INTRODUCTION
SUMMARY
Early in precursor development in the marrow, cells destined to be leukocytes of the granulocytic series—neutrophils, eosinophils, and basophils—synthesize proteins and store them as cytoplasmic granules. The synthesis of primary or azurophilic granules defines the conversion of the myeloblast, a virtually agranular, primitive cell that is the earliest granulocyte precursor identifiable by light microscopy, into the promyelocyte, which is rich in azurophilic granules. Synthesis and accumulation of secondary or specific granules follows. The appearance of specific granules marks the progression of the promyelocyte to neutrophilic, eosinophilic, or basophilic myelocytes. Thereafter, the cell continues maturation into an amitotic cell with a segmented nucleus, capable of chemotaxis, phagocytosis and microbial killing. The mature granulocytes also develop cytoplasmic and surface structures that permit them to attach to and penetrate the wall of venules. The mature granulocytes enter the blood from the marrow, circulate briefly, and move to the tissues to carry out their major function of host defense. Blood neutrophils exhibit the capacity for changes in phenotypic characteristics and life span depending on the stimulating milieu of cytokines and chemokines. Gene expression profiling studies indicate the neutrophil is a transcriptionally active cell, responsive to environmental stimuli, and capable of a complex series of early and late changes in gene expression.
Acronyms and Abbreviations
AML1, AML2, AML3, transcription factor for various hematologic lineages; C3a, serum complement fragment 3a; C5a, serum complement fragment 5a; CBFA1, CBFA2, core-binding factor subunit α-1 or -2; CCR, C-C chemokine receptor; C/EBPε, regulating factor of gene expression; CD11b/CD18, Mac-1 or integrin αmβ2; ECP, eosinophil cationic protein; EDN, eosinophil-derived neurotoxin; FcγRIIIB, receptor IIIB for the Fc region of IgG; GATA-1, lineage-specific transcription factor; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; GRO, growth-regulated protein; IFN, interferon; Ig, immunoglobulin; IL, interleukin; IP-10, interferon-γ–induced protein 10; JAK2, Janus-associated kinase 2; LPS, lipopolysaccharide; MBP, major basic protein; MMP-8, metalloproteinase-8, also called collagenase; MMP-9, metalloproteinase-9, also called gelatinase B; NADPH, reduced form of nicotinamide adenine dinucleotide phosphate; PAF, platelet-activating factor; PMN, polymorphonuclear neutrophil; RUNX1, RUNX2, RUNX3, runt-related transcription factor 1, 2, or 3; SNAP, soluble NSF (N-ethylmaleimide-sensitive factor) attachment protein; TGF, transforming growth factor; TNF, tumor necrosis factor; VAMP, vesicle-associated membrane protein.
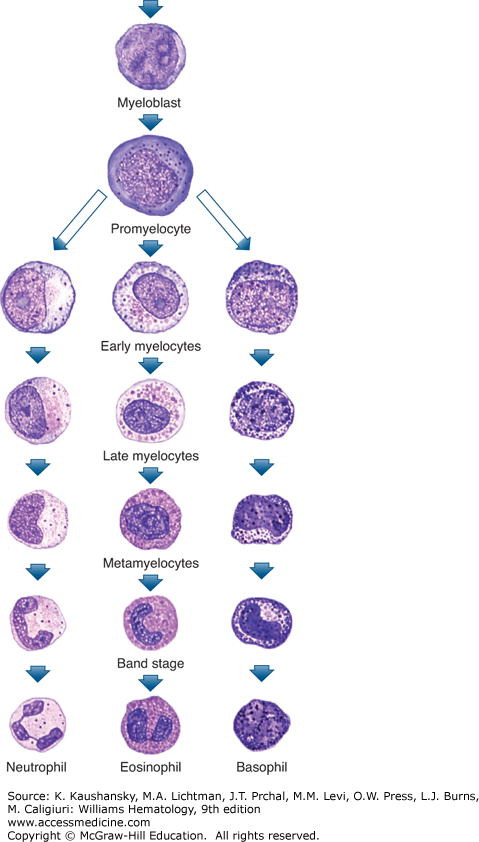
In the normal adult human, the life of granulocytes is spent in three environments: marrow, blood, and tissues. Marrow is the site of differentiation of hematopoietic stem cells into granulocyte progenitors and of proliferation and terminal maturation (Fig. 60–1). Precursor cell proliferation, which consists of approximately five divisions, occurs only during the first three stages of maturation (blast, promyelocyte, and myelocyte). After the myelocyte stage, the cells are no longer capable of mitosis and enter a large marrow storage pool from which they are released into the blood where they circulate for a few hours before entering tissues.
Figure 60–1.
Diagrammatic representation of neutrophil (polymorphonuclear neutrophil [PMN]) and stages of maturation (see text for discussion). Of every 100 nucleated cells in marrow, 0.5 percent are myeloblasts, 5 percent are promyelocytes, 12 percent are myelocytes, 22 percent are metamyelocytes and bands, and 20 percent are maturing and mature neutrophilic cells, yielding a total of approximately 60 percent of cells representing developing neutrophils in normal human marrow. (Reproduced with permission from Lichtman’s Atlas of Hematology. www.accessmedicine.com.)
The myeloblast is an immature cell with a large, oval nucleus, sizable nucleoli, and few or no granules. As the earliest precursor in the evolution of the neutrophil from the colony forming unit, it is an immature cell with a large nucleus and multiple nucleoli (Fig. 60–2). The nucleolus is the site of assembly of ribosomal proteins and ribosomal RNA, and is a prominent feature of early maturing cells. The scant cytoplasm contains reaction product for peroxidase within the rough-surfaced endoplasmic reticulum and Golgi cisternae and, sometimes, in early developing azurophilic granules. The dense product of the peroxidase reaction serves as a marker of azurophilic granules in human marrow and blood cells for electron and for light microscopy.1,2,3,4
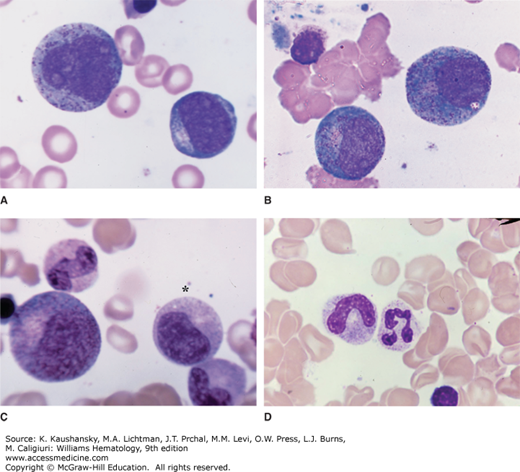
Figure 60–2.
Marrow films. A. Myeloblast is the smaller cell to the lower right. It is the first recognizable precursor in the granulocytic series. Relatively high nuclear-to-cytoplasmic ratio. Note nucleoli and agranular cytoplasm. Promyelocyte in upper left. This cell is the largest granulocyte precursor in the marrow. It often has overt nucleoli, usually more cytoplasm, and azurophilic (primary) granules scattered throughout the cytoplasm and overlying the nucleus. B. Two very early neutrophilic myelocytes. They are very similar to the promyelocyte in appearance with nucleoli and scattered azurophilic granules throughout the cytoplasm. The distinguishing feature is the burst of tan coloring at the site of the Golgi zone, indicating the initial synthesis of neutrophilic granules. C. Large cell to the left is an early neutrophilic myelocyte with more neutrophilic granules evident spreading from the Golgi zone at the hilus of the nucleus. It still has some features of the promyelocyte. The cell beneath the asterisk is a late neutrophilic myelocyte. The cell has decreased in size, the nuclear chromatin has condensed. Nucleoli are not evident and the cytoplasm is nearly filled with neutrophilic granules. Below the neutrophilic myelocyte is a neutrophilic metamyelocyte, characterized by its reniform nucleus and cytoplasm filled with neutrophilic granules. The cell above the large early myelocyte on the left is a band neutrophil. The nucleus has reached the shape of a sausage and is about equal in diameter through its length. D. A band neutrophil (left) and a segmented neutrophil (right). Neutrophilic granules, because of their small size, are not resolvable by the light microscope and are inferred by the characteristic tan staining quality of the cytoplasm. (Reproduced with permission from Lichtman’s Atlas of Hematology. www.accessmedicine.com.)
In the promyelocyte stage, the azurophilic or primary granules, large peroxidase-positive granules that stain metachromatically (reddish-purple) with a polychromatic stain such as Wright stain, are formed. Figure 60–3 shows that the promyelocyte produces and accumulates a large population of peroxidase-positive granules. Most of the granules are spherical and have a diameter of 500 nm, but ellipsoid, crystalline forms and small granules connected by filaments also are present.5 As with other secretory cells, peroxidase is present throughout the secretory apparatus of the promyelocyte, including cisternae of the rough endoplasmic reticulum, all Golgi cisternae, some vesicles, and all developing granules.2
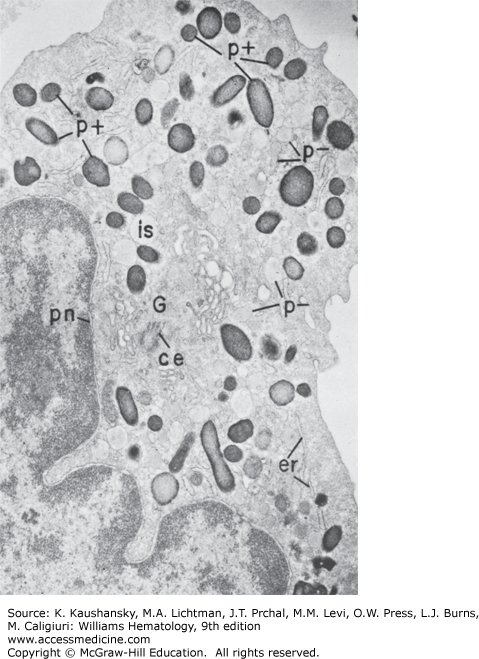
Figure 60–3.
Electron micrograph of a neutrophilic promyelocyte from normal human marrow reacted for peroxidase. This cell is the largest of the neutrophilic series. It has a sizable, slightly indented nucleus with a nucleolus, a prominent Golgi region (G), centriole (ce), and cytoplasm packed with dense peroxidase-positive (p+) azurophilic granules of varying shapes and sizes. Peroxidase reaction product is visible in less concentrated form within all compartments of the secretory apparatus—endoplasmic reticulum (er), perinuclear cisterna (pn), and Golgi cisternae (G), and there are peroxidase negative granules (p−). No reaction product is apparent in the cytoplasmic matrix or mitochondria. (×8000).
During the myelocyte stage of maturation, the specific or secondary granules, which are peroxidase negative, are formed (see Fig. 60–2). At the end of the promyelocyte stage, peroxidase abruptly disappears from rough endoplasmic reticulum and Golgi cisternae, and the production of azurophilic granules ceases. The myelocyte stage begins with production of peroxidase-negative specific granules.2
The only peroxidase-positive elements at this stage are the azurophilic granules. The specific granules are formed by the Golgi complex. The granules vary in size and shape but typically are spherical (approximately 200 nm) or rod shaped (130 × 1000 nm). Figure 60–4 shows the cell also labeled with immunogold particles to illustrate the presence of lactoferrin, a specific granule marker. Approximately three cell divisions occur at this stage of maturation. Mitoses can be observed (Fig. 60–5), and the two types of granules appear to be distributed to the daughter cells in fairly equal numbers.
Figure 60–4.
Portion of cytoplasm stained for peroxidase to mark the azurophil granules and then immunolabeled with gold particles to detect lactoferrin. The peroxidase-positive (p+) azurophil granules contain dense reaction product, whereas the lighter specific granules are peroxidase negative. Many of the peroxidase-negative granules (arrows) have gold label within their matrix (×70,000).
The metamyelocyte and band neutrophils are nonproliferating cells that precede the development of the mature neutrophil (see Fig. 60–2). The mature, segmented neutrophilic cells contain primary, peroxidase-positive granules and specific peroxidase-negative granules in a 1:2 ratio. The nucleus of the circulating neutrophil is segmented, usually into two to four interconnected lobes. The late stages of maturation consist of nondividing cells that can be distinguished by their nuclear morphology, mixed granule populations, small Golgi regions, and accumulations of glycogen particles. On average, an electron micrograph of a neutrophil displays 200 to 300 granules, and approximately one-third are peroxidase-positive (Fig. 60–6).
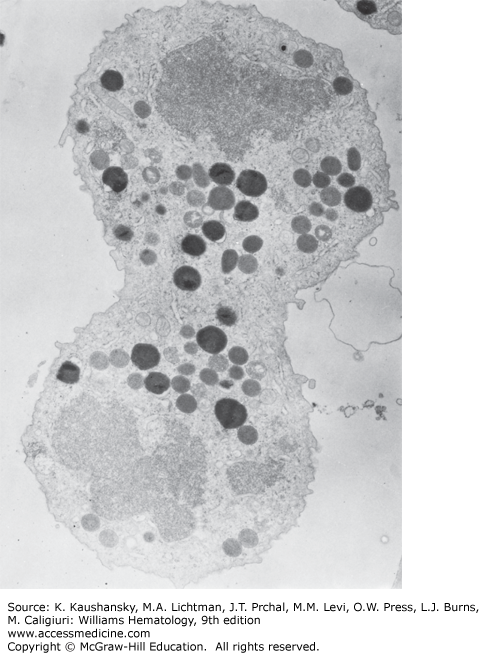
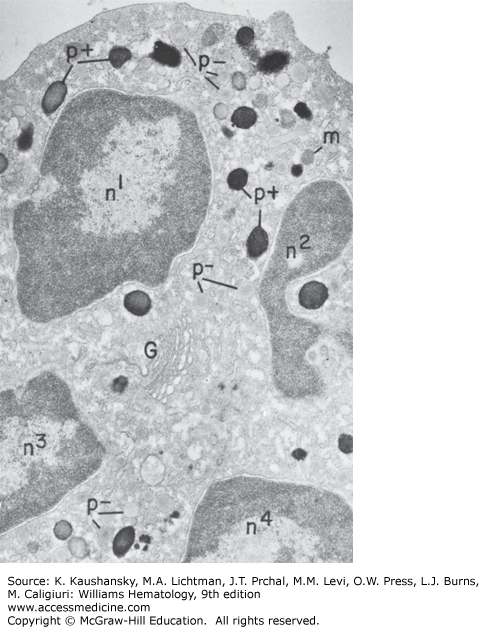
Figure 60–6.
Mature neutrophil from normal human marrow reacted for peroxidase. The cytoplasm is filled with granules of the two basic types: (1) the smaller, pale, peroxidase-negative granules (p−) and (2) the large, dense, peroxidase-positive granules (p+). The nucleus is condensed and lobulated (n1–n4), the Golgi region (G) is small and without any forming granules, the endoplasmic reticulum is scant, and mitochondria (m) are few (×21,000).
The violet-colored granules seen with light microscopy in mature neutrophils on Wright-stained blood films are azurophilic granules whose staining characteristics altered during maturation (Fig. 60–7). Therefore, with light microscopy, the most reliable method for identifying azurophilic granules on blood films is staining the cells for peroxidase. The size of most of the peroxidase-negative granules (approximately 200 nm) is at the limit of resolution of the light microscope. The granules cannot be distinguished individually but are responsible for the pink background color of neutrophil cytoplasm during and after the myelocyte stage.
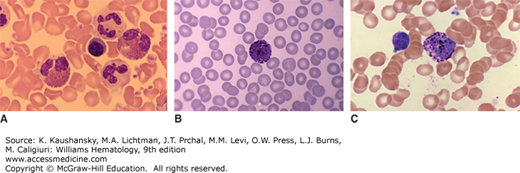
Figure 60–7.
Images of granulocytes in blood films. A. Image shows two neutrophils, two eosinophils with bilobed nuclei, and a single neutrophil. B and C. The images are of basophils showing densely stained metachromatic cytoplasmic granules. (Reproduced with permission from Lichtman’s Atlas of Hematology. www.accessmedicine.com.)
Peroxidase-negative granules are more numerous than peroxidase-positive granules during the myelocyte stage because peroxidase granule formation ceases after the promyelocyte stage, the number of oxidase-positive granules per cell is reduced by mitoses, and peroxidase-negative granules continue to be produced by each myelocyte generation.1
The purpose of nuclear segmentation is not known. Fluorescence in situ hybridization with chromosome-specific probes has shown that chromosomes are randomly distributed among the nuclear lobes.6 Some mature neutrophils in women have drumstick- or club-shaped nuclear appendages. These appendages contain the inactivated X chromosome. An X-chromosome–specific nucleic acid probe has confirmed the position of the X chromosomes in the drumstick structure of leukocyte nuclei by in situ hybridization.7
The diversity of neutrophil granules appears to be linked to the timing of biosynthesis during myelopoiesis. The hypothesis is that the different subsets of granules are the result of differences in the biosynthetic windows of the various granule proteins during maturation7 and not the result of specific sorting between individual granule subsets (Chap. 66). The control of biosynthesis is exerted by transcription factors that control the expression of the genes for the various granule proteins. Several transcription factors identified as important in the timing of granule protein synthesis, including the lineage-specific transcription factor GATA-1, the lineage-specific transcription factor PU.1, transcription factor for various hematologic lineages, AML1 (also known as runt-related transcription factor 1 [RUNX1] or core-binding factor subunit alpha-2 [CBFA2]), AML2 (also known as RUNX3), and AML3 (also known as RUNX2 or CBFA1), and regulating factor of gene expression C/EBPε.7,8,9 The importance of C/EBPε has been emphasized by the recognition of mutations in this protein in patients with the rare syndrome called “specific granule deficiency,”10,11,12 a condition that leads to increased susceptibility to bacterial infections. In neutrophils from these patients, total cellular content and release of the secondary and tertiary granule markers (e.g., lactoferrin, B12 binding protein, and lysozyme) are diminished, although levels of primary granule constituents (e.g., myeloperoxidase, β-glucuronidase) generally are normal.
The granular constituents are released from the membrane-enclosed granules into phagosomes or transported to the cell surface by a process of exocytosis following stimulation of the neutrophil.13 The signal cascade following stimulation of specific receptors on the cytoplasmic membrane results in elevated intracellular Ca2+, lipid remodeling, and protein kinase activation, which culminate in fusion of granules with phagosomes or the cell surface membrane. The process is rapid, highly efficient, and involves families of docking proteins related to those found in neurons (e.g., vesicle-associated membrane protein [VAMP]-2, syntaxin-4, soluble NSF (N-ethylmaleimide-sensitive factor)-attachment protein [SNAP]-23).14
The granule subsets appear to have a significant differential sensitivity to undergo exocytosis, ranging from secretory vesicles to tertiary, secondary, and primary granules, with primary granules being most resistant. The significance of this differential release is incompletely understood, but some aspects are apparent in the functions of the constituents within the granules and granular membranes. For example, secretory vesicles and tertiary granules contain receptors, such as CD11b/CD18 (adhesion molecule, Mac-1), formyl peptide receptor (chemotactic receptor), FcγRIIIB (Fc receptor), and gelatinase (metalloproteinase [MMP]-9), which potentially enhance extracellular interactions of the neutrophil. Primary granules contain microbicidal proteins and acid hydrolases, and the acidic environment of the phagolysosome creates an optimal pH for these enzymes.
Neutrophil granules are particularly rich in factors with antimicrobial activity. Some (e.g., myeloperoxidase) function in conjunction with the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, whereas others (e.g., defensins) exhibit activity independent of the oxidative burst. Table 60–1 lists the principal contents of the four granule types in neutrophils: primary (azurophilic), secondary (specific), tertiary, and secretory vesicles.15–56
| Granules | Membrane Markers | NADPH Oxidase | Receptors | Antimicrobial | Enzymes | Other Factors |
|---|---|---|---|---|---|---|
| Primary (azurophilic) | CD63 | BPI-protein | Elastase | Acid mucopolysaccharide | ||
| CD68 | Defensins (HNP 1–4) | Cathepsin G | α1-Antitrypsin | |||
| V-type H+-ATPase | CAP37 | Proteinase 3 | ||||
| Myeloperoxidase | α-Mannosidase | |||||
| Lysozyme | β-Glucuronidase | |||||
| β-Glycerophosphatase | ||||||
| Sialidase | ||||||
| N-Acetyl-β-glucosaminidase | ||||||
| Secondary (specific) | CD15 | gp91phox | Formyl peptide R | Lactoferrin | Gelatinase B (MMP-9) | β2-Microglobulin |
| CD66 | p22phox | CR3 (CD11b/CD18) | Lysozyme | Histaminase | Vitamin B12-binding protein | |
| CD67 | Rap1A | Fibronectin R | hCAP-18 | Sialidase | Plasminogen activator | |
| CD11b/CD18 | Rap2 | G-protein α-subunit | Collagenase (MMP-8) | |||
| Laminin R | Heparinase | NGAL (lipocalin) | ||||
| Thrombospondin R | ||||||
| TNF R | ||||||
| uPAR | ||||||
| VAMP-2 | ||||||
| Vitronectin R | ||||||
| Tertiary | CD11b/CD18 | gp91phox | Formyl peptide R | Lysozyme | Gelatinase B (MMP-9) | β2-Microglobulin |
| V-type H+-ATPase | p22phox | CR3 (CD11b/CD18) | Acetyltransferase | Oncostatin M | ||
| Rap1A | uPAR | Diacylglycerol-deacylating enzyme | ||||
| VAMP-2 | ||||||
| Secretory vesicles | CD11b/CD18 | gp91phox | Formyl peptide R | CAP37 | Proteinase 3 | Plasma proteins (e.g., albumin) |
| CD10 | p22phox | CR1 (CD35) | ||||
| CD13 | Rap1A | CR3 (CD11b/CD18) | Decay accelerating factor | |||
| CD45 | CR4 (CD11c/CD18) | |||||
| CD35 | C1qR | |||||
| CD14 | FcγRIIIB (CD16) | |||||
| uPAR |
The earliest morphologically identifiable form of an eosinophilic leukocyte is as a late myeloblast or early promyelocyte (see Fig. 60–1). This cell is approximately 15 µm in diameter and has a large nucleus with nucleoli and a few blue or azurophilic granules in intensely basophilic cytoplasm. The later eosinophilic promyelocyte and myelocyte contain mostly acidophilic granules. A lineage-committed eosinophil progenitor has recently been identified that expresses high levels of interleukin (IL)-5 receptor α and is negative for myeloperoxidase.57 The fully mature eosinophilic leukocyte has a bilobed nucleus (see Fig. 60–7), and its cytoplasm is filled with large eosinophilic granules whose rims stain for peroxidase and Sudan black. Multilobed nuclei, comparable to those of neutrophils, are rare. Eosinophils are susceptible to mechanical damage during preparation of blood films.
Eosinophils of the promyelocyte and myelocyte stages stain positively for peroxidase in all cisternae of the rough-surfaced endoplasmic reticulum, including transitional elements and the perinuclear cisterna; clusters of smooth vesicles at the periphery of the Golgi complex; all cisternae of the Golgi complex; and all immature- and mature-specific granules.4,58 Mature granules are completely filled with peroxidase except in areas occupied by centrally located crystals.
In the later stages of development, after granule formation has ceased, the eosinophils contain few of the organelles associated with the synthesis and packaging of secretory proteins. The endoplasmic reticulum is sparse or virtually nonexistent. The Golgi complex is small and inconspicuous. The cytoplasm of the mature eosinophil (Fig. 60–8) primarily contains granules and glycogen. Most of the granules are specific granules with crystals, which usually are centrally located. After the myelocyte stage, peroxidase can no longer be detected in the endoplasmic reticulum or Golgi elements of the eosinophil by any of the enzyme procedures; however, peroxidase can be found in the matrix of granules.1,58
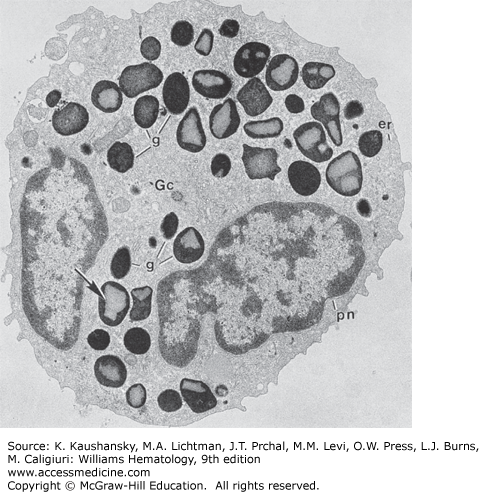
Figure 60–8.
Human mature eosinophil incubated for peroxidase. Reaction product is present only in granules (g). The rough endoplasmic reticulum (er), including the perinuclear cisterna (pn) and the Golgi cisternae (Gc), does not contain reaction product. Most of the granules (arrow) contain the distinctive crystalline bar (×8000).
As with neutrophils, eosinophils contain distinct granular organelles: primary granules, crystalloid granules, small granules and secretory vesicles.59 The crystalloid granules (see Fig. 60–8) are the largest, 0.5 to 0.8 µm in diameter, and contain much of the granular protein. The proteins packaged in these granules are highly basic proteins, with the crystalline core being mostly major basic protein (MBP).60,61 The granule matrix contains eosinophil peroxidase, eosinophil cationic protein (ECP), and eosinophil-derived neurotoxin (EDN). The primary granules contain Charcot-Leyden crystals. Charcot-Leyden crystals are bipyramidal crystals observed in fluids in association with eosinophilic inflammatory reactions. They possess lysophospholipase activity and compose 7 to 10 percent of total eosinophil protein.62,63 The ultrastructural localization of this protein is in a large, crystal-free granule and supports the presence of a distinct primary granule population in mature eosinophils.4,63,64 MBP consists of two homologues and is an abundant granular protein, 5 to 10 pg per cell. Mature eosinophils can no longer express this protein so all MBP is stored during development.65 Eosinophil peroxidase is an abundant heme-containing protein (approximately 15 pg per cell) that catalyzes the peroxidation of halides together with hydrogen peroxide forming bactericidal hypohalous acids.66,67 ECP is a bactericidal protein exists in two isoforms (ECP-1 and ECP-2) with activity toward helminthic parasites. EDN shares high sequence homology with ECP and is abundant, approximately 10 pg per cell. Other granule-stored proteins include several enzymes of potential importance in inflammation, including acid phosphatase, collagenase (MMP-8), matrix metalloproteases, histaminase, catalase, and phospholipase D.68,69,70 Chapter 62 discusses the functional aspects of these granular proteins. In addition, mature eosinophils retain the ability to synthesize a diverse array of proteins including cytokines and chemokines,71,72 adhesion molecules,73,74,75,76 receptors for cytokines, complement components, lipid mediators, and immunoglobulins.77,78,79,80,81,82