Abstract
It has been demonstrated that as a result of breast cancer and its treatment, survivors of the disease experience a high prevalence of functional impairments. Survivors may experience short-term, long-term or late effects causing impairment, which often leads to difficulties with daily living. Cancer rehabilitation focuses on the restoration of maximum ability in survivors and can be implemented at any point in the disease continuum. While working with the oncology team, a physiatrist-led rehabilitation team has the potential to prevent significant decline in function and improve quality of life of breast cancer survivors.
Keywords
cancer rehabilitation, physiatry, cancer-related fatigue, cardiopulmonary fitness, neuropathy
According to the National Cancer Institute and National Coalition for Cancer Survivorship, an individual is considered a cancer survivor from the time of cancer diagnosis through the balance of his or her life. With improved diagnostics and treatment plans, the “balance of life” has been steadily increasing with an estimated 2.975 million female breast cancer survivors in the United States in 2012. As a result of breast cancer and its treatment, survivors often develop impairments. Impairments may be classified as short-term when occurring during treatment, long-term when occurring at the time of diagnosis or treatment and then persisting, or as late effects that may manifest after treatment has completed or as currently unrecognized toxicities. A survivor’s experiences are unique and involve factors that are disease-specific (cancer type, location, presence of metastases), treatment-specific (surgery, chemotherapy, radiation, endocrine therapy), and individual-specific (precancer medical and functional status, precancer psychosocial status). Consequently, breast cancer survivors often do not feel quite the same as they have before diagnosis and may experience a decline or loss of function, impeding their ability to perform job-related, recreational, and other daily activities.
Cancer rehabilitation can be defined as any evaluation or intervention assisting in restoration of maximum function and ability in any survivor with cancer at any point in the disease continuum. The rehabilitation team is best led by a physiatrist, who is a specialist in physical medicine and rehabilitation (PM&R) and responsible for patient’s overall functional health. Although there is no formal subspecialty of cancer rehabilitation, there are physiatrists that focus their clinical and research interests specifically on this population, whereas many others often have experience working with cancer survivors. Other members of the rehabilitation team include physical therapists, occupational therapists, speech language pathologists, exercise physiologists, and rehabilitation nurses. Each member of the rehabilitation team brings a unique skill set and training background that survivors may benefit from. Not unlike an oncology team, individualized care should be developed based on an individual survivor’s current and future potential impairments.
Multiple studies have demonstrated a high prevalence of physical impairments amenable to rehabilitation in the breast cancer populations. Thus it is clear that cancer rehabilitation should be an important component of a survivor’s care plan. However, this is not always the reality. This chapter highlights the need for rehabilitation care to be involved in the care breast cancer survivors’ care from the time of diagnosis throughout the balance of his or her life.
Deconditioning
Deconditioning can be defined as multiple, potentially reversible changes in body systems brought about by physical inactivity and disuse. It is a cumulative multifactorial phenomenon, resulting in functional decline in multiple body systems. It is not an “all or nothing” decline but occurs across a spectrum depending on length and frequency of inactivity. It is important to understand that the changes that occur due to deconditioning are separate from any change due to other underlying medical or surgical issues. Breast cancer survivors often have a decrease in their activity level before, during, and after treatment secondary to the physical, psychological, and social stressors on their lives. It is important for all clinicians to be aware of potential effects that may occur due to those activity changes. This section reviews the major changes seen in the musculoskeletal and cardiovascular systems, and Box 83.1 highlights some of the other effects that can be seen throughout the body.
Increase risk of deep vein thrombosis
Impaired balance and coordination
Perceptual impairment
Restlessness
Decreased pain tolerance
Sleep disturbance
Insulin resistance
Decreased bone density
Urinary retention/incomplete bladder emptying
Decreased diaphragmatic movement with decreased strength and endurance of intercostal, axillary respiratory muscles leading to atelectasis, hypostatic pneumonia
Reflux esophagitis
Decreased peristalsis/constipation
Musculoskeletal System
With strict bedrest, skeletal muscle strength declines by 1% to 1.5% per day and with cast immobilization, up to 1.3% to 5.5% decline in strength per day can be seen. The loss of strength is noted to be greatest within the first week of immobilization, decreasing up to 40%.
Muscle atrophy is another complication with the reduction of muscle protein synthesis and whole body protein production as likely main contributors. Type 1 muscle fibers are affected more than type 2 muscle fibers, and the lower limbs are affected more that upper limbs. There is evidence that these processes can be minimized with activity, including as little as resisted leg exercise greater than 50% of maximum effort every second day. A potential treatment target is the inhibition of myostatin, which is a growth factor-beta protein that inhibits muscle synthesis and known to increase during bed rest. Although there are no current available treatments for humans, there are ongoing animal studies.
It is also important to be aware of sarcopenia, which is an age-related loss of muscle mass and strength. Despite society’s acceptance of loss of muscle mass and strength with age, sarcopenia has been shown to be reversible with high-intensity resistive exercise.
Cardiovascular System
With bedrest, immobilization tachycardia can occur, and with 3 weeks of bed rest, it has been shown that resting heart rate can increase 10 to 12 beats per minute. After just 24 hours of bed rest, plasma volume can decrease with a correlated drop in cardiac output and stroke volume. With these changes, and additionally a blunted sympathetic response with limited vasoconstriction, orthostasis can occur. The body’s normal response from rising up from supine may be completely lost after 3 weeks and make take weeks to months for recovery.
Cardiopulmonary Fitness
Cardiopulmonary fitness, which is a key predictor for mortality in all populations, can be measured in metabolic equivalents (METs) or maximal oxygen consumption (VO 2max = mL⋅kg −1 ⋅min −1 , 1 MET = 3.5 mL O 2 ⋅kg −1 ⋅min −1 ). In part, due to the effects already discussed, deconditioning causes a significant reduction of cardiopulmonary fitness. With 20 days of bed rest, VO 2max can decline by 27%. However, this may be counteracted with even low levels of physical activity (see Box 83.1 ).
Fatigue
Cancer-related fatigue (CRF) is one of the most common and concerning symptoms for breast cancer survivors during treatment, but often persists in disease-free survivors. CRF is defined by the National Comprehensive Cancer Network (NCCN) as a persistent, subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning. CRF may clinically present as sensory, physical, affective, and/or behavioral complaints with fatigue often further classified as peripheral or central in nature depending on a survivor’s symptoms. This multidimensional nature is evident in the International Classification of Diseases, 10th Revision (ICD-10) definition of CRF ( Box 83.2 ).
The following symptoms have been present every day or nearly every day during the same 2-week period in the past month:
- A.
Significant fatigue, diminished energy, or increased need to rest, disproportionate to any recent change in activity level, plus five or more of the following:
- 1.
Complaints of generalized weakness, limb heaviness
- 2.
Diminished concentration or attention
- 3.
Decreased motivation or interest to engage in usual activities
- 4.
Insomnia or hypersomnia
- 5.
Experience of sleep as unrefreshing or nonrestorative
- 6.
Perceived need to struggle to overcome inactivity
- 7.
Marked emotional reactivity to feeling fatigued
- 8.
Difficulty completing daily tasks attributed to feeling fatigued
- 9.
Perceived problems with short-term memory
- 10.
Postexertional fatigue lasting several hours
- 1.
- B.
The symptoms cause clinically significant distress or impairment in social, occupational, or other important areas of functioning
- C.
There is evidence from the history, physical examination, or laboratory findings that the symptoms are a consequence of cancer or cancer therapy
- D.
The symptoms are not primarily a consequence of comorbid psychiatric disorders such as major depression, somatization disorder, or delirium
ICD-10, International Classification of Diseases, 10th Revision.
The pathophysiology of CRF is not well understood. Recent research has begun to connect fatigue symptoms with inflammation and inflammatory pathways. It is still not clear what triggers the initial inflammatory process, although it is suspected to be related to the underlying malignancy and the various oncologic treatment interventions stimulating a neuroendocrine, immune, and/or biopsychological response. Studies have demonstrated increased inflammatory biomarkers in breast cancer survivors with fatigue initially posttreatment and persisting years after treatment has been completed.
Screening and Diagnosis
According to NCCN guidelines, the recommendation for standard of care includes all patients being screened for fatigue at the initial visit as well as regularly during and after cancer treatment with the use of interdisciplinary teams for management. Although there is an ICD-10 criterion for the diagnosis, there is no clear objective assessment tool. However, CRF can be evaluated and monitored by using one of the available patient reported outcome measures, such as Patient-Reported Outcomes Measurement Information System (PROMIS) Fatigue Short Forms or Brief Fatigue Inventory (BFI).
Treatment
Breast cancer survivors may also have other numerous potential contributing etiologies for CRF ( Box 83.3 ). Many of the underlying factors are treatable, including anemia, endocrine dysfunction, sleep disturbances, poor nutrition/hydration, pain, mood disturbances, and deconditioning. Beyond addressing these factors, pharmacologic interventions include the use of stimulants, such as methylphenidate and modafinil, although currently evidence is limited to survivors undergoing active treatment or with advanced disease. Cytokine antagonists, which disrupt initiation and continuation of the suspected underlying proinflammatory pathways, have also shown promise in improving CRF in cancer survivors, although data are quite limited thus far. There is better evidence that exercise interventions can improve CRF. In addition, exercise has well-accepted benefits on muscle strength, cardiopulmonary fitness, aerobic capacity, quality of sleep, pain, and mood disturbances. Studies also support psychological interventions, such as a cognitive behavioral therapy and relaxation therapy, as well as education. However, these positive research findings are limited in widespread applicability due to study population and intervention variability.
Malignancy
Cancer treatment
Medications
Cardiac dysfunction
Pulmonary impairment
Anemia
Neutropenia
Hormone deficiency or excess
Hypothyroidism
Dehydration
Nutritional deficiencies
Deconditioning
Cachexia
Mood disturbance
Sleep disturbances
Pain
In summary, breast cancer survivors with CRF should be evaluated for physical activity, rehabilitation, and psychological interventions as well as have treatable contributing factors and concurrent symptoms addressed.
Upper Quadrant Dysfunction
Breast cancer survivors are at heightened risk to develop upper quadrant (neck, upper thorax, axilla, and arm) dysfunction. This dysfunction can manifest during the treatment period and may persist for months to years. Studies have reported upper extremity pain and limited upper quadrant flexibility and strength at up to 10 years posttreatment completion. Upper extremity impairment and pain suggests an underlying musculoskeletal or neuromuscular disorder. Cited postsurgical effects include axillary web syndrome, lymphedema, postmastectomy pain syndrome, paresthesias, and decreased glenohumeral range of motion (ROM) due to rotator cuff disease or shoulder impingement syndrome. There is a significant association among pain, disability, and scapulothoracic dysfunction. Left unaddressed, these conditions reduce function; impede one’s ability to participate in daily activities and to reintegrate fully into the community. The following is a summary of upper extremity biomechanics, observed treatment-related effects, the impact on daily life, and methods to identify individuals with upper extremity limitations.
Pretreatment Upper Quadrant Function
Musculoskeletal disorders of the neck and shoulder are known to occur with age. Preoperative upper extremity ROM, level of activity, and pain may be predictors of long-term functional outcome.
The incidence of preoperative impairments such as mobility and pain varies. One study revealed that 40% of more than 2000 breast cancer survivors with shoulder symptoms at 5-year follow-up postsurgery and postradiation had also reported symptoms at baseline. Additionally, it has been demonstrated that survivors with pectoralis tightness either at preoperative baseline or 3 months after had a higher prevalence of rotator cuff disease at 1 year posttreatment. Because these survivors may be more susceptible to further injury after surgery and radiation therapy, appropriate screening before surgical intervention could prevent further decline.
Biomechanics: Range of Motion, Scapular Control, Muscle Strength
Breast cancer survivors often exhibit reduced shoulder flexion, shoulder abduction, and lateral rotation. Some have reported up to 68 degrees of decreased ROM reported in individuals posttreatment. A review of more than 5000 breast cancer survivors demonstrated decreased abduction, forward flexion at 1 month postsurgery and decreased abduction, internal rotation, and strength at 2 years. Furthermore, the limited mobility may persist for more than 5 years in 20% to 80% of survivors. Even in survivors with intact range of motion, a thorough strength assessment often reveals weakness with dynamic movement.
The preceding findings suggest that cancer and its treatment alter shoulder mechanics. Three-dimensional motion analysis has revealed significant asymmetry in humeral movement and differences in the anterior and posterior tilt between the treated and the normal side. Changes in muscle size and recruitment likely contribute to this reduced scapular stability. Dynamic studies have shown an ipsilateral weakened and smaller pectoralis minor muscle as well as decreased recruitment of the upper trapezius, rhomboid and serratus anterior. The pectoral shortening can cause anterior posturing. Interestingly, the decreased activity of upper trapezius and rhomboids suggests altered muscle activity in areas indirect to the treatment field. Weakened, stiffened shoulder girdle muscles are associated with increased susceptibility to rotator cuff injury, winged scapula, brachial plexopathy and axillary web syndrome and dropped shoulder syndrome. As already described, the weakened shoulder stabilizing muscles affect upper extremity function.
Correlation of Surgical Intervention and Upper Quadrant Dysfunction
Proposed causative factors for altered upper quadrant biomechanics include the type of surgical procedure, use of radiation therapy and decreased use of the treated side. Depending on the degree of tissue removal, the resulting asymmetry in mass alters scapulohumeral movement as previously described. Furthermore, tumor resection or lymph node removal involves interventions proximal to critical neural structures. Axillary nerve injury during the axillary lymph node dissection (ALND) even without nerve dissection can result in symptoms in addition to potentially affecting the nearby thoracodorsal, long thoracic, and intercostal brachial nerves.
The type of surgery affects impairment. In a systematic review, women had a 5.67 times greater odds for shoulder restriction postmastectomy. Additionally, ALND appears to be associated with greater shoulder morbidity than sentinel lymph node biopsy (SLNB). Studies have found that 10% to 80% of participants post ALND demonstrated decreased ROM versus 0% to 41% post SLNB. These individuals also exhibit a higher prevalence of pectoral tightness. Although many improve or return to premorbid function after 6 months to 1 year, a growing number of studies show reduced mobility in the ALND group after 5 to 10 years. Individuals with SLNB typically present with less arm pain swelling, and decreased incidence of paresthesias compared with those post-ALND.
It should be considered that individuals who undergo ALND likely had more extensive disease and may have undergone additional treatments affecting physical health. Regardless, the finding serves as a way to identify individuals at risk and presents an objective way to measure function.
Impact of Impairments on Daily Activities
Many daily activities require shoulder mobility and coordination. Overall, impaired mobility, altered mechanics, and weakness affects activities requiring some force or impact or free arm movement. Decreased upper trapezius and serratus muscle activity is significantly associated with pain while performing activities such as placing an object on a shelf or pushing an object. Individuals acknowledged difficulty also with opening a jar, ironing, and carrying groceries.
Relationship to Physical Activity Level
Pain when using the arm and ROM restriction has been found to be significant predictor of activity level up to years posttreatment. Multiple long-term analyses cite grip strength, elbow strength, and shoulder abduction mobility as predictors of subsequent impairment and function. These predictors persist over a time, with one study citing significance at up to 7 years. Conversely, less physically active individuals before surgery have a propensity to develop shoulder morbidity or limitations. Older, obese, and less educated are also more likely to report limitations ( Fig. 83.1 ).
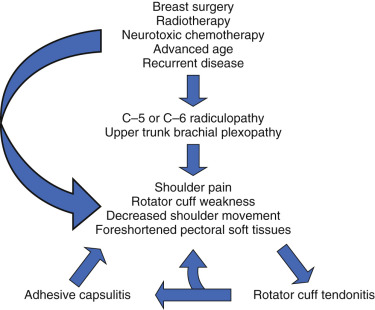
Screening
Upon evaluation of a breast cancer survivor, given the prevalence and associated comorbidities, screening should be performed for upper quadrant dysfunction. The history should attempt to elicit any history for upper quadrant or shoulder injuries and dysfunction. The physical examination should at least include range of motion assessment with shoulder flexion, extension, abduction as well as internal and external rotation, observation for signs of scapular asymmetry or winging at rest and with range of motion activities, grip strength, and assessing for signs of lymphedema or axillary cording. In addition, several validated self-reported questionnaires area available to assess upper quadrant dysfunction in breast cancer survivors. The questions address the ability to carry out daily activities, level of pain, and quality of life ( Box 83.4 ).
American Shoulder and Elbow Surgeons Shoulder Score
DASH (Disabilities of the Arm, Shoulder, and Hand questionnaire)
Kwan’s Arm Problem Scale
Pennsylvania Shoulder Score
Shoulder Disability Questionnaire
Treatment
Treatment for upper quadrant dysfunction depends on the diagnosis. Generally, a multimodality approach is used that includes medications, physical and/or occupational therapy. Evaluation by a physiatrist will guide the individualized treatment plan to restore function. Specific therapy interventions involve soft tissue scar massage, stretching, scapular stabilization and postural exercises, strength training, and myofascial release. Strategies to retrain upper quadrant proprioception improve coordination. For individuals whose pain limits mobility and strength, nonsteroidal antiinflammatory drugs are the recommended first-line agent. For more severe or nonremitting pain, individuals should undergo evaluation for a steroid injection; however, this should be in conjunction with physical therapy. For those with lymphedema, massage, compression wrapping, and special garments help to redirect fluid. Individuals would benefit from a referral to a certified lymphedema specialist.
In summary, upper quadrant dysfunction is a combination of reduced mobility, decreased strength and endurance and altered mechanics. Although some of the impairments may not be caused solely by cancer treatment, it is important to assess for underlying musculoskeletal conditions to determine who may be susceptible to further progression. Because of the effects of upper quadrant limitations on daily activities and physical activity, the interplay of musculoskeletal pathology, function, and quality of life have emerged as key issues in survivorship care.
Neuropathy
The treatment of breast cancer can cause both short- and long-term effects on the peripheral nervous system, leading to pain and loss of function.
Mononeuropathies
Upper extremity mononeuropathies are common in the general population and also affect breast cancer patients, both during and after treatment.
Carpal tunnel syndrome (CTS) is classified by compression of the median nerve at the wrist level. Affected individuals initially present with paresthesias within the median nerve distribution of the palmar surface of the hand; symptoms can progress to include sensory changes and motor weakness. It has been previously proposed that lymphedema related to breast cancer is a risk factor for development of CTS, but a recent retrospective study by Stubblefield and coworkers found that there was no association between presence or severity of lymphedema and CTS.
Other mononeuropathies, such as cubital tunnel syndrome (ulnar nerve entrapment at the elbow) or radial neuropathy can also be seen breast cancer patients but are overall less common.
Radiculopathy
Cervical radiculopathy is a commonly encountered diagnosis in both the general and breast cancer populations, leading to neck and arm pain, as well as arm weakness. Compression occurs most often secondary to herniation of the intervertebral disc, but in breast cancer patients, other etiologies such as instability or tumors should also be considered. Symptomatic radiculopathies can also develop or worsen during chemotherapy treatment.
Patients typically present with neck pain radiating into the upper extremity, with symptoms typically following a dermatomal or myotomal distribution. Weakness is expected but may not be present if only the dorsal nerve root is affected. On physical examination, cervical spine range of motion should be assessed; lateral rotation or bending toward the affected side may reproduce symptoms. Evaluation of strength, sensation, and reflexes will also assist in the identification and localization of affected nerve roots.
Brachial Plexopathy
Brachial plexopathy may be seen in survivors of breast cancer, but is typically not seen at time of initial diagnosis unless disease is already advanced. Brachial plexopathy is frequently seen from either local tumor invasion or as a complication of radiation therapy.
In all cancer survivors, the frequency of neoplastic brachial plexopathy is 0.43%, but in breast cancer survivors, this number can be as high as 1.8% to 4.9%. Tumors have a tendency to invade the lower plexus initially and present with pain (localized to shoulder and axilla with radicular symptoms into the arm/hand). Horner syndrome (ptosis, miosis, and anhidrosis) may also be present.
In breast cancer specifically, radiation therapy (RT) can lead to radiation-induced brachial plexopathy (RIBP), with injury localized to the axillary-supraclavicular region. Delayed progressive RIBP can occur months to decades after treatment. The overall incidence of RIBP has drastically declined with advances in radiation therapy; from 15% of those receiving total dose of 57.75 Gy and 73% of those receiving total dose of 63 Gy, to current incidence of less than 2% of women irradiated for breast cancer.
Survivors typically present with sensory changes such as paresthesias, with eventual progression to hypoesthesia; neuropathic pain is rare. Survivors develop motor weakness, which progressively worsens, and can eventually lead to upper limb paralysis. Skin and muscle atrophy may also occur. The overall prognosis for delayed progressive RIBP is poor.
Less commonly, survivors can develop early transient RIBP within the first year after irradiation. Those with early transient RIBP have better prognosis; 80% will have complete resolution of symptoms. Symptoms are initially similar to delayed progressive RIBP, with distal paresthesias but also with proximal pain. Motor impairments can occur immediately or within a few months and progressively worsen. Symptoms typically regress within 3 to 6 months.
Chemotherapy-Induced Peripheral Neuropathy
Chemotherapy is a frequently used treatment modality in breast cancer, and chemotherapy-induced peripheral neuropathy (CIPN) is a common adverse effect experienced by cancer survivors. CIPN is classified by damage or dysfunction to the peripheral nerves and can include motor, sensory, and autonomic nerves of the upper and lower extremities. Survivors often present with sensory symptoms such as paresthesias, numbness, cold sensitivity, or pain; less commonly, motor symptoms such as weakness may be seen. Several chemotherapy agents have been implicated in the development of CIPN.
Taxanes
Taxanes are an established treatment regimen for both early and metastatic breast cancer but are well known to cause neuropathy; incidence of taxane-associated CIPN ranges from 11% to 64% overall for docetaxel to 57% to 83% for paclitaxel. The mechanism of action of taxanes is to affect microtubules, an essential component of both mitotic spindles and also axonal structures of nerves; axonal damage, therefore, is a common side effect. The solvents used in various taxanes can be an additional source of neurotoxicity; the solvent of paclitaxel, for example, has been shown in preclinical studies to lead to axonal degeneration and demyelination. The neurotoxic effects of taxanes appear to be dose dependent, with higher or more frequent doses associated with higher incidence of neuropathy. Persistent neuropathy can be a dose-limiting factor for treatment of breast cancers with taxanes, in turn affecting survival rates. Taxane-related CIPN is managed with dose delays or reductions; discontinuation is recommended for patients who develop severe neuropathy. Symptoms can present as early as within the first 24 hours of infusion, and may improve or resolve within 3 to 6 months of discontinuation in mild to moderate cases, but more severe cases of neuropathy are less likely to resolve.
Platinum Derivatives
Platinum derivative chemotherapy agents have also been implicated in causing CIPN, primarily because platinum compounds deposit within dorsal root ganglion cells, leading to neurotoxicity. The incidence of CIPN from cisplatin, one platinum derivative, ranges from 10% to 28%. Symptoms include numbness, tingling and paresthesias in the upper and lower extremities as well as ataxia. Platinum-induced CIPN can be associated with worsening neuropathy symptoms after discontinuation of treatment, known as “coasting.”
Vinca Alkaloids
Vinca alkaloids can also lead to CIPN. Similar to taxanes, these chemotherapy agents target the microtubules, and lead to adverse effects in axons. The incidence of CIPN secondary to vinca alkaloids ranges from 30% to 47%. Vinca alkaloids generally lead to a sensory neuropathy but can also cause ataxia (although less frequently than platinum derivatives). These agents can also lead to severe autonomic impairment, including bladder dysfunction and constipation.
Risk factors for developing CIPN regardless of agent used include diabetes mellitus, prior exposure to neurotoxic chemotherapy, and radiculopathy.
Diagnosis
Many neuropathies can be diagnosed through history and physical alone. Electrodiagnostic studies (electromyography and nerve conduction studies) can also be helpful for diagnosing mononeuropathies (such as CTS), brachial plexopathy, and peripheral neuropathy. Advanced imaging such as magnetic resonance imaging can be used for evaluation for cervical radiculopathy (to evaluate for disc herniation or tumor invasion) as well as brachial plexopathy (to rule out tumor recurrence).
Treatment
Unfortunately, no agents exist to prevent the development of neuropathies associated with breast cancer. Treatment is targeted toward symptomatic management. Recent guidelines from the American Society of Clinical Oncology recommend use of duloxetine for management of pain associated with chemotherapy-induced peripheral neuropathy. Other agents, such as tricyclic antidepressants (such as amitriptyline or nortriptyline), gabapentin, antiepileptics (such as lamotrigine), may be considered but do not have robust evidence at this time ( Table 83.1 ).