INTRODUCTION
SUMMARY
Collectively, the erythroid progenitors, terminally differentiating erythroblasts (precursors), and adult red cells are termed the erythron to reinforce the idea that they function as an organ.* The widely dispersed cells comprising this organ arise from pluripotential hematopoietic stem cells. Following commitment to the erythroid lineage (unipotential progenitor), further maturation gives rise to the erythroid progenitors, burst-forming unit–erythroid and, subsequently, colony-forming unit–erythroid (CFU-E), that can be identified by their development into representative clonal colonies of red cells in vitro. The CFU-E then undergoes terminal differentiation, progressing through four to five morphologic stages, each having characteristic light microscopic and ultrastructural features. During terminal erythroid differentiation there is an increasing amount of hemoglobin synthesis accompanied by nuclear chromatin condensation and at the final stage of differentiation there is nuclear extrusion to generate an anucleate polychromatophilic macrocyte (reticulocyte with supravital staining). The human polychromatophilic macrocyte (reticulocyte) matures over 2 to 3 days, first in the marrow and then in circulation into the discoid erythrocyte. During reticulocyte maturation, cytoplasmic inclusions including residual mitochondria and ribosomes are degraded and the reticulocyte loses surface area to achieve the mean cell volume and surface area of a discoidal erythrocyte. Mature erythrocytes are approximately 7 to 8 μm in diameter and undergo extensive deformation to pass through 3-μm diameter capillaries and the 1-μm wide and 0.5-μm thick endothelial slits in the red pulp of the spleen. The ability of the red cell to undergo extensive reversible deformation is essential for both its function and its survival. Red cell deformability is a function of its geometry, the viscosity of the cytoplasm, largely determined by the concentration of hemoglobin. Decreased deformability is a feature of red cells in various pathological states. The erythrocyte is unique among eukaryotic cells in that its principal physical structure is its cell membrane, which encloses a concentrated hemoglobin solution. Thus, all of the structural properties of this cell are in one way or another linked to the cell membrane. In contrast to other cells, the erythrocyte has no cytoplasmic structures or organelles. Only, red cells and platelets do not have a nucleus.
Acronyms and Abbreviations:
BFU-E, burst-forming unit–erythroid; CFU-E, colony-forming unit–erythroid; cP, centipoise; DIC, disseminated intravascular coagulation; EMP, erythroblast macrophage protein; ICAM-4, intercellular adhesion molecule-4; IL, interleukin; MCH, mean cell hemoglobin content; MCHC, mean corpuscular hemoglobin concentration; MCV, mean cell volume; MDS, myelodysplastic syndrome; SA:V, surface area-to-volume ratio; TTP, thrombotic thrombocytopenic purpura.
*This chapter contains text written for previous editions of this book by Brian Bull, Paul Herrmann, and Ernest Beutler.
ERYTHRON
The mass of circulating erythrocytes constitutes an organ responsible for the transport of oxygen to tissues and the removal of carbon dioxide from tissues for exhalation. Collectively, the progenitors, precursors, and adult red cells make up an organ termed the erythron, which arises from pluripotent hematopoietic stem cells. Following commitment to the erythroid lineage, unipotential progenitors mature into the erythroid progenitors, the burst-forming unit–erythroid (BFU-E) and, subsequently, the colony-forming unit–erythroid (CFU-E), which then undergoes further maturation to generate anucleate polychromatophilic macrocytes (reticulocytes on supravital staining). The BFU-E and CFU-E are identified by their development into morphologically identifiable clonal colonies of red cells in vitro. The reticulocyte further matures, first in the marrow for 2 to 3 days and, subsequently, in the circulation for approximately 1 day, to generate discoid erythrocytes.1,2,3,4,5 The proerythroblast, the first morphologically recognizable erythroid precursor cell in the marrow undergoes four to five mitoses prior to maturation to an orthochromatic erythroblast, which then undergoes nuclear extrusion. A feature of erythropoiesis is that following each cell division the daughter cells advance in their state of maturation as compared to the parent cell and, ultimately, become functional as mature erythrocytes.4 In this process, they acquire the human blood group antigens, transport proteins, and all components of the erythrocyte membrane.4,6
In the adult stage of development, the total number of circulating erythrocytes is in a steady state, unless perturbed by a pathologic or environmental insult. This effect is not so during growth of the individual in utero, particularly in the early stages of embryonic development and also during neonatal development as the total blood volume increases markedly. Consequently, erythrocyte production in the embryo and fetus differs markedly from that in the adult.
In the very early stages of human growth and development, there are two forms of erythroid differentiation: primitive and definitive.7,8,9,10 Chapters 5 and 7 provide detailed descriptions of embryonic and fetal hematopoiesis. The primitive erythron supplies the embryo with oxygen during the phase of rapid growth before the definitive form of maturation has had a chance to develop and seed an appropriate niche. The hallmark of this primitive erythron is the release of nucleated erythroid precursors containing embryonic hemoglobin. Although primitive in the sense that the cells contain nuclei when released into the circulation, this form of maturation differs from avian and reptilian erythropoiesis in that the nucleus is eventually expelled from the mammalian cells as they circulate. The transient presence of a nucleus in the cells of the circulating primitive erythron can decrease the efficiency of gas exchange in the lungs and microvasculature because the nucleus prevents the red cell from behaving as a fluid droplet.11 The definitive stage of maturation makes its appearance around week 5 of embryogenesis when multipotential stem cells develop and seed the liver which maintains the erythron for most of fetal life. In later fetal life, skeletal development provides marrow niches to which erythropoiesis relocates being sustained in the form of erythroblastic islands, a central macrophage with circumferential layers of developing erythroid cells.12 The definitive stage of erythroid maturation predominates during the remainder of fetal development and is the only type of erythroid maturation present through childhood and adult life. All of normal human erythropoiesis occurs in the marrow in the form of erythroblastic islands.13
The earliest identifiable progenitor committed to the erythroid lineage is the BFU-E (see Chap. 32, Fig. 32–1). A BFU-E is defined in vitro by its ability to create a “burst” on semisolid medium—that is, a colony consisting of several hundred to thousands of cells by 10 to 14 days of growth, during which time smaller satellite clusters of cells form around a larger central group of erythroid cells, giving rise to the designation of a “burst.” The generation of BFU-E from hematopoietic stem cells requires interleukin (IL)-3, stem cell factor, and erythropoietin for differentiation, proliferation, prevention of apoptosis, and maturation (Chap. 18).5,13
As erythroid maturation progresses, a later progenitor, the CFU-E, derived from the BFU-E, can be defined in vitro. The CFU-E is dependent on erythropoietin for its development and can undergo only a few cell divisions.5,14 Thus, the CFU-E forms a smaller colony of morphologically recognizable erythroid cells in 5 to 7 days (see Chap. 32, Fig. 32–1). Adhesion between erythroid cells and macrophages occurs at the CFU-E stage of maturation.
Using cell-surface markers, IL-3 receptor, CD34, and CD36, highly purified populations of BFU-E and CFU-E can be isolated from human marrow.5 Gene expression profiling show distinctive changes in gene expression profiles in hematopoietic stem cells, BFU-E, and CFU-E.5 Some of the marrow failure syndromes are the result of defects in differentiation of stem cells into erythroid progenitors.
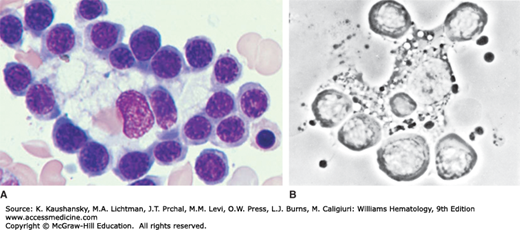
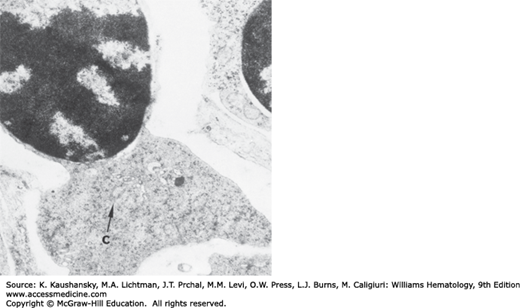
The anatomical unit of erythropoiesis in the normal adult is the erythroblastic island or islet.13,16,17 The erythroblastic island consists of a centrally located macrophages surrounded by maturing terminally differentiating erythroid cells (Fig. 31–1A). A number of binding proteins are implicated in the cell–cell adhesions important to this process. These include α4β1 integrin, erythroblast macrophage protein (EMP), and intercellular adhesion molecule-4 (ICAM-4) on the erythroblasts and vascular cell adhesion molecule (VCAM-1), EMP, αV integrin on macrophages.16 Additional macrophage receptors include CD69 (sialoadhesin) and CD163, but the counterreceptors for these on erythroblasts remains to be defined.16 Phase-contrast microcinematography reveals that the macrophage is far from passive or immobile. Evidence suggests that either the erythroblastic islands migrate or that erythroid precursors move from island to island, as islands near sinusoids are composed of more mature erythroblasts while islands more distant from the sinusoids are composed of proerythroblasts.18 The macrophage’s pseudopodium-like cytoplasmic extensions move rapidly over cell surfaces of the surrounding wreath of erythroblasts. On phase contrast micrographs, the central macrophage of the erythroblastic island appears sponge-like, with surface invaginations in which the erythroblasts lie (Fig. 31–1B). As the erythroblast matures, it moves along a cytoplasmic extension of the macrophage away from the main body. When the erythroblast is sufficiently mature for nuclear expulsion, the erythroblast makes contact with an endothelial cell, passes through a pore in the cytoplasm of the endothelial cell and enters the circulation as a polychromatophilic macrocyte (reticulocyte).19,20,21 The nucleus is ejected prior to egress from the marrow, phagocytized, and degraded by marrow macrophages.22 In addition to the unique cytologic features described above, the macrophage of the erythroblastic island is also molecularly distinct as demonstrated by a unique immunophenotypic signature.23 In addition, the macrophage of the erythroblastic island appears to play a stimulatory role in erythropoiesis independent of erythropoietin. The anemia of chronic inflammation and of the myelodysplastic syndrome (MDS) may result, at least in part, from inadequate stimulation of erythropoiesis by these macrophages (Chap. 5).
Figure 31–1.
Erythroblastic island. A. Erythroblastic island as seen in Wright-Giemsa–stained marrow. Note central macrophage surrounded by a cohort of attached erythroblasts. B. Erythroblastic island in the living state examined by phase-contrast microscopy. The macrophage shows dynamic movement in relation to its surrounding erythroblasts. (A, reproduced with permission from Lichtman’s Atlas of Hematology, www.accessmedicine.com.)
Despite the central role of erythroid islands in erythropoiesis in vivo, morphologically normal development of erythroid cells can be recapitulated in vitro without these structures as long as developing cells are provided with supraphysiologic concentrations of appropriate cytokines and growth factors. Such growth, however, occurs at a much slower rate than that observed in vivo, when erythroblasts form erythroblastic islands.24 The erythroblastic island is a fragile structure. It is usually disrupted in the process of obtaining a marrow specimen by needle aspiration but can be seem in marrow biopsies.
Macrophages in erythroblastic islands not only affect erythroid differentiation and/or proliferation, but also perform other functions, including rapid phagocytosis (<10 min) of extruded nuclei as a result of exposure of phosphatidylserine on the surface of the membrane surrounding the nucleus.22 This phagocytosis is the reason for the inability to find extruded nuclei in marrow aspirates in spite of the fact that 2 million nuclei are extruded every second during steady-state erythropoiesis. A protective macrophage function linked to efficient phagocytosis has been described. In normal mice, DNase II in macrophages degrades the ingested nuclear DNA but in DNase II knockout mice the inability to degrade DNA results in macrophage toxicity with resultant decrease in number of marrow macrophages and in conjunction with severe anemia.25 Macrophages can play both positive and negative regulatory roles in human erythropoiesis but the mechanistic basis for these regulatory processes are not completely understood.16,24 These processes may play a role in the ineffective erythropoiesis in disorders such as MDS, thalassemia, and malarial anemia.
Another potentially important role originally proposed for the central macrophage is direct transfer of iron to developing erythroblasts mediated by ferritin exchange between macrophages and erythroblasts (Chap. 42).13 Although this is an interesting concept, there is no definitive evidence for this exchange.
A “progenitor” in the hematopoietic system is defined as a marrow cell that is a derivative of the pluripotent hematopoietic stem cell through the process of differentiation and is antecedent to a “precursor” cell, the latter being identifiable by light microscopy by its morphologic characteristics (see Chap. 83, Fig. 83–2). In erythropoiesis, the earliest precursor is the proerythroblast. Erythroid progenitor cells are identified as marrow cells capable of forming erythroid colonies in semisolid medium in vitro under conditions in which the appropriate growth factors are present. Progenitor cells also may be identified by characteristic profiles of surface CD antigens using flow cytometry. Numerically, erythroid progenitors, BFU-E and CFU-E represent only a minute proportion of human marrow cells. BFU-E range from 300 to 1700 × 106 mononuclear cells and CFU-E range from 1500 to 5000 × 106 mononuclear cells.5 In vitro cultures using CD34+ cells from blood, cord blood, and marrow as the starting material have identified the critical cytokines required for erythroid differentiation and maturation and enabled the identification and isolation of pure cohorts of erythroid progenitors and erythroblasts at all stages of terminal erythroid maturation.4,5
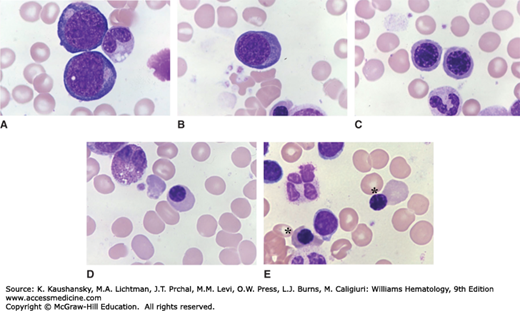
Figure 31–2 shows the sequence of precursors as seen in marrow films. Figure 31–3 shows the marrow precursors as isolated by flow cytometry.
Figure 31–2.
Human erythrocyte precursors. Light microscopic appearance. Marrow films stained with Wright stain. There are five stages of erythroblast development recognizable by light microscopy. A. Proerythroblasts. Two are present in this field. They are the largest red cell precursor, with a fine nuclear chromatin pattern, nucleoli, basophilic cytoplasm, and often a clear area at the site of the Golgi apparatus. B. Basophilic erythroblast. The cell is smaller than the proerythroblast, the nuclear chromatin is slightly more condensed and cytoplasm is basophilic. C. Polychromatophilic erythroblasts. The cell is smaller on average than its precursors. The nuclear chromatin is more condensed with a checkerboard pattern developing. Nucleoli are not apparent, usually. The cytoplasm is gray, reflecting the staining modulation induced by hemoglobin synthesis, which adds cytoplasmic content that takes an eosinophilic stain, admixed with the residual basophilia of the fading protein synthetic apparatus. D. Orthochromic normoblast. Smaller on average than its precursor, increased condensation of nuclear chromatin, with homogeneous cytoplasmic coloration approaching that of a red cell. E. Late orthochromatic erythroblasts (asterisks). The orthochromatic erythroblast to the right is undergoing apparent enucleation. The other three mononuclear cells are lymphocytes. A degenerating four-lobed neutrophil is also present. (Reproduced with permission from Lichtman’s Atlas of Hematology, www.accessmedicine.com.)
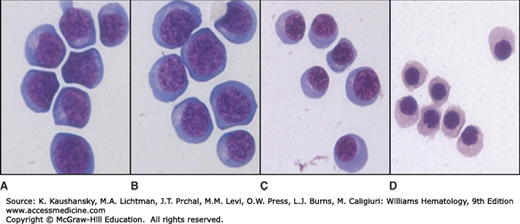
Figure 31–3.
Human erythroblast precursors as isolated by cell flow cytometry. Images of populations of human erythroblast precursors at stages of erythroid maturation when sorted from human marrow by flow cytometry. A and B. Proerythroblasts and early basophilic erythroblasts; (C) polychromatic erythroblasts; and (D) orthochromatic erythroblasts.
On stained films, the proerythroblast appears as a large cell, irregularly rounded or slightly oval.13 The nucleus occupies approximately 80 percent of the cell area and contains fine chromatin delicately distributed in small clumps. One or several well-defined nucleoli are present. The high concentration of polyribosomes gives the cytoplasm of these cells its characteristic intense basophilia. At very high magnification, ferritin molecules are seen dispersed singly throughout the cytoplasm and lining the clathrin-coated pits on the cell membrane (see Figs. 31–2 and 31–4) Diffuse cytoplasmic density on sections stained for peroxidase indicates hemoglobin is already present. Dispersed glycogen particles are present in the cytoplasm.
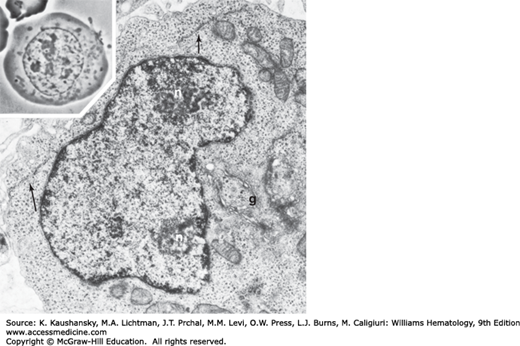
Figure 31–4.
Proerythroblast. Phase-contrast micrograph (inset) of a proerythroblast showing the immature nucleus with nucleoli and finely dispersed nuclear chromatin. The centrosome (juxtanuclear clear zone) is apparent with its dense accumulation of mitochondria. Electron microscopic section of the proerythroblast shows nucleoli (n) in contact with the nuclear membrane. Chromatin is finely dispersed and forms small aggregates in the fixed nuclear membrane. The perinuclear canal is narrow but well defined. Polyribosome groups, many in helical configuration, are dispersed throughout the cytoplasm. The Golgi apparatus (g) is well developed, and regions of endoplasmic reticulum (arrows) are seen.
Basophilic erythroblasts are smaller than proerythroblasts. The nucleus occupies three-fourths of the cell area and is composed of characteristic dark violet heterochromatin interspersed with pink-staining clumps of euchromatin linked by irregular strands.13 The whole arrangement often resembles wheel spokes or a clock face. The cytoplasm stains deep blue, leaving a perinuclear halo that expands into a juxtanuclear clear zone around the Golgi apparatus. Cytoplasmic basophilia at this stage results from the continued presence of polyribosomes (see Figs. 31–2 and 31–5).
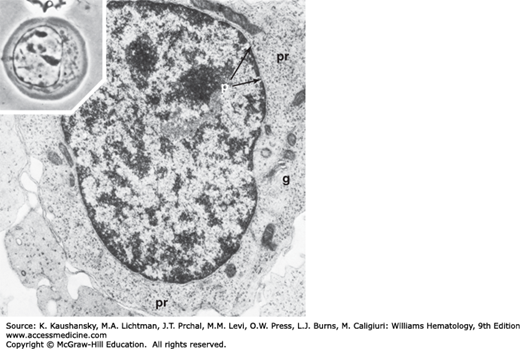
Figure 31–5.
Basophilic erythroblast. Phase-contrast photomicrograph (inset) shows increased clumping of the nuclear chromatin and further rounding of the cell, with aggregation of the mitochondria and centrosome into the regions of nuclear indentation. Electron microscopic section shows clumping of the nuclear chromatin, nuclear pores (p), organization of the nucleoli, increased density of polyribosomes (pr), well-developed Golgi apparatus (g), and a decrease in smooth endoplasmic reticulum.
Following the mitotic division of the basophilic erythroblast, the cytoplasm changes from deep blue to gray, as hemoglobin dilutes the polyribosome content. Cells at this stage are smaller than basophilic erythroblasts. The nucleus occupies less than half of the cell area. The heterochromatin is located in well-defined clumps spaced regularly about the nucleus, producing a checkerboard pattern. The nucleolus is lost, but the perinuclear halo persists.13 It is at this point that erythroblasts lose their mitotic potential. Electron microscopy of the polychromatophilic erythroblast reveals increased aggregation of nuclear heterochromatin.13 Active ferritin transport across the cell membrane is always evident, and siderosomes along with dispersed ferritin molecules can be identified within the cytoplasm (see Figs. 31–2 and 31–6).
Figure 31–6.
Polychromatophilic erythroblast. Phase-contrast micrograph (inset) demonstrates diminished size of this cell compared with its precursor. Further clumping of nuclear chromatin gives the nucleus a checkerboard appearance. The centrosome is condensed, and a perinuclear halo has developed. Electron microscopic section demonstrates relative reduction of the density of polyribosomes and dilution by the moderately osmiophilic hemoglobin in the cytoplasm. Nuclear chromatin shows a marked increase in clumping, and nuclear pores (P) are enlarged.
After the final mitotic division of the erythropoietic series, the concentration of hemoglobin increases within the erythroblast. Under the light microscope, the nucleus appears almost completely dense and featureless. It is measurably decreased in size. This cell is the smallest of the erythroblastic series.13 The nucleus occupies approximately one-fourth of the cell area and is eccentric. Cell movement can be appreciated under the phase-contrast microscope. Round projections appear suddenly in different parts of the cell periphery and are just as quickly retracted.13 The movements probably are made in preparation for ejection of the nucleus. The cell ultrastructure is characterized by irregular borders, reflecting its motile state. The heterochromatin forms large masses. Mitochondria are reduced in number and size (see Figs. 31–2, 31–7, and 31–8).
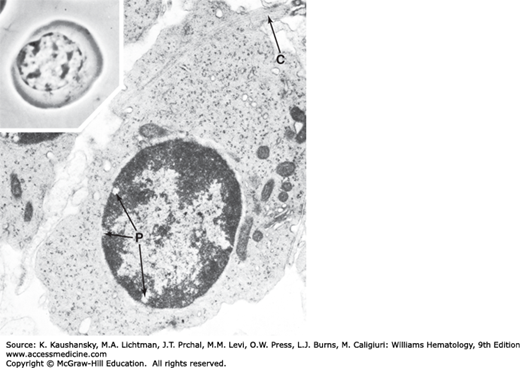
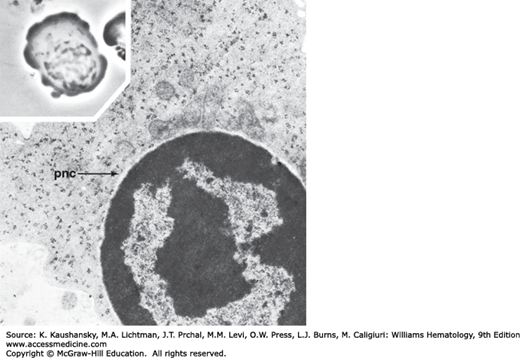
Figure 31–7.
Orthochromic erythroblast. Phase-contrast appearance of this cell in the living state (inset) shows the irregular borders indicative of its characteristic motility, the eccentric nucleus making contact with the plasmalemma, further pyknosis of the nuclear chromatin, and condensation of the centrosome. Electron microscopic section shows further dilution of polyribosomes, some of which appear to be disintegrating into monoribosomes, by the increasing hemoglobin. The number of mitochondria is decreased, and some mitochondria are degenerating. Nuclear chromatin is clumped into large masses, and a perinuclear canal (pnc) is seen.
All normal erythroblasts are sideroblasts in that they contain iron in structures called siderosomes, as evident by transmission electron microscopy. These structures are essential for the transfer of iron for heme (hemoglobin) synthesis. By light microscopy, under the usual conditions of Prussian blue staining for iron, a minority of normal erythroblasts (approximately 15 to 20 percent) can be identified as containing siderosomes and those that can be so identified have very few (one to four) small Prussian blue–positive granules.
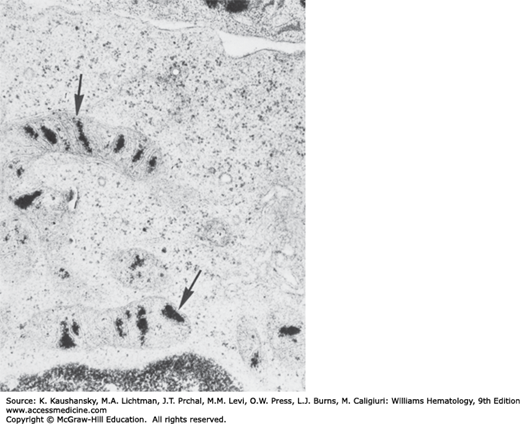
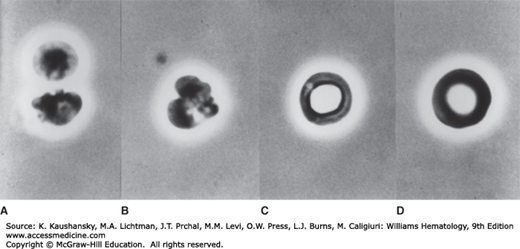
A heterogeneous group of erythrocyte disorders is accompanied by ineffective erythropoiesis, abnormal erythroblast morphology and hyperferremia. These disorders include acquired megaloblastic anemia (Chap. 41), congenital dyserythropoietic anemias (Chap. 39), thalassemias (Chap. 48), the inherited and acquired sideroblastic anemias, pyridoxine-responsive anemia, alcohol-induced sideroblastic anemia, and lead intoxication (Chaps. 52 and 59). Some of these conditions are characterized by the presence of pathologic sideroblasts. Pathologic sideroblasts are of two types. One type is an erythroblast that has an increase in number and size of Prussian blue–stained siderotic granules throughout the cytoplasm. Another type is the erythroblast that shows iron-containing granules that are arranged in an arc or a complete ring around the nucleus (Fig. 31–8). These pathologic sideroblasts are referred to as ring or ringed sideroblasts.26,27 Electron microscopic studies show that granules in ringed sideroblasts are iron-loaded mitochondria. In cells with iron-loaded mitochondria, many ferritin molecules are deposited between adjacent erythroblast membranes.
Prior to enucleation at the late orthochromatic erythroblasts stage, intermediate filaments and the marginal band of microtubules disappear. Enucleation is a highly dynamic process that involves coordinated action of multiple mechanisms.28,29,30 Tubulin and actin become concentrated at the point where the nucleus will exit. These changes, accompanied by microtubular rearrangements and actin polymerization, play a role in nuclear expulsion. Expulsion of the nucleus in vitro is not an instantaneous phenomenon; it requires a period of 6 to 8 minutes. The process begins with several vigorous contractions around the midportion of the cell, followed by a division of the cell into unequal portions. The smaller portion consists of the expelled nucleus surrounded by a thin ring of hemoglobin and plasma membrane (Fig. 31–9). In vivo, expulsion of the nucleus may occur while the erythroblast is still part of an erythroblastic island or the nucleus may be lost during passage through the wall of a marrow sinus as the nucleus, which cannot traverse the small opening, remains in the marrow. The outer leaflet of the bilaminar membrane surrounding the expelled nucleus is high in phosphatidylserine, a signal for macrophage ingestion (Fig. 31–10).22 It is not clear what fraction of the expelled nuclei is ingested by the macrophage of the erythroblastic island or by other macrophages resident in marrow. Two hypotheses have been proposed to explain how the reticulocyte exits the marrow.19,20,21 The reticulocyte may actively traverse the sinus epithelium to enter the lumen. More likely, however, the reticulocyte may be driven across by a pressure differential because it appears incapable of directed amoeboid motion. The precise mechanism is yet to be defined.
Following nuclear extrusion, the reticulocyte retains mitochondria, small numbers of ribosomes, the centriole, and remnants of the Golgi apparatus. It contains no endoplasmic reticulum. Supravital staining with brilliant cresyl blue or new methylene blue produces aggregates of ribosomes, mitochondria, and other cytoplasmic organelles. These aggregates stain deep blue and, arranged in reticular strands, give the reticulocyte its name. Maturation of the reticulocyte requires 48 to 72 hours. During this period, approximately 20 percent of the membrane surface area is lost and cell volume decreases by 10 to 15 percent and the final assembly of the membrane skeleton is completed.31,32,33 Living reticulocytes observed by phase-contrast microscopy are irregularly shaped cells with a characteristically puckered exterior and a motile membrane. Examined by electron microscopy, reticulocytes are irregularly shaped and contain many remnant organelles.13 The organelles, small smooth vesicles, and an occasional centriole are grouped in the region of the cell where the nucleus is expelled. In “young” reticulocytes, the vast majority of ribosomes dispersed throughout the cytoplasm are in the form of polyribosomes. As protein synthesis diminishes during maturation, the polyribosomes gradually transform into monoribosomes. During reticulocyte maturation there is significant remodeling of the membrane, including loss of membrane proteins that include transferrin receptors, Na-K adenosine triphosphatase (ATPase), and adhesion molecules, as well as loss of tubulin and cytoplasmic actin.33 During the remodeling process the membrane becomes more elastic and acquires increased membrane mechanical stability.32
“Stress” reticulocytes are released into the circulation during an intense erythropoietin response to acute anemia or experimentally in response to large doses of exogenously administered erythropoietin.34 These cells may be twice the normal volume, with a corresponding increase in mean cell hemoglobin (MCH) content. Whether the increase results from one less mitotic division during maturation or from some other process such as changes in cell cycle is not clear. It is interesting to note that mice do not have the ability to produce stress reticulocytes with increased mean cell volume (MCV) and MCH. In contrast, even under moderate erythropoietic stress, some reticulocytes in the marrow pool shift to the circulating pool. These “shift” reticulocytes with normal MCH contain a higher-than-normal RNA content and now can be quantified. Quantification is commonly performed by applying a fluorescent stain to tag RNA and then dividing reticulocytes into high-, medium-, and low-fluorescence categories using a fluorescence-sensitive flow cytometer. The “stress” reticulocytes of the older literature likely fall in the high- and medium-fluorescence categories. Unfortunately, at present little attention is being paid to discriminate stress and shift reticulocytes.
The reticulocyte may show pathologic alterations in size or staining properties. The reticulocyte may contain inclusions visible by light microscopy or identifiable only on ultrastructural analysis. Most pathologic inclusions usually attributed to erythrocytes are actually found within reticulocytes and are nuclear or cytoplasmic remnants derived from late-stage erythroblasts. In splenectomized patients, they may also be found in mature erythrocytes.
See Fig. 31–11 for images of red cell inclusions.
Figure 31–11.
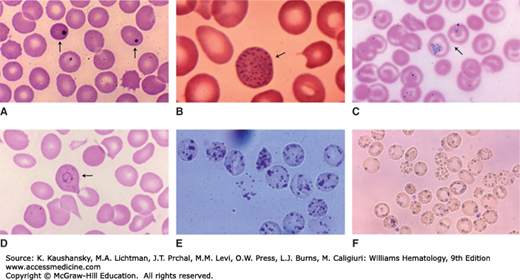
Red cell inclusions. Blood films. A. Red cells with Howell-Jolly bodies (arrows) postsplenectomy. The crisp circular border, dark blue color, and peripheral location are characteristic. B. Basophilic stippling. These basophilic inclusions may be fine or coarse. In this case, the cell contains coarse stippling seen in lead poisoning (arrow). C. Siderocyte. These cells contain purple granules when stained with Wright stain (Pappenheimer bodies). Compared to basophilic stippling, siderotic granules are usually fewer in number and sometimes clustered. These Prussian blue–stained cells confirm that the granules contain iron (blue reaction product). Arrow points to two siderocytes. D. Cabot ring. Rare red cell inclusion (arrow). See text for further description. E. Heinz bodies. These cells from a patient with glucose-6-phosphate dehydrogenase deficiency were incubated with a supravital dye, which stains the denatured globin precipitates. F. Red cells from a patient with hemoglobin H disease (α-thalassemia). The hemoglobin precipitates are stained with brilliant cresyl blue. (Reproduced with permission from Lichtman’s Atlas of Hematology, www.accessmedicine.com.)
Howell-Jolly bodies are small nuclear remnants that have the color of a pyknotic nucleus on Wright-stained films and give a positive Feulgen reaction for DNA.35,36 They are spherically shaped, randomly distributed in the red cell and usually no larger than 0.5 μm in diameter. Howell-Jolly bodies may be numerous, although generally only one is present. In pathologic situations, they appear to represent chromosomes that have separated from the mitotic spindle during abnormal mitosis, and contain a high proportion of centromeric material along with heterochromatin. More commonly, during normal maturation they arise from nuclear fragmentation or incomplete expulsion of the nucleus. Howell-Jolly bodies are pitted from the reticulocytes during their transit through the interendothelial slits of the splenic sinus. They are characteristically present in the blood of splenectomized persons and in patients suffering from megaloblastic anemia, and hyposplenic states.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree