Gregory P. DeMuri, Ellen R. Wald
Sinusitis
Sinusitis is defined as an inflammatory disorder of the paranasal sinuses. This condition is one of the most common reasons for a patient to seek care from a primary care physician and is responsible for more than 20 million antibiotic prescriptions per year in the United States.1 Sinusitis may be classified as acute or chronic. Because most cases of acute bacterial sinusitis are secondary to viral upper respiratory infection (URI) or allergic inflammation, nasal inflammation is a common finding. Some experts refer to this complex as rhinosinusitis. However, in these instances of bacterial superinfection of the paranasal sinuses, the nose is merely a conduit for the secretions originating in the sinuses. The preferred term is acute community-acquired bacterial sinusitis.2
Evidence of maxillary sinusitis has been found in human archeologic specimens discovered in Africa, North America, and Europe.3 Hippocrates recognized the association between high arched palate, nasal obstruction, headache, and discharging ears, likely rhinosinusitis associated with otitis media. Medieval physicians believed nasal discharge emanated from fluid at the base of the brain. In fact, the pituitary gland is named from the Latin word for slime or mucus because it was believed that the source of yellow discharge from the nose came from the hypophysis. The first accurate description of the paranasal sinuses was by Vesalius in the 16th century, and the first documented cases of suppurative sinusitis by Antonio Molinetti in Venice in 1697.4 The unique and troublesome drainage of the maxillary sinus was recognized by William Cowper, who in 1707 eloquently described how “the antra have small openings situated high up in the cavity so that peccant humors could not escape into the nose unless the antrum were full to the top or the head held to one side.”4 Early treatment of sinusitis was mainly surgical, often involving either puncture of the sinus with a trocar or the removal of a molar with drainage through alveolar bone (Fig. 63-1).
Anatomy and Physiology of the Paranasal Sinuses
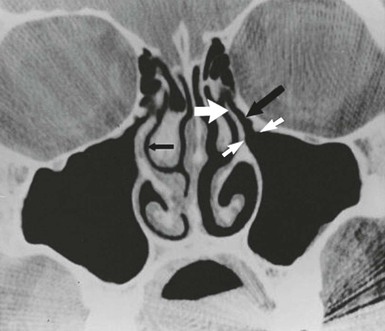
Infections of the paranasal sinuses are a direct consequence of their unique anatomy and physiology. An appreciation of the anatomic relationships of the sinuses is necessary to understand the pathogenesis and complications of sinusitis. The paranasal sinus cavities consist of the maxillary, ethmoid, frontal, and sphenoid sinuses. The maxillary and ethmoid sinuses develop during the third month of gestation and thus are present, albeit small, at birth.5 The maxillary sinus originates as a slitlike cavity parallel to the middle turbinate and elongates into a pyramidal shape with a final volume of 15 to 30 mL in the adult. The floor of the maxillary sinus lies over the alveolar ridge of the maxilla, which holds the upper dentition. The walls of the maxillary sinus extend from the lateral wall of the nasal cavity to the zygomatic arch, and the roof of the sinus is the floor of the orbit. The proximal location of the paranasal sinuses to structures such as the teeth (maxillary sinus) and eye (ethmoid and frontal sinuses) explains many of the clinical manifestations in the affected patient. The outflow tract of the maxillary sinus is located at the highest part of the medial wall of the sinus where it opens into the nasal cavity. This unfortunate positioning inhibits gravitational drainage of secretions and requires an intact mucociliary apparatus to move secretions and debris from the body of the sinus to the nose, thereby predisposing to infection. The ostium of the sinus empties via the hiatus semilunaris into the nasal cavity via a 7- to 11-mm-long passage called the infundibulum, which drains into the middle meatus.6 The anterior ethmoid and frontal sinuses also empty into this same location, known as the osteomeatal complex (Fig. 63-2). The ethmoid sinuses are a complex group of 5 to 15 tiny air cells separated from one another by thin bony partitions. The lamina papyracea, named for its paper-like thinness, comprises the medial wall of the orbit (lateral wall of the ethmoid) and provides a minimal barrier for spread of infection from the sinuses to the orbit. The ethmoid sinuses are divided into anterior and posterior cells. The larger anterior group empties into the middle meatus, and the posterior cells empty into the superior meatus. The frontal sinuses develop from an anterior ethmoid cell and are present above the orbital ridge by the fifth or sixth birthday. Up to 5% of individuals lack one or both frontal sinuses.7 The posterior wall of the frontal sinus is the anterior wall of the cranial fossa, and the floor of the frontal sinus forms the roof of the orbit. The frontal sinuses drain into the middle meatus via the frontonasal duct. The location of the frontal sinuses allows for the easy and rapid spread of infection from the sinus cavity to the central nervous system and/or the orbits. The sphenoid sinuses are located just anterior to the pituitary fossa and are surrounded by several vital structures including the optic nerve, internal carotid arteries, and cavernous sinuses. The sphenoid sinus drains via a narrow duct into the superior nasal meatus. The sphenoid sinuses are infrequently the only site of infection; rather, they accompany a pansinusitis. The sphenoid sinus, like the frontal sinus, is a source for the spread of infection from the sinuses to the central nervous system.
The paranasal sinuses are lined with a pseudostratified columnar (respiratory) epithelium, which also lines much of the nasal cavity. This epithelial lining contains four types of cells: basal cells, which adhere to the basement membrane; columnar cells, which possess cilia; goblet cells, which produce mucus to protect and lubricate the epithelial surface; and inflammatory cells. These inflammatory cells consist of T and B lymphocytes, as well as antigen recognition cells.6,7
Mucus and other material produced in the maxillary sinus cavity are transported by ciliary action in a spiral direction up to and through the infundibulum into the middle meatus. These cilia beat at a frequency of 1000 times per minute and move material at a rate of 3 to 25 mm per minute.8 The mucus blanket in the sinus turns over two to three times per hour, and mucus normally does not accumulate in the sinus cavity.9
The paranasal sinuses are sterile under normal conditions, unlike the nasal passages, which are heavily colonized with bacteria.10–12 Sterility is maintained in the sinus by mechanisms that are not fully understood but are believed to include mucociliary clearance, the immune response, and possibly antibacterial concentrations of nitric acid in the sinus cavity.13 Nasal secretions contain the immunoglobulins IgA, IgG, IgM, and IgE; enzymes such as lysozymes; and proteins such as lactoferrin and complement, all of which exert an antibacterial effect.14
Although the exact function of the paranasal sinuses in humans is unknown, multiple roles have been suggested. These include contributing to the resonance of the voice, warming and humidifying inspired air, and acting as a shock absorber for the brain by absorbing energy during trauma.6
Pathogenesis
The pathogenesis of rhinosinusitis involves three key elements: the patency of the sinus ostia, the function of the ciliary apparatus, and the character of sinus secretions. The narrow caliber of the sinus ostia sets the stage for obstruction to occur. Factors that predispose the ostia to obstruction include those that result in mucosal swelling and those that cause direct mechanical obstruction. Table 63-1 lists the most common factors that predispose to ostial obstruction. Of these multiple causes, viral infection of the upper respiratory tract and allergic inflammation are the most frequent and most important. During episodes of acute rhinitis, a completely patent ostia is present only 20% of the time.15
TABLE 63-1
Factors That Predispose to Sinus Ostial Obstruction
| MUCOSAL SWELLING | MECHANICAL OBSTRUCTION |
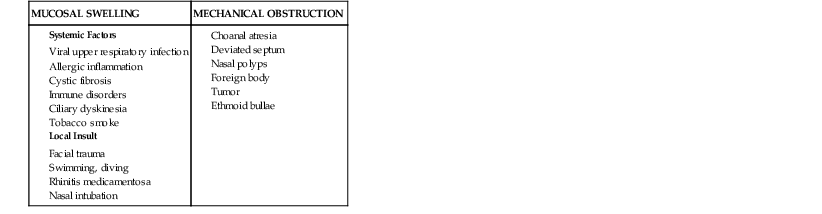
When obstruction of the sinus ostium occurs, there is a transient increase in pressure within the sinus cavity. As oxygen is depleted in this closed space, the pressure in the sinus becomes negative relative to atmospheric pressure. This negative pressure may allow the introduction of nasal or nasopharyngeal bacteria into the sinuses during sniffing or nose blowing.16 When obstruction of the sinus ostia occurs, secretion of mucus by the mucosa continues, resulting in accumulation of fluid in the sinus. A study of adult volunteers investigated the role of nose blowing in introducing nasal fluid and thus possibly microbes into the sinus cavities.17 Serial computed tomography (CT) scans showed that up to 1 mL of viscous fluid was propelled into the sinus when volunteers blew their noses. This would serve as one potential mechanism for nasal fluid and flora to contaminate the sinuses, particularly during a common cold. It should be noted, however, that young children who do not blow their noses still develop acute bacterial sinusitis. Thus, there must be multiple factors that play a role in the development of acute infection.
Dysfunction of the mucociliary apparatus also contributes to the pathogenesis of sinusitis. During viral colds, both the structure and function of the mucociliary apparatus are impaired. In a study of children with viral URI, subjects had nasal mucosal biopsies performed for examination of the ultrastructure of the cilia. Dysmorphic ciliary forms involving microtubular abnormalities were observed during the acute phase (7 days) of illness. Progressive loss of ciliated cells was noted throughout the illness in a patchy pattern.18 In a study of documented viral URIs in adults, mucociliary clearance was measured using a solution of blue-colored saccharin. Mucociliary clearance times, measured by taste and color, were significantly slower during the acute phase of illness.19 Presumably, these same changes in structure and function of the nasal mucosa during viral URI occur in the sinus mucosa. This contributes to the reduced clearance of fluid and material, which increases the likelihood of infection of the sinus cavity.
The quality and character of sinus secretions also plays a role in the pathogenesis of sinusitis. Cilia can only beat in a fluid media. The mucus blanket in the respiratory tract consists of two layers. The sol phase is a thin, low-viscosity layer that envelops the shaft of the cilia and allows the cilia to beat freely. A more viscous layer, the gel phase rides on the sol phase. Alterations in the mucus layer, which occur in patients with cystic fibrosis or allergy, may impair ciliary function. The presence of inflammatory debris, which is found in an infected sinus, may further impair ciliary movement.
Historically it has been stated that a reduction in airflow through the nasal passages contributes to the development of rhinosinusitis. An extensive review of this hypothesis, however, found no convincing evidence that diminished airflow is a factor in sinus pathology.20
Except in experimental animal models, the histologic findings during acute sinusitis had not been well characterized until recently. In a rabbit model of acute sinusitis, histologic changes include epithelial desquamation, edema, and goblet cell hyperplasia. Of note is the distinct loss of ciliated cells from the epithelium.21,22 Berger examined biopsies of 11 humans who had acute sinusitis.23 Surprisingly, the epithelial layer of the sinus remained intact. In contrast, the lamina propria showed edema and massive infiltration of neutrophils and mononuclear cells including lymphocytes and plasma cells. Occasionally, aggregates of inflammatory cells with microabscesses were also detected. Thrombosed blood vessels and deep necrotic foci were observed in patients with complications of acute sinusitis. Immunohistologic staining showed T lymphocytes scattered throughout the lamina propria with dense aggregates of B lymphocytes. An analysis of cytokine production in sinusitis has shown that IL-8, a potent chemoattractant for neutrophils, is upregulated in the sinus during acute infection.24
In patients with acute sinusitis, healing of the mucosa occurs over a period of weeks after infection. In a study in which serial magnetic resonance images were performed in patients with acute bacterial sinusitis, clinical symptoms resolved within 3 days of treatment in most patients. Radiographic changes took much longer, however, to show improvement with only half of the sinuses showing resolution of opacification by 10 days. It took up to 56 days for 80% of the sinuses to be aerated.25
Microbiology
Knowledge of the microbiology of acute community-acquired sinusitis is essential in choosing appropriate antimicrobial therapy. Studies performed to examine this issue require close attention to the method of specimen collection. The nasal cavity is heavily colonized with respiratory flora, which may easily contaminate material obtained from the paranasal sinuses. Classic studies of the bacteriology of sinusitis have obtained a specimen of sinus secretions by puncture of the maxillary antrum to reduce the risk of nasal contamination. In this method, the maxillary sinus is accessed by puncture through a transnasal approach. A trocar is placed beneath the inferior nasal turbinate through the lateral nasal wall. Meticulous efforts to sterilize the mucosal area through which the trocar is placed avoid contaminating the specimen with nasal flora. In a further effort to discriminate true infection from contamination, quantitative methods are used to enumerate the density of microbes. Infection is defined as a colony count of at least 104 colony-forming units per milliliter (CFUs/mL) of aspirated material.26
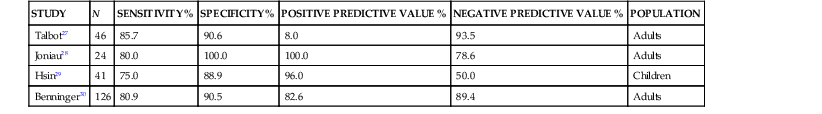
Because of the invasive nature of this procedure, there has been considerable interest in obtaining cultures of sinus material through less invasive means. Cultures obtained via an endoscope have been compared with those obtained by sinus aspiration. In this method, the sample is obtained from the middle meatus adjacent to the sinus ostia via swab or aspiration through the endoscope. Because the endoscope is passed through the nonsterile anterior nasal cavity, the potential for contamination is great. Many studies correlating sinus puncture with middle meatal culture attempt to improve the results of cultures obtained endoscopically by analyzing data only for Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis while dismissing other bacteria. This may ignore potential pathogens such as β-hemolytic streptococci and anaerobes. Most studies of the performance of endoscopic middle meatal culture in adult patients show a sensitivity of approximately 80%, specificity of 90%, positive predictive value of 80% to 90%, and negative predictive value of 80% to 90% with maxillary sinus aspiration considered the gold standard (Table 63-2).27–30 It should be noted, however, that the sensitivity and specificity of cultures obtained by endoscope are much lower in children. This is likely due to the smaller nasal cavity and more difficult technical aspects of the procedure.29 Furthermore, middle meatal cultures obtained from a group of normal children without respiratory symptoms frequently showed the presence of the sinus pathogens, S. pneumoniae and H. influenzae.31 Accordingly, sinus puncture remains the gold standard in investigating the microbiology of sinusitis in children.
TABLE 63-2
Comparison of Endoscopic Middle Meatal Culture vs. Sinus Puncture in Acute Sinusitis
| STUDY | N | SENSITIVITY % | SPECIFICITY % | POSITIVE PREDICTIVE VALUE % | NEGATIVE PREDICTIVE VALUE % | POPULATION |
| Talbot27 | 46 | 85.7 | 90.6 | 8.0 | 93.5 | Adults |
| Joniau28 | 24 | 80.0 | 100.0 | 100.0 | 78.6 | Adults |
| Hsin29 | 41 | 75.0 | 88.9 | 96.0 | 50.0 | Children |
| Benninger30 | 126 | 80.9 | 90.5 | 82.6 | 89.4 | Adults |
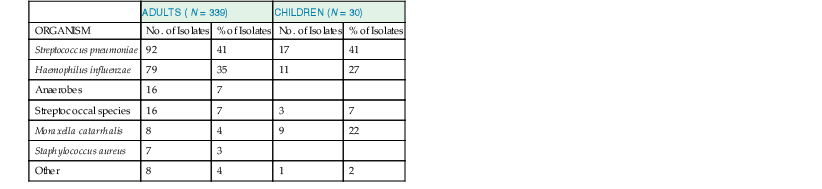
In studies of acute community-acquired bacterial sinusitis in which sinus puncture has been performed with attention to reducing contamination and analyzing results quantitatively, S. pneumoniae is the most frequently isolated organism, followed by nontypeable H. influenzae and M. catarrhalis. Streptococci, Staphylococcus aureus, and anaerobes are isolated much less frequently (Table 63-3). In children with acute bacterial sinusitis, M. catarrhalis is isolated with a greater frequency than in adults.
The predominance of pneumococci, Haemophilus, and Moraxella as pathogens in cases of acute sinusitis in children has not changed in more than 50 years.12,32,33–38 However, the relative role of pneumococci has diminished since the introduction of universal infant immunization with pneumococcal conjugate vaccine and the serotypes have shifted.39 This has not been confirmed by direct sinus aspirates, which are rarely performed in children with uncomplicated acute sinusitis. Rather, inferential data may be obtained by performance of tympanocentesis in children with acute otitis media. The similarity in pathogenesis of acute otitis media and acute bacterial sinusitis in children permits this inference to be made. The middle ear is, after all, a paranasal sinus.40
Accompanying the decrease in the isolation of S. pneumoniae has been an increase in the rate of isolation of nontypeable H. influenzae from middle ear isolates. The most recent study from a center performing tympanocentesis in the United States demonstrates a dramatic decrease in the recovery of S. pneumoniae. In contrast, H. influenzae and M. catarrhalis have increased in frequency to account for up to 90% of isolates. Surprisingly, the prevalence of β-lactamase-producing organisms is higher than expected, in the range of 70% to 80%.41 Studies of nasopharyngeal colonization have demonstrated a similar decrease in carriage of pneumococcal serotypes contained in the 13-valent pneumococcal conjugate vaccine.42 These shifts in microbiology have important implications for the selection of appropriate antimicrobial agents for the treatment of sinusitis.
The role of S. aureus in the etiology of sinusitis has been controversial. Some authors have purported that this organism should be considered a major pathogen of the sinuses. This would have important implications for antimicrobial selection because most guidelines do not recommend agents that are directed at this organism.30,43,44 However, when examined carefully, the role of S. aureus as an etiologic agent of acute bacterial sinusitis is doubtful.45 In sinus aspiration studies of adults done over a 15-year period, S. aureus was detected in only 7 of 226 (3%) positive specimens obtained from 339 patients.36 In the two studies in which maxillary aspirates were performed in 50 children with acute bacterial sinusitis, no isolates of staphylococci were detected.33,46 Most studies that argue that S. aureus is a pathogen in this setting are based on middle meatal cultures. To critically analyze these studies, it is imperative to understand the microbiology of the nose and middle meatus in healthy individuals. The nasal vestibule is a location that is frequently heavily colonized with S. aureus. In a study of healthy children in a community setting, S. aureus was detected in more than 65% of children from the anterior nares.47 Any study of the microbiology of the middle meatus will involve passage of the endoscope through this highly colonized region. Even with antiseptic measures, contamination of the specimen is possible. Studies of the middle meatus have also shown similarly high levels of detection of S. aureus. Gordts and colleagues48 performed middle meatal cultures on healthy children undergoing surgery for reasons unrelated to the sinuses. S. aureus was found in 20% of children, indicating that this region is also frequently colonized.48
Thus, caution must be exercised in interpreting studies that highlight the role of S. aureus as a major pathogen in acute bacterial sinusitis because there is serious concern that these studies have a high rate of contamination of specimens with normal nasal flora. Nonetheless, although S. aureus is an infrequent cause of acute bacterial sinusitis in children, it is a frequent etiologic agent when complications (intracranial and/or orbital) occur. The reason for this discrepancy is unknown.
Despite their prominent role in the pathogenesis of acute community-acquired bacterial sinusitis, viruses have been infrequently isolated from patients with acute sinusitis. This may relate, at least in part, to the timing of sinus aspiration, which is usually done when the patient has been symptomatic for at least 7 to 10 days, by which time the viral infection may be waning. Respiratory viruses such as adenovirus, parainfluenza, and rhinovirus have been recovered from approximately 10% of sinus aspirates.12,33 Approximately 30% to 40% of sinus aspirates in patients with acute sinusitis do not yield bacteria. It is presumed that many of these infections are, in fact, viral. However, a detailed analysis using modern molecular techniques, such as polymerase chain reaction, to establish a viral etiology is lacking.
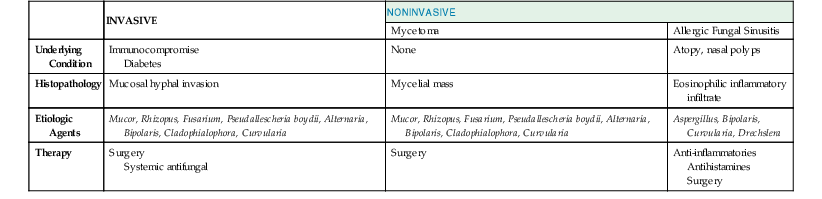
Fungi have long been recognized as important pathogens in selected patients with either acute or chronic sinusitis. Fungal sinusitis may take one of three forms (Table 63-4). Invasive fungal sinusitis is a fulminant disease often with dissemination. Most cases occur in patients with serious underlying diseases, such as diabetes mellitus, malignancy and associated neutropenia, or those using high-dose corticosteroids. Local invasion of the sinus cavity and surrounding structures of the skull occurs and is often accompanied by systemic fungal infection. The second form is mycetoma or fungus ball, which typically presents in the maxillary or sphenoid sinuses. A dense accumulation of hyphae is present, and local invasion may occur but dissemination is rare. Allergic fungal sinusitis occurs in patients who are immunocompetent but atopic and is the third form of fungal infection. An exuberant immune response to fungal spores mediated by IgE results in mucinous material collecting in the sinus. Local invasion is not present, but a mucoid inflammatory infiltrate fills the sinus cavity. The causal agents in invasive fungal sinusitis are the filamentous fungi including Aspergillus, Mucor, Rhizopus, Fusarium, Pseudallescheria boydii, and the dematiaceous fungi such as Alternaria, Bipolaris, Cladophialophora, and Curvularia spp.49,50–56 Aspergillus, Bipolaris, Curvularia, and Drechslera have been associated with allergic fungal sinus disease. Sinus zygomycosis refers to infection with members of the phylum Zygomycota, of which Mucor and Rhizopus spp. are the most common.
Nosocomial sinusitis is a relatively frequent complication of medical care, particularly in the critical care setting. The cumulative incidence of sinusitis in critically ill patients has been estimated to be 7.7% to 32%.57,58 Nasal intubation has long been recognized as a risk factor for this infection. In addition, the use of a nasal-enteric tube increases the risk of sinusitis. A prospective study found an incidence rate of 15.7 cases of sinusitis per 1000 patient days in patients with nasoenteric tubes versus 1.6 cases per 1000 patient days in patients without such tubes.58 It is hypothesized that the presence of a tube in the nose irritates the nasal mucosa, resulting in inflammation, edema, and subsequent obstruction of the sinus ostia. Other risk factors include nasal colonization with gram-negative enteric bacilli, sedative use, and a Glasgow coma score below 8. S. aureus, gram-negative organisms including Pseudomonas, and S. pneumoniae are frequently isolated in patients with nosocomial sinusitis (Table 63-5). Ventilator-associated sinusitis has been described in critically ill patients and may be a cause of unexplained fever. Nasotracheal intubation increases the risk for this complication.59
TABLE 63-5
Microorganisms Associated with Nosocomial Sinusitis Based on Sinus Puncture
| MICROORGANISM | % OF ISOLATES |
| Pseudomonas spp. | 10.7 |
| Escherichia coli | 5.9 |
| Proteus mirabilis | 5.9 |
| Klebsiella spp. | 7.2 |
| Enterobacter spp. | 7.2 |
| Other gram negatives | 8.4 |
| Staphylococcus aureus | 9.5 |
| Viridans streptococci | 8.3 |
| Streptococcus pneumoniae | 2.4 |
| Other gram positives* | 22.7 |
| Anaerobic bacteria | 3.6 |
| Candida spp. | 8.4 |
| Total | 100 |
* Organisms of low pathogenicity such as coagulase-negative Staphylococci and Corynebacterium spp.
From Robinson MR, Fine HF, Ross ML, et al. Sino-orbital-cerebral aspergillosis in immunocompromised pediatric patients. Pediatr Infect Dis J. 2000;19:1197-1203.
Chronic Sinusitis
Chronic rhinosinusitis, which is often referred to as chronic sinus disease, is defined as symptoms and signs of sinus inflammation that persist for at least 12 weeks. Despite the prevalence of this disorder in the general population, its pathogenesis is incompletely understood. However, many of the same factors that play a role in acute bacterial sinusitis are found in chronic sinus disease, namely obstruction of the sinus ostia, mucociliary impairment, and thickening of secretions. In addition, the risk factors for chronic disease are similar to those of acute sinusitis (see Table 63-1).60 The histopathology of the sinus in biopsies of patients with chronic sinusitis demonstrates an infiltration of T and B lymphocytes, macrophages, and eosinophils into the mucosa.61 The microbiology of chronic sinusitis has been studied extensively, but the exact role of microorganisms is unclear. The same bacterial species (S. pneumoniae and H. influenzae) that are found in acute sinus disease are occasionally found in chronic sinusitis, especially in patients with acute exacerbations of chronic sinusitis. Other bacteria such as S. aureus, gram-negative enteric organisms, and anaerobes have been isolated with a greater frequency in sinus puncture studies.62–66 Despite the isolation of such bacteria, there has been doubt in the medical literature as to their pathogenic role.36,67 It is possible that in chronic sinusitis, mucociliary clearance and host defenses are impaired to the point that the sinus cavity loses its normal sterility and becomes colonized with nasal flora. Thus, chronic sinusitis may not truly be an “infectious process” but an aberration of the normal anatomy responsible for drainage and damage to the mucosa of the sinus cavity.67 The frequent isolation of bacteria of low pathogenicity such as Corynebacterium species and coagulase-negative Staphylococci, as well as the unsatisfactory response to antimicrobials, support this hypothesis.
Biofilms, in which bacteria form specialized communities of microorganisms encased in complex extracellular polymeric substances, have been found in many patients with chronic rhinosinusitis and may play a role in chronic inflammation and in exacerbations, although their exact role is not defined.68 Biofilms offer important survival advantages to bacteria. They are more resistant to the effects of antibiotics than free-floating planktonic bacteria. This is accomplished by several mechanisms: (1) greater cell-cell contact to facilitate plasmid exchange for the evolution of resistance, (2) production of β-lactamases, (3) slow bacterial growth resulting in decreased effectiveness of antibiotics that rely on cell growth and turnover for killing, and (4) the presence of “persister” cells that re-form the biofilm when the antibiotic is discontinued.69 Although biofilms have been demonstrated on the mucosa of patients with chronic sinusitis, their precise role remains to be determined because they are not present in all cases of chronic rhinosinusitis and limited biofilms are present in some healthy controls.
In addition, it is also important to mention that although most cases of chronic sinusitis are not thought to be an “infectious process,” when patients with chronic sinusitis develop central nervous system complications, these are often caused by S. aureus and respiratory anaerobes.
Recently there has been intense interest in the role of fungi in the pathogenesis of chronic sinus disease. One study reported the isolation of multiple species of fungi in 95% of patients with chronic sinusitis. However, similar rates of isolation of fungi were present in control patients.70 It has been hypothesized that chronic rhinosinusitis may be the result of the allergic response to fungi present in the sinus cavity. Immunotherapy in these patients has been disappointing, as have been trials of topical or systemic antifungal agents.71,72
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree