visits (COLOFOL) improves outcomes.
Frequency and Duration
Two thirds of patients with colon cancer undergo curative intent therapy, including adjuvant therapy.1 Up to 50% of these patients develop recurrent disease,34 and most recurrences are within the first 2 years (60%).6 Although the pace of recurrence decreases at this point, a significant number of patients continue to have recurrence within the next few years, with 80% of recurrences happening within the first 3 years and 90% within the first 5 years.6,14,15,34 No definitive evidence exists to support the optimal frequency and duration of surveillance to detect these recurrences. Given the heterogeneity of follow-up strategies, the suggestion in several studies that more intense follow-up allows detection of a greater number of resectable recurrences, and the conclusion of several meta-analyses that more intense surveillance provides a survival benefit, several societies have designed a rationale design for follow-up based on the timing of recurrence (see Table 17-1). This rationale is based on scattered strategies of the several studies previously discussed; however, ultimately a cogent plan can still be distilled from these attempts at defining the optimal surveillance strategy.
Considering the wide variety of strategies and combination of tests, it is difficult to analyze the meta-analyses and make a conclusion about the optimal frequency and duration of follow-up (see Tables 17-1 and 17-2). The review of a single randomized prospective trial demonstrating a survival benefit associated with intensive or conventional follow-up compared with minimal or no follow-up, in the background of a similar intensity and combination of tests in each arm, may provide some foundation for this determination. Unfortunately, only one of the randomized prospective studies (Pietra and associates28) revealed an overall survival benefit to more intensive surveillance in the background of a similar combination of tests in each arm. Interpretation of this study in the more broad application of determining the optimal frequency and duration of evaluations is complicated by the inclusion of patients with rectal cancer (whose earlier detection of local recurrence in the more intensive follow-up arm provided a substantial portion of the overall measured survival benefit seen in the study). Nonetheless, patients with a high risk for recurrence fared better in the more intensive follow-up arm, in which patients underwent evaluation every 3 months for the first 2 years and then every 6 months for the next 3 years. This frequency of testing is in comparison to the minimal follow-up arm, in which patients underwent evaluations every 6 months for the first year and then annually.28
In both groups, clinical examination, ultrasound, and serum CEA measurements were performed at each visit, and chest radiography and colonoscopy were performed annually. In the more intensive arm, patients also underwent annual CT scans, but these scans detected recurrence in only 1 of 104 asymptomatic patients when not previously detected by another method.28 Given a similar combination of tests in study arms of differing frequency of follow-up, the survival benefit attributed to the arm with more frequent evaluations should give some credence to this particular schedule. Unfortunately, the study can be criticized on several other fronts. The survival benefit, attributed mostly to an asymptomatic CEA-directed detection of recurrence, is solely ascribed to local, not distant, recurrence. Both CT and CEA should be useful primarily for detecting asymptomatic resectable recurrences in the liver and lung; this should provide the bulk of the measurable survival benefit of detecting recurrent disease.35 The identification of the less common event of local recurrence in colon cancer should contribute less to the survival benefit of surveillance, and it should certainly not be the sole contributor to such a benefit. It is likely that not just the incorporation of a significant portion of patients with rectal cancer (who have higher rates of local recurrence) but the performance of the study in the age before high-quality CT scans and before expanded criteria for resection of pulmonary and hepatic metastases contributed to the skewed findings. Nonetheless, it provides the only evidence of a survival benefit attributed to a particular frequency and schedule of follow-up seen in a single trial, and this particular frequency of follow-up has been independently adopted by many of the other studies. As such, it provides a rational schedule of follow-up.
Although the meta-analyses all support a survival benefit associated with more intense surveillance, the frequency of surveillance varied widely among studies; the intense and more frequent surveillance arm of one study could be interpreted to equate with the minimal and less frequent arm in another study. With this caveat, six of the eight prospective randomized controlled trials had a similar frequency of early follow-up in the higher-intensity arm of surveillance, with every-3-month evaluations in the first 2 years of follow-up and every-6-month evaluations through year 5.17,21,26–28,30 In one of the remaining two studies, Secco and associates29 attempted to risk-stratify frequency of follow-up and is therefore not neatly included in these six studies. In comparison, Secco and associates provided a similar level of frequency of follow-up only to the highest-risk patients who were randomized to the more intense follow-up strategy; the remainder of the study participants—including the low-risk patients randomized to the more intensive strategy and both the high-risk and low-risk patients randomized to the less intensive arm—did not undergo such frequent follow-up in the first 2 years.29 This variation of strategies is further complicated by the fact that three studies varied the combination and performance of tests, but not the frequency or duration of follow-up, to provide an intensive arm and a minimal arm of surveillance.17,21,26
When comparing the higher-intensity surveillance arm in these trials, only four of the studies continued with every-6-month evaluations through the 5-year follow-up point without regard to risk of recurrence.17,21,26,30 In one of these studies, Wattchow and associates30 randomized follow-up for patients between general practitioners and surgeons; the researchers only suggested surveillance guidelines, including frequency of evaluations, which by study design were not enforced. Other studies provided less frequent follow-up in their higher-intensity surveillance arm in different combinations. The majority of differences in the schedule were seen after year 3. After this time point, at either year 4 or year 5, some investigators progressed from every-6-month visits to annual evaluations. Despite this significant variation, a specific frequency and schedule (every 3 months for the first 2 years and every 6 months through year 5) clearly dominates the studies analyzed, though without a valid comparison to other schedules. In meta-analysis, when combined with the more intensive tests, this schedule appears to provide a survival benefit.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree