Abstract
Controversy has long existed regarding the biological implications and surgical treatment of regional lymph node metastasis in invasive breast cancer. Several factors have resulted in a renewed evaluation of axillary node dissection. First is the continuing biological controversy that axillary lymph node metastases are “indicators but not governors” of outcome in breast cancer. Indeed in all human cancers with few exceptions, this biological concept has been proved repeatedly, and in most studies addressing this issue, lymph node metastases have proved to be indicators only. Another factor that has led to resurgence of interest in the role of axillary node dissection is the downward trend in tumor size secondary to mammographic screening and the resulting decrease in proportion of patients with lymph node metastasis. Use of primary tumor characteristics and genomic patterns to aid in decisions to administer systemic chemotherapy, the failure of high-dose therapy with bone marrow support, and the increasing indications for systemic adjuvant therapy in most cases have also challenged the need for axillary node dissection. Finally, with the advent of sentinel lymph node biopsy and its widespread application, the need for complete axillary evaluation has been questioned. This chapter summarizes the role of the lymphatic system in breast cancer and factors that have led to the decreased need for surgical axillary evaluation. Alternatives to axillary lymph node dissection, including axillary observation only in patients with small tumors, treatment of the axilla with tangential whole breast radiotherapy fields or axillary radiotherapy, lymph node evaluation by four- and five-node sampling, sentinel node biopsy, and the use of ultrasound to stage the axilla are also discussed. The continuing controversy surrounding the potential value of axillary dissection in breast cancer patients is explored.
Keywords
axillary lymph node dissection, management of axilla in breast cancer, targeted axillary dissection, axillary anatomy
Controversy has long existed regarding the biological implications and surgical treatment of regional lymph node metastasis in invasive breast cancer. Several factors have resulted in a renewed evaluation of axillary node dissection. First is the continuing biologic controversy that axillary lymph node metastases are “indicators but not governors” of outcome in breast cancer. Indeed in all human cancers with few exceptions, this biological concept has been proved repeatedly, and in most studies addressing this issue, lymph node metastases have proved to be indicators only. Another factor that has led to resurgence of interest in the role of axillary node dissection is the downward trend in tumor size secondary to mammographic screening and the resulting decrease in proportion of patients with lymph node metastasis. Use of primary tumor characteristics and genomic patterns to aid in decisions to administer systemic chemotherapy, the failure of high-dose therapy with bone marrow support, and the increasing indications for systemic adjuvant therapy in most cases have also challenged the need for axillary node dissection. Finally, with the advent of sentinel lymph node biopsy and its widespread application, the need for complete axillary evaluation has been questioned. This chapter summarizes the role of the lymphatic system in breast cancer and factors that have led to the decreased need for surgical axillary evaluation. Alternatives to axillary lymph node dissection, including axillary observation only in patients with small tumors, treatment of the axilla with tangential whole breast radiotherapy fields or axillary radiotherapy, lymph node evaluation by four- and five-node sampling, sentinel node biopsy, and the use of ultrasound to stage the axilla are also discussed. The continuing controversy surrounding the potential value of axillary dissection in breast cancer patients is explored.
Lymphatic Function and Nodal Metastases
A brief analysis of anatomy, physiology, biological function, and metastatic specificity of the lymphatic system adds to our understanding of lymph node metastasis and its impact on survival. The lymphatic system was first described by Asselius in Pavia in 1622. It was not until 1863, however, that the relationship between the lymphatic system and lymph nodes was made by His. The lymphatic system serves four purposes:
- 1.
Return of interstitial fluids and proteins to the blood and the conduction of absorbed fats of the intestinal tract to the vascular system by way of intestinal lacteals and the thoracic duct
- 2.
Exposure of foreign antigens to lymph node lymphocytes for generation of acquired specific immunity
- 3.
Production and dissemination of antigen-specific T lymphocytes
- 4.
Production and maturation of plasma cells that produce antigen-specific antibodies
Lymphatic fluid contents can either pass through the lymph node or bypass the lymph node entirely to enter the hematogenous system. The predominant pattern is for lymph to flow through the afferent lymphatic channels to the lymph node for maximum exposure of foreign antigens to lymphocytes. Because lymph fluid can either pass through the lymph node or bypass the lymph node directly into the hematogenous system, it is not surprising that studies show that metastatic cancer cells arriving at the lymph node by afferent lymphatic channels may transit through or bypass the lymph node entirely through lymphatic channels or lymphatic venous anastomoses. Metastatic cancer cells that drain directly into the lymph node may lodge and remain without progressive growth (this may be exemplified by isolated tumor cells), be destroyed by physiologic processes that occur in the lymph node, or lodge and grow in the lymph node and become metastases. The presence of metastatic cancer cells in the lymph node is therefore only an indication of the ability of the cancer cell to metastasize from the primary tumor site and not necessarily proof of later nodal metastatic growth. As clinical trials show, many patients with lymph node metastases may harbor systemic cells or micrometastases; however, significant proportions of patients develop systemic disease without any evidence of lymph node metastases. In these patients, it is conceivable that the metastatic cancer cell bypassed the lymph node and entered the hematogenous system directly or transited the node without residual evidence of its presence. Another major subgroup of patients has lymph node metastases but never develops systemic disease. The number of lymph node metastases is the major factor in this statistical relationship to later systemic metastatic disease; patients with only one-node metastases have an excellent prognosis, whereas those with more than 10-node metastases have a very poor prognosis.
The advent of sentinel lymph node biopsy has furthered our knowledge of the lymphatic system of the breast. Sentinel lymphadenectomy has proven that drainage of lymph from the breast is orderly through one or two initial draining nodes, or sentinel lymph nodes. These sentinel lymph nodes tend to be close to the breast. Injection of blue dye or radioactive tracer in the breast parenchyma around the primary tumor, in the overlying skin, or around the nipple all drain to the same sentinel lymph nodes. These studies have shown that the breast lymphatic drainage is orderly, and thus the distribution of lymph, metastatic cells, and nodal metastases are not random events but rather highly structured, defined, and sequentially based on the anatomy and physiology of the lymphatic system.
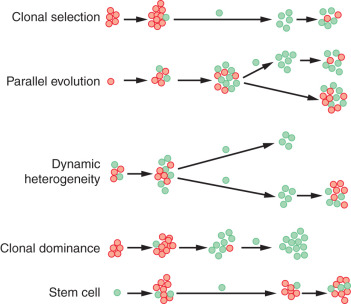
There are five models of when and how tumors develop metastasis ( Fig. 41.1 ). In clonal selection, metastasis develops as the final step in a multistep process. The Halstedian view of breast cancer follows this model. In parallel evolution, metastasis occurs early in tumor progression and is independent of the primary tumor. This model is in agreement with the Fisherian model of breast cancer. Tumor cells develop metastasis at varying rates in the dynamic heterogeneity model. In clonal dominance, clonal cells with the most metastatic potential outgrow other tumor cells to dominate the primary tumor mass and metastatic deposits. The stem cell model suggests that only the stem cells have the capacity to metastasize. Regardless of the model, a cancer cell has to detach from the primary tumor mass (loss of cell-to-cell adhesion), invade the blood and lymphatic vasculature, extravasate into parenchymal tissue, and colonize that tissue.
Extensive research work is being conducted on the specificity of the interaction between metastatic cancer cell and recipient organ. Cancer cells, selected by sequential harvesting of specific organ site metastasis and reinjected intravenously into animal models, display an inability to lodge or grow in organs other than the source from which they were obtained. It has been shown that metastatic subclones with different gene expression profiles developed metastases in different organs despite being derived from the same breast cancer cell line. Brodt and colleagues demonstrated lymph node specificity of human lymphatic metastatic cells grown in nude mice and provide further evidence for organ specificity of lymph node metastases. This distinct organ-specific metastatic behavior has been eloquently proven by animal studies in which individual cells from the effusion of a breast cancer patient differed in their ability to metastasize to the bone, lung, or adrenal organs. These observations provide evidence for Paget’s “seed and soil theory.” Unknown as yet are the exact physiologic mechanisms that permit or prevent lodging and progressive growth of organ-specific metastatic cells, but they may be related to cytokine function, electrical charge, structural aspects of the metastatic cell surface, or other tissue features in the receptor organ. Metastatic cancer cells have to be able not only to detach from the primary organ and invade the lymphatic system but also to attach to the lymphatic metastatic site via specific interaction between cell and endothelial or lymph node structural element receptors. Once attachment has occurred, these cancer cells have to evade possible immune system rejection and produce angiogenic factors that are essential for growth. These cells that lodge and grow in lymph nodes may have no ability to lodge and grow in other organs or other organ endothelial cells.
Patients can harbor metastases in different organs such as the lymph node, liver, or bone. What is unclear is whether some of these metastases are directly derived from the primary tumor (synchronous seeding) or if some of the metastatic deposits metastasized to other organs (metachronous seeding). The current concept of lymph nodes as indicators, not governors, of survival would support synchronous seeding. Overwhelming data from randomized trials do support this concept. However, the recent overview of outcomes after radiotherapy for breast cancer does suggest a survival benefit in those patients who received radiotherapy and had better locoregional control. If metachronous seeding can occur, axillary clearance of lymph nodes that harbored metastatic disease should result in better outcome. These differing concepts of seeding are further complicated by self-seeding in which disseminated tumor cells can return to the primary tumor site and grow.
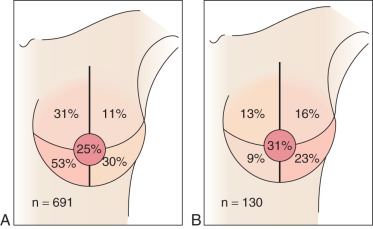
Most breast cancers drain to the axilla irrespective of the quadrant location for the primary index lesion. Tumors located in the inner quadrants of the breast have the greatest likelihood of draining to the internal mammary nodes ( Fig. 41.2 ). However, even in these patients, the primary tumor also drains to the axilla. The few patients with metastasis to the internal mammary nodes are more likely to have larger tumors, more aggressive disease, and multiple positive axillary lymph nodes. Indeed, in a recent analysis of 6000 breast cancer patients, the incidence of internal mammary lymph node recurrence was 0.1%, with almost of all these patients also having systemic recurrence.
Bone marrow micrometastasis in breast cancer patients in the absence of lymph node involvement supports the concept of direct hematogenous spread. Braun and associates reported that one-third of node-negative breast cancer patients have bone marrow micrometastasis and that their presence increased relapse. Combined data from nine studies involving 4703 breast cancer patients suggested that the presence of bone marrow micrometastasis was a significant prognostic factor for both overall and breast cancer–specific survival. Presence of micrometastasis increased twofold the likelihood of disease recurrence and dying of breast cancer and was a significant prognostic factor after controlling for tumor size, lymph node involvement, tumor grade, and hormone receptor status.
Axillary Anatomy and Evaluation
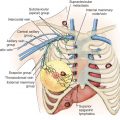
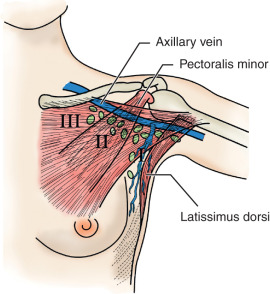
The axillary space is defined by the axillary vein superiorly, the serratus anterior medially, and the latissimus dorsi laterally. The pectoralis muscles are anterior to the medial portion of the axilla. The pectoralis minor is used to divide the axilla into three levels: level I nodes are lateral to the pectoralis minor, level II nodes are behind the pectoralis minor, and level III nodes are medial to the pectoralis minor ( Fig. 41.3 ). The interpectoral (Rotter) nodes are found between the pectoralis major and minor muscles along the lateral pectoral nerve.
There has been considerable debate regarding appropriate extent of axillary node dissection. A complete axillary dissection removes all three levels of lymph nodes, whereas a partial axillary dissection may refer to the removal of level I and II nodes or only level I nodes. Most axillary metastases involve either level I or II nodes. Indeed, sentinel node mapping has shown that low level I lymph nodes are frequently a site of metastatic disease. Veronesi and coworkers reported that the likelihood of “skip metastases” (i.e., involvement of level III nodes in the absence of level I and II involvement) was less than 1%. Similarly, Rosen and colleagues studied 429 patients with axillary node metastases and found isolated disease in level III in only 0.2% of the specimens. Sentinel node mapping has shown that the breast can bypass level I nodes to drain into level II nodes. It is not surprising that the reported rate of skip metastases to level II nodes is as high as 25%. Contemporary axillary dissection in breast cancer refers to the removal of level I and II lymph nodes.
The axilla can be evaluated by clinical examination, noninvasive imaging, minimally invasive surgery, and axillary clearance. Axillary node dissection is considered the gold standard, and it is the modality to which all methods are compared. Clinical examination of the axilla is not an accurate method to evaluate the axilla. Sentinel lymphadenectomy has proved that axillary metastases can be present in more than 30% of patients with a clinically negative axilla. Alternatively, about 25% of patients with axillary adenopathy have no metastatic disease. This is especially valid if the patient has had a recent intervention in the breast.
Axillary lymph nodes can often be seen on mammograms. They are not clinically suspicious unless they are greater than 2 cm in size or have loss of the fatty hilum. Ultrasound is the best noninvasive method to assess lymph nodes. Lymph nodes that are less than 1 cm in size and have a centrally located hilum are most likely benign. Conversely, lymph nodes that are large and have a thick, eccentric hilum are suspicious for metastatic disease. Sonographic diagnosis of lymph nodes will be accurate in approximately 70% of cases. Accuracy of axillary ultrasound can be improved when coupled with fine-needle aspiration cytology (FNAC). FNAC of axillary lymph nodes is very sensitive, and the false-negative rate is less than 1%. Several centers have shown that proceeding directly to axillary node dissection based on a positive FNAC is cost-effective and spares up to 40% of patients the intermediate step of a sentinel node biopsy. Patients with negative FNAC proceed to sentinel node biopsy because of the known sampling error, especially in patients with micrometastasis.
The likelihood of nodal involvement correlates directly with increasing tumor size. Although the exact numbers varied, in general, the likelihood of nodal involvement with a T 1a tumor was 5% to 10%, with T 1b 10% to 20%, and with T 1c greater than 20%. However, these percentages were based on conventional axillary node dissections, often on registry data with their inherent discrepancies. The advent of sentinel lymphadenectomy has resulted in intense histologic examination of lymph nodes and an increased detection of smaller-sized metastasis and upstaging. The incidence of positive lymph nodes based on tumor size and detected by sentinel lymphadenectomy is depicted in Table 41.1 . Because most breast cancer patients with clinically negative axilla are treated with sentinel lymphadenectomy, results based on this surgical approach and method of lymph node evaluation are included. The likelihood of lymph node metastases in T 1a cancers is 10% to 18%. Higher rates are encountered if immunohistochemistry is used to evaluate the lymph nodes. Lymph node metastases are observed in up to 24% of patients with T 1b tumors and approximately one third of patients with T 1c cancers. Similar to T 1a cancers, a higher incidence of lymph node metastases is seen when immunohistochemistry is used to evaluate the lymph node. Approximately 50% of patients with T 2 tumors have nodal involvement. It is clear that intense histologic evaluation of sentinel lymph nodes has resulted in increased identification of nodal metastasis, especially isolated tumor cells and micrometastasis. It has been shown that in countries that have adopted sentinel lymphadenectomy in the management of patients with early breast cancer, lymph node metastasis has increased 13%. For example, intense histologic analysis of sentinel lymph nodes has resulted in an increased detection of metastases in microinvasive breast cancer from 2% to almost 10%. Most of the metastases identified in these patients are micrometastases or isolated tumor cells, and the clinical significance of these small tumor deposits is debated. In addition to tumor size, prognostic factors associated with increased incidence of lymph node metastasis include tumor grade, age, hormone receptor status, and presence of lymphovascular invasion. The special subtypes of ductal carcinoma such as colloid, papillary, and tubular have been reported to have a low likelihood of lymph node metastasis. For example, in a meta-analysis of pure tubular carcinomas, positive nodes were seen in 7% of patients and none in patients with tumors less than 1 cm. However, the advent of sentinel lymphadenectomy has also increased nodal positivity. Leikola and coworkers reported that almost 30% of patients with tubular carcinomas had a positive sentinel lymph node, including tumors less than 1 cm. The average size of the metastasis was small (0.17 mm), and in most patients only the sentinel lymph node was positive for disease.
| Series | No. | Evaluation | T 1mic | T 1a | T 1b | T 1c | T 2 |
|---|---|---|---|---|---|---|---|
| Memorial Sloan Kettering Cancer Center b | Overall includes IHC | 11% | 14% | 22% | 36% | 58% | |
| Serial section | 5% | 10% | 18% | 32% | 53% | ||
| H&E alone | 2% | 5% | 13% | 25% | 45% | ||
| University of California, Davis c | 577 | NS | — | 15% | 31% | 56% | |
| European Working Group d | 2929 | Overall includes IHC | 6.5% | 10% | 16% T < 1.5 | — | |
| Mayo Clinic e | 77 | Overall includes IHC | 7.8% | — | — | — | — |
| Mayo Clinic, University of Pennsylvania f | 222 | Overall includes IHC | — | 4% | 20% | 24% | 49% |
| University of Istanbul g | 400 | Overall includes IHC | — | 18% | 24% | 33% | 52% |
a Immunohistochemistry has resulted in an increased detection of nodal disease.
c Data from Vanderveen KA, Schneider PD, Khatri VP, et al. Upstaging and improved survival of early breast cancer patients after implementation of sentinel node biopsy for axillary staging. Ann Surg Oncol. 2005;13:1450-1456.
d Data from Cserni G, Bianchi S, Vezzosi V, et al. Sentinel lymph node biopsy in staging small up to 15 mm breast carcinomas. Results from a European multiinstitutional study. Pathol Oncol Res. 2007;13:5-14.
e Data from Gray RJ, Mulheron B, Pockaj BA, et al. The optimal management of the axillae of patients with microinvasive breast cancer in the sentinel lymph node era. Am J Surg. 2007;194:845-848, discussion 848–849.
f Data from Reynolds C, Mick R, Donohue JH, et al. Sentinel lymph node biopsy with metastasis: can axillary dissection be avoided in some patients with breast cancer? J Clin Oncol. 1999;17:1720-1726.
Surgical techniques for evaluating the axilla include sentinel lymphadenectomy, limited axillary clearance, and axillary node dissection. For many decades, axillary node dissection was an important tool in the management of breast cancer because of its ability to determine the number of positive nodes involved with disease and because it had a low rate of regional recurrence. Axillary recurrence after a level I and II dissection is less than 3%. However, axillary node dissection has significant morbidity, including lymphedema, paresthesias, and nerve damage. The widespread application of mammographic screening and smaller tumor size at presentation has resulted in approximately only 25% of women having nodal disease at the time of presentation. An axillary node dissection in node-negative women provides no therapeutic benefit other than prognostic information, and the advent of sentinel lymphadenectomy to stage the axilla has relegated axillary node dissection to the treatment of patients with known positive nodes. Despite the complications associated with axillary node dissection, it remains the gold standard for surgical evaluation of the axilla in breast cancer patients with large tumors (>T2) and/or with known axillary node metastatic disease.
Sentinel lymphadenectomy is a minimally invasive technique that can stage the axilla in breast cancer patients. Since its initial description in 1994, sentinel lymphadenectomy has been rapidly incorporated into clinical practice. The advantage of sentinel lymphadenectomy is that patients who have no nodal metastases can avoid a formal axillary node dissection. The ability to detect at least one sentinel lymph node is in the high 90th percentile. In most patients, only two sentinel lymph nodes are removed, and therefore a disadvantage is that those patients who have positive sentinel lymph nodes may need to have a completion axillary node dissection to determine the number of positive nodes. An international consensus conference agreed that sentinel node biopsy was a suitable replacement for axillary node dissection in early breast cancers.
The early studies of sentinel lymphadenectomy reported false-negative rates in patients who had immediate completion axillary node dissections. The false-negative rate, defined as node-positive patients with negative sentinel lymph nodes divided by all patients with positive axillary lymph nodes, ranged from 9.6% to 11.4% in early studies. The excellent results obtained with this technique led to early abandonment of a completion axillary node dissection in patients with negative sentinel lymph nodes. There have been multiple institutional studies that have reported outcomes of patients with negative sentinel lymph nodes who did not have a formal axillary node dissection. Clinical axillary recurrence rates from single institutions in patients with negative sentinel node biopsy alone are negligible and less than 1.5%. A similar low axillary recurrence of 0.6% was reported by the Swedish Multicenter Cohort Study of 3534 patients after 3 years of follow-up. In the European Institute of Oncology randomized trial of sentinel lymphadenectomy and axillary node dissection, patients with negative sentinel lymph nodes did not have completion axillary node dissection. There have been 2 axillary recurrences out of 167 patients with a negative sentinel lymph node who did not have completion axillary dissection after a median follow-up of 102 months.
Patients undergoing sentinel lymphadenectomy alone have less morbidity compared with those who have a completion axillary node dissection. The European Institute of Oncology study randomized 516 patients to sentinel node biopsy followed by immediate axillary node dissection or axillary node dissection only if the sentinel lymph node was positive for metastatic disease. Besides the results previously mentioned, less morbidity, as measured by axillary pain, paresthesias, arm mobility, and arm swelling was observed in the sentinel node group. The Axillary Lymphatic Mapping Against Nodal Axillary Clearance (ALMANAC) trial randomized patients with operable breast cancer to sentinel lymphadenectomy or axillary node dissection. The primary outcome measures were arm and shoulder morbidity and quality of life. Patients randomized to sentinel lymphadenectomy had a decreased risk of lymphedema and sensory loss and perceived better quality of life than those undergoing axillary dissection. National Surgical Adjuvant Breast and Bowel Project (NSABP) B-32 randomized patients to sentinel node biopsy followed by immediate axillary node dissection or sentinel lymphadenectomy without formal axillary dissection if the sentinel lymph nodes were negative. The primary end points of this trial were survival, regional control, and morbidity. This trial randomized 5611 patients between 1999 and 2004. Findings demonstrated overall survival, disease-free survival, and regional control to be similar between groups with mean time on study for SLN negative patients of 95.6 months. The American College of Surgeons Oncology Group (ACOSOG) Z0011 randomized patients with a positive sentinel lymph node to observation or completion axillary node dissection; women in the latter group had more wound infections, seromas, and paresthesias. Women in the completion axillary node dissection arm were more likely to have lymphedema. An Italian trial (Sentinella-GIVOM) and a UK trial (Cambridge/East Anglia) are designed in a similar manner to NSABP B-32. Both of these studies have reported arm morbidity and better quality of life in patients randomized to the sentinel lymphadenectomy arm alone. In summary, sentinel lymphadenectomy is a minimally invasive surgical technique that can accurately stage the axilla in breast cancer patients with less morbidity than a level I/II axillary node dissection.
Sentinel lymphadenectomy can provide information about whether breast cancer patients have nodal disease, but because there is limited sampling of nodes, it cannot be used to determine the number of positive lymph nodes. There has been much debate about the need for a completion axillary node dissection in patients with a sentinel lymph node positive for metastatic disease. Total number of positive nodes is important prognostic information because an increasing number of positive nodes correlates with decreased survival. Furthermore, additional positive axillary lymph nodes may influence decisions regarding adjuvant treatment. However, as discussed earlier, axillary node metastases are indicators, and not governors, of survival, and therefore the therapeutic benefit of further surgery may be minimal. Most patients with a positive sentinel lymph node have no further axillary disease, and there is clearly no benefit to removing negative axillary lymph nodes. Delayed axillary dissection is also associated with increased operative time. Furthermore, adjuvant chemotherapy is seldom altered by information gathered from the completion axillary dissection.
These arguments have resulted in discussions of whether patients with a positive sentinel lymph node need to have a completion axillary node dissection and methods to predict which patients will have a positive non–sentinel lymph node. Patients with positive sentinel lymph nodes but no residual nodal disease would derive little benefit from a completion axillary node dissection. Size of metastasis is most predictive of likelihood of non–sentinel node metastasis. Micrometastases are defined as having a size of more than 0.2 mm but not greater than 2 mm. Isolated tumor clusters are not greater than 0.2 mm, have no evidence of proliferation or stromal reaction, and are located in lymphatic sinuses. As shown in Table 41.2 , about 50% of patients with metastatic deposits greater than 2 mm (macrometastases) have residual disease identified on completion axillary node dissection. However, patients with micrometastases, especially isolated tumor clusters, are less likely to have further positive sentinel lymph nodes after completion axillary dissection. An exception is the series from University of Helsinki in which 26% of patients with a sentinel lymph node micrometastasis had tumor deposits in non–sentinel lymph nodes. Immunohistochemical analysis of non–sentinel lymph nodes may partially explain the high rate of non–sentinel lymph node involvement in the Helsinki series. In addition to size of metastasis, tumor features may be used to predict patients likely to have non–sentinel lymph node metastases. Patients with a macrometastasis, lymphovascular invasion in the primary tumor, extranodal extension, or a high ratio of involved to noninvolved sentinel lymph nodes are more likely to metastasize to non–sentinel lymph nodes. Patients with a solitary positive sentinel lymph node accompanied by additional negative sentinel lymph nodes are unlikely to have residual disease.
| Size of Metastasis | Series | No. | Incidence of non-SLN Metastasis (%) |
|---|---|---|---|
| Macrometastasis | John Wayne Cancer Institute b | 101 | 63 |
| European Institute of Oncology c | 794 | 50 | |
| Rush-Presbyterian-St. Luke’s-Roosevelt d | 63 | 46 | |
| University of Texas MD Anderson Cancer Center e | 92 | 48 | |
| University of Ferrara f | 38 | 43 | |
| Netherlands Cancer Institute g | |||
| Mayo Clinic/University of Pennsylvania h | 24 | 80 | |
| Istanbul University i | 130 | 56 | |
| Micrometastasis | John Wayne Cancer Institute b | 93 | 26 |
| European Institute of Oncology c | 318 | 21 | |
| Rush-Presbyterian-St. Luke’s-Roosevelt d | 30 | 20 | |
| Netherlands Cancer Institute e | 106 | 19 | |
| Mayo Clinic/University of Pennsylvania f | 36 | 25 | |
| Micrometastasis and isolated tumor cluster | Mayo Clinic j | 28 | 7 |
| University of Ferrara f | 58 | 14 | |
| Istanbul University i | 18 | 11 | |
| Helsinki University k | 84 | 26 | |
| Univ. of Texas MD Anderson Cancer Center e | 30 | 17 | |
| Isolated tumor cluster | John Wayne Cancer Institute l | 61 | 5 |
| Rush-Presbyterian-St. Luke’s-Roosevelt d | 31 | 19 | |
| European Institute of Oncology c | 116 | 15 |
a Patients with SLN containing micrometastases and isolated tumor clusters are less likely to have residual disease after completion axillary node dissection.
b Data from Turner RR, Chu KU, Qi K, et al. Pathologic features associated with nonsentinel lymph node metastases in patients with metastatic breast carcinoma in a sentinel lymph node. Cancer. 2000;89:574-581.
c Data from Viale G, Maiorano E, Pruneri G, et al. Predicting the risk for additional axillary metastases in patients with breast carcinoma and positive sentinel lymph node biopsy. Ann Surg. 2005;241:319-325.
d Data from Menes TS, Tartter PI, Mizrachi H, et al. Breast cancer patients with pN0i + and pN1mi sentinel nodes have high rate of nonsentinel node metastases. J Am Coll Surg. 2005;200:323-327.
f Data from Carcoforo P, Maestroni U, Querzoli P, et al. Primary breast cancer features can predict additional lymph node involvement in patients with sentinel node micrometastases. World J Surg. 2006;30:1653-1657.
h Data from Reynolds C, Mick R, Donohue JH, et al. Sentinel lymph node biopsy with metastasis: Can axillary dissection be avoided in some patients with breast cancer? J Clin Oncol. 1999;17:1720-1726.
l Data from Calhoun KE, Hansen NM, Turner RR, Giuliano AE. Nonsentinel node metastases in breast cancer patients with isolated tumor cells in the sentinel node: implications for completion axillary node dissection. Am J Surg. 2005;190:588-591.
These risk factors are difficult to apply to the individual patient who may be unsure if she should proceed to a completion axillary dissection. Nomograms have been designed to assist physicians and patients in this decision-making process. The first computerized model for prediction of positive non–sentinel lymph nodes in patients with a positive sentinel lymph node was reported from the Memorial Sloan Kettering Cancer Center (MSKCC). This nomogram was created using the data from 702 patients who had had a sentinel lymphadenectomy followed by completion axillary dissection. Factors used in calculating risk in this nomogram included tumor size, histology and grade, lymphovascular invasion, multifocality, hormone receptor status, method used to analyze the sentinel lymph nodes, and the number of positive and negative sentinel lymph nodes. The model was subsequently applied to 371 patients. The MSKCC nomogram has been validated by several centers. Although it has been shown to be a good predictive tool, limitations include overestimation of risk in patients with sentinel lymph node that contain micrometastasis only, differences in pathologic examination of the node, and volume of metastasis and presence of extracapsular extension. The MSKCC nomogram has been shown to be more accurate than clinical judgment in predicting likelihood of non–sentinel lymph node metastasis and need for completion axillary node dissection. Although the MSKCC nomogram has been validated by other institutions, caution should be used when applied to patients who have a preoperative axillary ultrasound showing a normal-appearing sentinel lymph node and a positive sentinel lymph node. In this group of patients, the MSKCC nomogram may overestimate the risk of non–sentinel lymph node metastasis.
A mathematical scoring system using clinical and pathologic factors to predict likelihood of metastases in non–sentinel lymph nodes has been developed at the MD Anderson Cancer Center. This algorithm was based on 131 breast cancer patients who had a positive sentinel node and underwent completion axillary dissection. Factors predictive of a positive non–sentinel node were a primary tumor greater than 2 cm, presence of a macrometastasis (i.e., metastatic deposit >2 mm), and lymphovascular invasion. Patients with a score of 4 all had further axillary metastases after completion axillary dissection.
A drawback of the MSKCC nomogram is that size of metastasis is not included in the model. The presence of immunohistochemistry metastasis is used as a surrogate for micrometastasis. Although the MD Anderson model does include size of metastasis as a factor, only four variables are used in their mathematical algorithm. The ability of both of these models has been compared, and in a retrospective series of 186 sentinel lymph node–positive patients, the MSKCC was found to be more useful in individualizing surgical management of the axilla in sentinel lymph node–positive patients. These models may be helpful in counseling patients who are unlikely to have non–sentinel lymph node metastasis or who are poor surgical candidates. The S classification of sentinel lymph nodes may be used to predict non–sentinel lymph node metastasis in patients with a positive sentinel lymph node. This classification system was initially described in patients with malignant melanoma and measures the maximum distance between tumor cells and the interior margin of the respective lymph node capsule for each positive sentinel lymph node. The S classification system used depth of invasion as a surrogate for size of metastasis. SI is defined as subscapular tumor cells no deeper than 0.3 mm, SIII has tumor cells deeper than 1 mm below the capsule, and SII has a depth between SI and SIII. In a recently published study of 36 patients with a positive sentinel lymph node, there were no non–sentinel lymph nodes positive for metastasis in patients classified as SI, whereas 61% of patients with SIII had non–sentinel lymph nodes positive for metastasis. A study by the same group retrospectively looked into the capability of predicting presence of metastases and overall survival in a population of 236 patients with a positive sentinel node followed by axillary lymph node dissection. This study concluded that this system allowed adequate prediction of the likelihood of the involvement of non-SLN and with a good OS prognosis with a mean follow-up of 65 months. Although encouraging, the S classification of sentinel lymph nodes would still need to be validated in a larger cohort of breast cancer patients. In summary, it is difficult to accurately predict which patients with a positive sentinel lymph node will have residual disease after completion axillary node dissection. Patients with immunohistochemistry-positive sentinel lymph nodes or who fit ACOSOG Z0011 trial population profile do not need to undergo completion axillary node dissection. Within this group, caution should be reserved for patients with T 3 and T 4 tumors because these are the patients most likely to have non–sentinel lymph node involvement.
It has been argued that axillary clearance is not needed in patients with positive sentinel lymph nodes because tangential fields from breast radiotherapy cover the axilla. Studies have looked at whether tangential breast radiotherapy fields overlap in breast cancer patients treated with breast conservation. Initial studies were difficult to interpret because the extent of the axilla was difficult to define using plain films. The advent of sentinel lymphadenectomy allowed marking of the sentinel lymph node with a clip. Radiotherapy fields in relation to this clip could be determined and the radiation dose to the clip calculated. In a small study of 36 women, the clip marking the sentinel lymph node fell within the tangential breast radiotherapy fields 94% of the time with a radiation dose greater than 4400 cGy in 50% of the patients. A larger study of 106 patients confirmed that the sentinel lymph node clip was within the tangential fields used for breast radiotherapy but surprisingly was not covered by standard axillary radiation fields. These results suggest that residual axillary disease within the axilla would be treated in patients undergoing breast radiotherapy and therefore that completion axillary dissection may not be necessary in patients with a positive sentinel node.
Two large randomized studies were designed to address the need for completion axillary dissection in breast cancer patients with a positive sentinel lymph node. The ACOSOG Z0011 study randomized breast cancer patients with a positive sentinel lymph node to completion axillary dissection or observation. Patients in the observation arm did not have radiotherapy fields altered to include the axilla. Decisions regarding adjuvant systemic chemotherapy were based on primary tumor characteristics. This study randomized 891 patients with 446 patients undergoing lumpectomy and radiation for T1 or T2 tumors and clinically negative axilla. At a median follow-up of 6.3 years, there were no differences in local recurrence or regional recurrence in the two comparison groups, suggesting that sentinel lymph node procedure without axillary node dissection offers adequate regional control in patients with up to 2 positive sentinel lymph node. The European Organization for Research and Treatment of Cancer 10981 to 22023 After Mapping of the Axilla: Radiotherapy or Surgery (AMAROS) trial was designed to compare regional control in patients with a positive sentinel lymph node randomized to delayed axillary dissection or axillary radiotherapy. This intergroup noninferiority phase III study addressed whether axillary radiation therapy provides equivalent regional control with fewer side effects compared with further axillary node dissection in cases where the sentinel lymph node is positive. Patients with tumors up to 5 cm and clinically negative axillary lymph nodes were eligible. The type of breast treatment was either breast conservation followed by whole breast radiation or mastectomy followed or not by chest wall radiation. Primary end point for patients with a positive sentinel lymph node was axillary recurrence in 5 years with secondary measured outcomes including recurrence-free, disease-free, and overall survival; shoulder mobility; lymphedema; and quality of life. A total of 4823 patients were enrolled from 2001 to 2010, with 4806 of them being eligible for randomization to either receive axillary node dissection or axillary radiotherapy. A positive sentinel lymph node was found in 1425 patients with 244 of them assigned to receive axillary node dissection and 681 axillary radiotherapy (intention-to-treat patient population). With 6.1 years of median follow-up it was seen that the 5-year axillary recurrence was 0.43% and 1.19% respectively, a difference without statistical significance, whereas lymphedema in the ipsilateral arm was significantly more frequently seen in the axillary dissection arm at 1, 3, and 5 years. Ipsilateral arm range of motion and quality of life were not significantly different between groups.
Although a level I and II dissection has been part of treatment practice worldwide, many surgeons in the United Kingdom have preferentially used four-node sampling for many decades. Four-node sampling involves removal of palpable lymph nodes by surgical exposure beginning at the axillary tail until four axillary lymph nodes have been identified. Chetty and colleagues reported the Edinburgh Breast Unit experience with this technique in their prospective study of 466 patients randomized to level III axillary dissection or four-node sampling and axillary radiotherapy or, in the latter half of the trial, observation only of patients with negative nodes. There was no statistical difference in the proportion of women with positive lymph nodes or in the regional nodal recurrence rate between the two arms. Long-term regional control was excellent, with relapse rates of 5% at 10 years with no detriment in survival. A similar prospective study of four-node sampling in 237 women demonstrated that this surgical approach to the axilla accurately predicted axillary status in 98% of patients. These results should not be surprising because in a comparative study of four-node sampling and sentinel lymph node biopsy, the sentinel lymph node was contained within the four nodes sampled 80% of the time.
Five-node biopsy has also been suggested as an alternative to axillary clearance in women with breast cancer. This technique involves removal of five lymph nodes with dissection beginning at the axillary tail. The accuracy of five-node sampling to evaluate nodal status has been reported in a prospective trial of 415 breast cancer patients. The sensitivity of five-node biopsy was 97.3% (95% confidence interval 97.1%–97.5%), with a negative predictive value of 98.5% (95% confidence interval 98.4%–98.6%). The accuracy of five-node sampling was similar for cancers detected clinically or mammographically (97.9% and 95.8%, respectively). There were four false-negative cases in this study, resulting in an overall false-negative rate of 1%. If only patients with a positive lymph node were included in the denominator, the false-negative rate was 2.7% (4/149). The sensitivity of sampling only four nodes was negligibly lower than that reported for five-node biopsy (96%). This study concluded that five-node sampling was an accurate test for staging the axilla in breast cancer. Because all patients in this study had an axillary node dissection after five-node sampling, the morbidity of this surgical technique could not be evaluated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree