Abstract
Breast conservation therapy is the complete removal of the breast cancer with a margin of normal tissue surrounding the tumor. This is usually followed by radiation therapy. The primary goals of breast conservation therapy are to provide a survival equivalent to mastectomy, an acceptable rate of local recurrence, and a cosmetic outcome that is acceptable to the patient.
Keywords
breast conservation therapy, lumpectomy, margins, radiation, biopsy
Breast conservation therapy is the complete removal of breast cancer with a margin of normal tissue surrounding the tumor. This is standardly followed by radiation therapy (RT). The primary goals of breast conservation therapy are to provide a survival equivalent to mastectomy, an acceptable rate of local recurrence, and a cosmetic outcome that is acceptable to the patient.
Historical Perspective
Breast conservation therapy (BCT) for carcinoma was first described in 1924 by Sir Geoffrey Keynes, an English surgeon at St. Bartholomew Hospital in London. Keynes, who used radium seeds as an adjunct to surgery, reported a 5-year survival rate of 77% for women with clinically negative lymph nodes and 36% for those with clinically enlarged lymph nodes (N 1 , N 2 ). In 1939 M. Vera Peters, a radiation oncologist in Toronto, began treating patients who had undergone conservative surgery with radiation. By the 1950s and 1960s, breast conservation became increasingly accepted in clinical practice, and in some institutions this therapeutic approach was considered standard. In the early 1970s, several trials were undertaken in an effort to confirm the equivalency of mastectomy and BCT. Six randomized trials and two meta-analyses conducted in the United States and Europe documented the equivalence of lumpectomy and radiation with mastectomy; this was achieved with a low rate of local recurrence while preserving cosmetic and functional outcome. Four clinical trials now have more than 20 years of follow-up, which further confirm long-term results.
At the time of the initial National Surgical Adjuvant Breast and Bowel Project (NSABP) report in 1985 by Fisher and associates, which documented the 5-year equivalence of breast-conserving surgery compared with mastectomy, only 23.9% of stage I breast cancer patients in the United States eligible for breast conservation underwent the procedure. After Dr. Fisher’s report, the use of breast conservation increased to 34.6% in the United States for stage I patients according to the Surveillance, Epidemiology, and End Results (SEER) data. In 1990, the National Institutes of Health Consensus Development Conference recommended that breast conservation treatment is an appropriate method of primary therapy for the majority of women with stage I and II breast cancer. Furthermore, it suggested that the procedure is preferable because it provides survival rates equivalent to those of total mastectomy and axillary dissection while preserving the breast. After this landmark recommendation, 53.4% of eligible breast cancer patients in the United States underwent conservative treatment. In an American College of Surgeons Commission on Cancer National Cancer Database (NCDB) study of 16,643 women with stage I or II breast cancer who were treated in 1994, Bland and coworkers confirmed that only 42.6% were treated with a breast-conserving approach. A recent query of the NCDB of stage 0 to II breast cancer included 553,593 patients and showed lumpectomy rates of 66.4%, not significantly changed from 67.7% in 2003. In the group of women who were 45 years old or younger, lumpectomy rates dropped from 61.3% in 2003 to 49.4% in 2010. Most reports indicate that the majority of women who present with breast cancer do not have contraindications to conservative surgery. Reasons for underutilization of breast conservation include patient preference, age, and poor prognostic factors. Patient preference is influenced by complex issues regarding access to care, concerns for cancer recurrence, and the impact of surgery on body image and sexuality. Medical comorbidity is rarely a major factor in the underutilization of breast-conserving therapy.
Survival
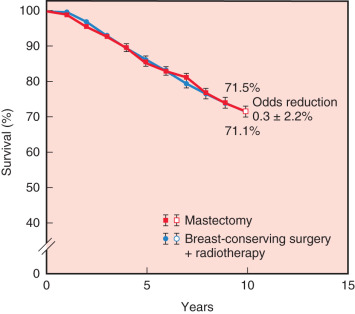
The first and primary goal of breast-conserving surgery is to achieve survival that is equivalent to mastectomy. Six randomized prospective trials comparing breast conservation surgery with mastectomy have been conducted (1972–1989). The specifics of treatment protocols are depicted in Table 32.1 . In 2013 van Hezewijk performed a seventh randomized prospective trial evaluating hormonal therapy with a secondary outcome that demonstrated a significantly higher recurrence rate in mastectomy alone but equivalent survival in patients undergoing mastectomy with RT or lumpectomy with RT. No significant differences in overall or disease-free survival rates were evident in any of the trials. The 20-year follow-up has been reported for several of these trials, confirming the long-term survival equivalence. Moreover, the Early Breast Cancer Trialists’ Collaborative Group performed a meta-analysis, including 3100 patients from seven randomized trials. No difference in 10-year survival was observed ( Fig. 32.1 ). The more recent outcomes data published between 2007 and 2015 have actually demonstrated survival advantage of BCT compared with mastectomy, but most of these studies have been retrospective ( Table 32.2 ).
| Trial | No. of Cases | Follow-Up (yr) | Tumor Size (cm) | BCT Surgical Margins | RT Boost | LOCAL RECURRENCE | OVERALL SURVIVAL | ||
|---|---|---|---|---|---|---|---|---|---|
| BCT (%) | Mx (%) | BCT (%) | Mx (%) | ||||||
| NSABP | 1851 | 20 | 4 | Tumor free | No | 14 | 10 | 46 | 47 |
| Milan | 701 | 20 | 2 | — | Yes | 9 | 2 | 42 | 41 |
| NCI | 247 | 18 | 5 | Grossly free | Yes | 22 | 6 | 59 | 58 |
| EORTC | 868 | 10 | 5 | 1-cm gross | Yes | 20 | 12 | 65 | 66 |
| Danish | 793 | 20 | Any | Grossly free | Yes | NR | NR | 58 | 51 |
| Gustave-Roussy | 179 | 15 | 2 | 2-cm gross | Yes | 9 | 14 | 73 | 65 |
| Author/Year | Center/Country | Treatment Period | No. of Pts. | Study Type | Comparison of Survival | Comparison of Local Control | BCT Rate | Results/Comments |
|---|---|---|---|---|---|---|---|---|
| Hwang et al. 2013 | Duke University, USA | 1990–2004 | 112,154 Stage I, II | Retrospective, California Cancer Registry | HR for OS BCS + RT: 0.72 (0.68–0.76) Mx: 1.0 | NR | 55% | BCS + RT associated with higher breast cancer specific survival at almost 10-yr follow-up. For every potential confounding factor related to mortality evaluated, women with Mx more likely to die within 3 years. |
| Agarwal et al. 2014 | University of Michigan, USA | 1998–2008 | 132,149 <4 cm <4 LN+ | Retrospective, SEER | HR for survival ( p < .001) BCS + RT: 1.0 Mx: 1.31 (1.25–1.39) Mx + RT: 1.47 (1.34–1.61) | NR | 70% | Patients with BCT improved breast cancer specific survival |
| Van Hezewijk et al. 2013 | Multicenter trial, various countries (TEAM trial) | 2001–2006 | 9231 ER, PR+ | Prospective | HR 5-yr OS: ( p < .001) BCS + RT: 1.0 Mx: 1.22 (1.02–1.47) | HR for LRR ( p = .01) BCS + RT 1.0 Mx 1.53 (1.10–2.11) | 54.1% | Significantly higher LRR in patients with Mx only |
| Martin et al. 2007 | Australian National University | 1995–1999 | 2787 | Retrospective | Hazard of death reduced by 55.88% | NR | 51.5% | Patients with BCS better survival than mastectomy |
| Hofvind et al. 2015 | Cancer Registry of Norway | 2005–2011 | 9547 | Retrospective, Norway Registry | HR of death at 6 yr BCT: 1.0 Mx: 1.7 (1.3–2.4) | NR | 61.1% | Women treated with BCT have significantly better breast cancer-specific survival |
Initiated in the late 1970s, the NSABP B-06 study prospectively randomized 1851 women to receive total mastectomy, lumpectomy, and lumpectomy with radiation. Patients enrolled in this seminal study by Fisher and coworkers had stage I or II breast cancer with tumor diameters that were less than 4 cm. For the lumpectomy candidates, tumors were resected with the intent of removing adequate surrounding tissue that would ensure negative pathologic margins and an acceptable cosmetic outcome. However, approximately 10% of patients in the breast preservation arm had positive margins. Those with positive margins underwent total mastectomy and continued to be followed ( Figs. 32.2 and 32.3 ). Axillary node dissection was performed in all patients. All patients with positive axillary lymph nodes received adjuvant chemotherapy. Overall cumulative survival at 20-year follow-up was 47% in women treated with mastectomy, 46% in women treated with lumpectomy alone, and 46% in those treated with lumpectomy followed by radiation. The cumulative incidence of death from any cause was 47.7% in women with negative nodes and 63.3% in women with positive nodes ( Fig. 32.4 ).
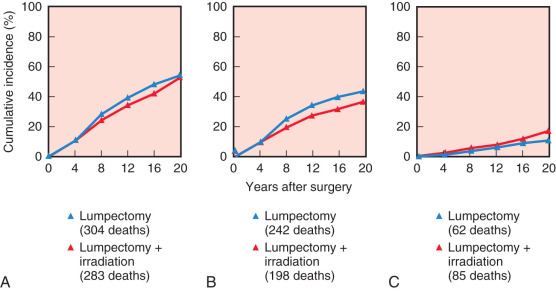
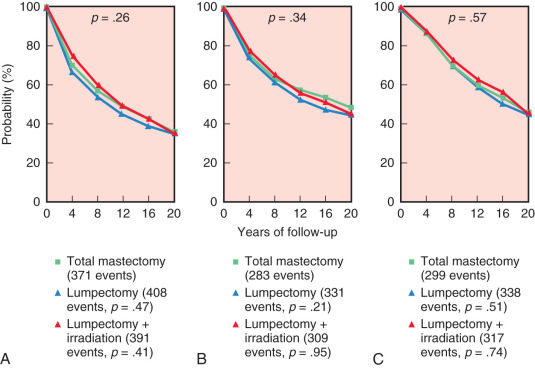
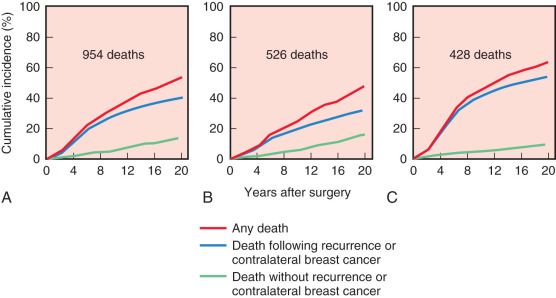
At 15 years’ evaluation, independent prognostic determinants of poor survival were identified from the NSABP B-06 trial. These factors included ipsilateral breast tumor recurrence (IBTR), race, positive nodal status, nuclear grade, histologic type, and patient age. Women younger than 40 years of age and older than 65 years of age had a decreased overall survival compared with women between 40 and 65 years of age. At 20-year follow-up, the IBTR was 39.2% in women who underwent lumpectomy and 14.3% in women who underwent lumpectomy and breast irradiation, but no significant differences in terms of disease-free survival, distant disease–free survival, or overall survival between the three groups.
In a retrospective analysis from the MD Anderson Cancer Center, 1043 women with stage I or II breast carcinoma who were treated with BCT were evaluated with respect to survival. The 5- and 10-year disease-specific survival rates were 94% and 87%, respectively. On multivariate analysis, independent predictors of poor disease-specific (cancer-related) survival included tumor size greater than 2 cm, positive surgical margins, and ipsilateral breast tumor recurrence. Ipsilateral breast tumor recurrence and positive surgical margins have been shown in other studies to be associated with a decreased survival. These data suggest that aggressive local therapy to minimize ipsilateral breast tumor recurrence is important.
Local Recurrence
The second goal of BCT is to maintain local control with an acceptable rate of local recurrence. Most clinicians agree that a 10-year local recurrence rate of 5% to 10% (<1% per year) is acceptable. Tumor recurrence is defined by most investigators as recurrent tumor at or near the primary site of the index quadrant of therapy. In the randomized and retrospective trials comparing breast conservation with mastectomy, ipsilateral recurrence rates were similar in both groups; local recurrence rates of 5% to 22% have been reported for breast conservation and local recurrence rates of 2% to 14% have been reported for mastectomy (see Table 32.1 ). Modern NSABP protocols of systemic therapy for patients undergoing BCT have demonstrated local recurrence rates of 3% to 6% and 5% to 10% in node-negative and node-positive patients, respectively.
In the NSABP B-06 trial, 70% of all ipsilateral breast recurrences occurred within the initial 10 years of therapy (40% in the first 5 years; 30% in the second 5 years) ( Fig. 32.5 ). These data of the NSABP and other institutions demonstrate a persistent risk of local recurrence through 20 years of follow-up. In contrast, most local recurrences after mastectomy are evident within the first 36 months.
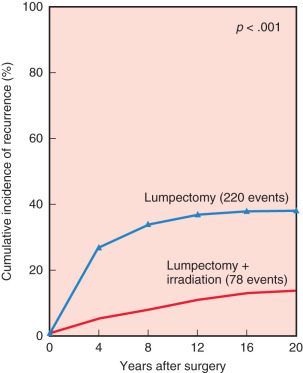
Risk factors for the development of local recurrence have been evaluated in multiple trials. Paramount in reduction of risk for recurrence in patients undergoing BCT is adequate surgery and the application of RT. Several studies, randomized and retrospective, have confirmed a significantly higher rate of local recurrence when RT is omitted after breast conservation (see Fig. 32.5 ).
Several patient- and tumor-related issues have been identified as risk factors for local recurrence. Young age is commonly associated with poor pathologic features and typically conveys a higher rate of local recurrence. Kurtz and coworkers and Vrieling and associates have related the increased rate of local recurrence to non–age-related adverse pathologic variables; however, other analyses confirm that young age is an independent risk factor for increased local recurrence.
Extensive intraductal component (EIC) has been associated with an increased rate of local recurrence because of a higher likelihood of residual disease and because tumor-free margins are less likely. Tumors are considered to contain an EIC component when more than 25% of the tumor is composed of ductal carcinoma in situ (DCIS), both within the tumor and in the periphery. However, a number of studies have confirmed that when negative margins are achieved, as opposed to close or positive margins, the presence of EIC is not associated with an increased rate of local recurrence and does not preclude breast conservation.
In addition to the preceding patient- and tumor-related factors, there are treatment-related factors that can decrease the incidence of local recurrence. The addition of adjuvant chemotherapy and hormonal therapy can further decrease the rate of local recurrence. One retrospective study demonstrated a 66% decrease in local recurrence when patients were given adjuvant hormonal therapy. In the NSABP B-13 trial, patients with node-negative, estrogen receptor (ER)–negative disease were randomized to a chemotherapy arm or a no-treatment arm. The 8-year rate of recurrence in the ipsilateral breast was 13.4% in the nontreatment arm but only 2.6% in the chemotherapy arm of the study.
For ionizing RT to be effective in tumor control, the primary tumor must be completely excised with pathologically confirmed negative margins. RT is not delivered as an adjunctive therapy to compensate for inadequate surgery; rather this modality is an adjunct to sterilize the operative field of the index quadrant of therapy. Pathologic margin status is the most important factor associated with ipsilateral breast tumor recurrence. Patients with positive surgical margins may have an associated increased risk of local recurrence (5%–25%). For many years, the recommended margin of tissue surrounding tumor remained in question, but a conference was held in 2014 and, based on a meta-analysis, found that no ink on tumor cells was adequate, and in fact, additional margin distance did not significantly improve ipsilateral breast tumor recurrence or survival. This conference held by the Society of Surgical Oncology (SSO) and American Society for Radiation Oncology (ASTRO), in collaboration with the American Society of Clinical Oncology (ASCO) and the American Society of Breast Surgeons (ASBS). Adherence to the consensus recommendation of no ink on tumor provides a means to decrease reexcision rates, improve cosmetic results, and decrease health care costs. It is understood that local recurrence is likely influenced by the interaction of factors and not one factor alone. Wider excision may be necessary with extensive EIC, young age, lack of systemic therapy, or delayed RT to ensure low rates of local recurrence according to Horst and colleagues ; however, the SSO/ASTRO consensus reports no decrease in IBTR for young patients, unfavorable tumor biology, lobular cancers, or tumors with EIC when wider margins were taken. The standard treatment of a local recurrence after BCT is mastectomy; however, some have adopted local excision alone for recurrence (e.g., ductal carcinoma in situ or for small invasive tumors), consequently accepting an increased risk of additional recurrence. There are now many studies in support of repeat breast-conserving therapy for IBTR as a safe option in a select group of patients. Although there are no prospective trial results comparing breast-conserving surgery with or without RT to mastectomy for IBTR, several retrospective studies have concluded that salvage lumpectomy followed by partial breast irradiation is equal to mastectomy as treatment for IBTR in selected patients—most favorably those >50 years old, those with recurrences (≤2 cm), late recurrences (>48 months), single-site recurrences—and would provide acceptable cosmetic results as suggested by Vila and colleagues. The most commonly used method of partial breast irradiation applied after salvage lumpectomy for IBTR is multicatheter interstitial brachytherapy, but balloon catheter brachytherapy, conformal external beam RT, and intraoperative RT can also be used.
Cosmetic Outcome
An acceptable cosmetic appearance is the third goal of breast conservation surgery. Cosmetic outcome depends principally on the patient’s view of the cosmetically acceptable breast; however, many analyses use the radiation oncologist’s and/or surgeon’s determinants of outcome results ( Fig. 32.6 ). Factors that may affect cosmetic outcome include the volume of tissue removed compared with the breast size and the location of the tumor. Tumors in the medial and inferior breast quadrants, when resected, may result in adverse cosmetic outcome compared with tumors in other quadrants of the breast. In one study of patients’ appraisal of cosmetic outcome, 96% of patients found their operated breasts to have an acceptable appearance, and most viewed their operated breasts to have an acceptable (“good”) outcome.
In a retrospective review from the Joint Center for Radiation Therapy, the examining physician evaluated the cosmetic outcome. Good or excellent cosmetic outcome was noted in 94% of 655 patients. In this cohort, the extent (volume) of breast tissue resection correlated with the patient’s assessment of cosmetic outcome. When the amount of resected breast tissue was less than 35 cm 3 , 85% of patients had excellent scores and 96% had good or excellent scores. When the resected breast tissue was larger than 85 cm 3 , only 51% had excellent scores and 94% had either excellent or good scores. In the study by Cochrane and associates comparing patient satisfaction versus an independent panel assessment of cosmesis, the volume of breast tissue removed was an accurate predictor of cosmesis and patient satisfaction. Other studies examining cosmetic outcome after lumpectomies confirm similar results.
A consensus conference, the Collaborative Attempt to Lower Lumpectomy Reoperation Rates (CALLER), was held by the ASBS in 2015 to reduce lumpectomy reoperations and improve cosmetic outcomes. The recommendations reported by Landercaster and colleagues included preoperative imaging with full field digital mammography with ultrasound as needed, minimally invasive breast biopsies for diagnosis, multidisciplinary breast teams, localization techniques for nonpalpable cancers, oncoplastic techniques, specimen orientation of three or more margins, intraoperative surgeon-reviewed specimen radiograph, consideration of shave margins and pathology assessment of margins intraoperatively, compliance with the previously mentioned SSO-ASTRO margin guideline, and routine use of patient reported outcomes specific to breast surgery when feasible.
A randomized controlled trial by Chagpar and colleagues randomized 235 patients intraoperatively who were undergoing partial mastectomy for stage 0 to III breast cancer to have further cavity shave margins or no further margins. The positive specimen margin in both groups was similar before randomization, but the shave group showed a significantly lower positive margin rate than the no shave group (19% and 34%, respectively). In addition, there was a reduction in the rate of reexcision surgery for margin clearance from 21% in the no shave group to 10% in the shave margin group ( p = .02). Minimizing the need for reexcision to obtain clear margins will be beneficial in terms of cosmetic result after lumpectomy.
Patient Selection
Careful patient selection is essential to ensure a low rate of local recurrence and an acceptable cosmetic outcome. A thorough history, physical examination, and mammographic examination are vital in choosing suitable candidates for BCT. Improvement in our understanding of risk factors for local recurrence and improvement in imaging technology have significantly reduced the incidence of local recurrence since the mid-1980s. Patients are not candidates for breast conservation if they have a high probability of recurrence, possess a high probability of complications from RT, have an unacceptably poor cosmetic result, or prefer mastectomy.
Several factors prohibit the use of BCT ( Box 32.1 ). Pregnancy is an absolute contraindication to RT because of radiation scatter and its potential for fetogenesis maturation injury. Although the breast-conserving surgical procedure may be performed during pregnancy, RT should not be delivered in any gestational period. Clearly, the gestational period of greatest fetal risk is the first trimester. Patients with extensive malignant-appearing microcalcifications are not suitable candidates for breast conservation. This mammographic presentation typically suggests diffuse ductal carcinoma in situ. A history of prior therapeutic radiation to the breast region or mediastinum/lung in most circumstances can be prohibitive to conservative surgery with radiation. Finally, patients who have persistently positive margins are unsuitable for breast conservation. Two or more primary tumors in separate quadrants (multicentric disease) of the breast may represent a contraindication to breast-conserving surgery. Multifocal (multiple tumors within the same quadrant) disease is typically not a contraindication to breast conservation, if it is possible to perform complete removal of the lesions with negative margins while maintaining a cosmetically acceptable outcome.
Absolute Contraindications
Pregnancy
Persistent positive margins
Inflammatory breast cancer
Diffuse malignant-appearing calcifications
Relative Contraindications
History of collagen vascular disease
Multiple gross tumors or indeterminate calcifications within the same quadrant
Two or more primary tumors in separate quadrants
History of prior radiation to the breast area
Breast size to tumor size ratio
Other factors that may contraindicate breast-conserving surgery include a history of collagen vascular disease and a diminutive breast size relative to tumor size. Collagen vascular disease may result in increased toxicity from RT. Neoadjuvant chemotherapy may be offered as an alternative in patients whose primary tumor is too large to undergo breast conservation at initial presentation. Cytoreduction with neoadjuvant chemotherapy does not improve survival but may render some patients who would otherwise have had mastectomy to be candidates for BCT. Family history, positive lymph nodes, bilateral breast cancer, and lobular histology should not exclude patients for breast conservation if otherwise eligible. Paget disease of the breast also should not be excluded from BCT as long as a careful preoperative imaging assessment of the breast is performed, often including breast magnetic resonance imaging (MRI) due to the frequency of underlying mammographically occult breast cancer as well as multicentricity and multifocality associated with Paget disease. With negative surgical margins and application of adjuvant radiotherapy, a systematic review including 43 studies reported by Helme and colleagues found oncologic outcomes of BCT for Paget to be equivalent to mastectomy. A central lumpectomy with excision of the entire nipple-areolar complex is required for cases of Paget disease of the nipple that qualify for BCT ( Fig. 32.7 ).
Magnetic Resonance Imaging
Several studies have been published indicating that MRI may be a useful adjunct to traditional breast imaging due to its increased sensitivity and specificity compared with traditional breast imaging. MRI has the imaging capability to detect mammographically and clinically occult tumor foci within the ipsilateral breast in approximately 25% of patients with known breast cancer. A 2012 European radiologic systematic review and meta-analysis reports MRI detected additional disease in 20% of women on the ipsilateral side and 5.5% in the contralateral breast. In addition, Plana and colleagues reported 12.8% of women with true positive MRI findings converted to a more extensive surgery, most commonly from BCT to bilateral mastectomies. Review of the available literature states clear guidelines for the use of breast MRI in patients with at least a 20% to 25% lifetime risk of the development of breast cancer as a screening tool, but no clear guidelines exist for the use of MRI as a perioperative tool for patients diagnosed with breast cancer. The results of a literature review by Pilewskie and King in 2014 showed little to no statistically significant decrease in IBTR rates, contralateral breast cancer rates, or improvement in survival rates with use of breast MRI. There does appear to be some benefit to MRI for lobular cancers, mammographically occult primary cancers, and measurement of tumor response to neoadjuvant chemotherapy to determine potential for BCT. The role of MRI as a surveillance strategy to follow women post-BCT has not been determined but may have clinical benefit in some high-risk situations and will require additional investigation.
Operative Technique
The goal of breast-conserving surgery is to achieve a low rate of local recurrence while preserving cosmetic outcome. Lumpectomy (segmental mastectomy, partial mastectomy, tylectomy) is the most common form of breast-conserving surgery internationally. Several aspects of technique are emphasized.
Localization
With advances in breast imaging, we have seen an increase in the number of small, nonpalpable breast lesions requiring surgical intervention. A Cochrane review by Chan and colleagues reported that wire-guided localization (WGL) of breast lesions remains the gold standard and in survey results is the predominantly used tool to localize nonpalpable breast lesions for excision. Eleven randomized controlled trials were included with the majority of comparisons of WGL, radioguided occult lesion localization (ROLL), and radioactive iodine ( I) seed localization (RSL). There was no statistically significant difference in terms of successful localization, positive margin rate, and reexcision rate among these options. These are all invasive methods of localization that require an additional procedure before surgery, leading to scheduling conflicts and patient discomfort and inconvenience. Arentz and colleagues reported on their 10-year experience with Hematoma-directed ultrasound-guided (HUG) breast lumpectomy in 2010. They reported a statistically significant decreased positive margin rate on lumpectomies for malignancy using intraoperative ultrasound (IOUS) for HUG compared with performing WGL. A recent review by Volders and colleagues compares the data on IOUS guidance for localization compared with previously mentioned methods. The positive margin rates with IOUS guidance range from 0% to 17% as opposed to the more commonly utilized techniques with rates up to 40% in some studies. In addition, IOUS prevents patients from needing an additional procedure before surgery, thereby alleviating scheduling conflicts and decreasing anxiety and discomfort for the patient.
Incision
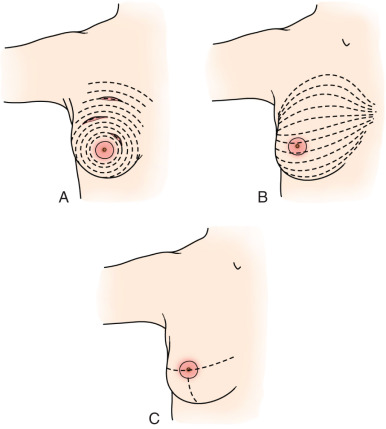
An incision corresponding to Langer or Kraissl lines is usually used; although some surgeons prefer radial incisions at the 3, 6, or 9 o’clock positions ( Fig. 32.8 ). In the past, an incision remote from the tumor was inadvisable; however, with the acceptance of oncoplastic techniques, this has become more acceptable. An incision directly over the tumor can always be used, but often can lead to a poor cosmetic result, especially in the upper inner quadrant. Tumors that are very near to the skin surface also typically require an incision directly over the tumor to ensure a negative anterior margin. Today we use a variety of incisions with cosmetic end result in mind, often utilizing a circumareolar incision or in a natural skin fold, where the scar will be somewhat hidden.
Tumor Removal
The tumor is removed so that it is completely surrounded by normal breast parenchyma. The surgeon’s objective is to remove an amount of tissue adequate to achieve negative specimen margins. An attempted resection amount of 0.5 to 1 cm of grossly normal breast tissue resulted in histologically negative margins in 95% of 239 patients in the experience of Kearney and Morrow. Larger resections may be necessary for invasive ductal carcinomas with EIC and for infiltrating lobular carcinomas; these clinical presentations have convincingly greater probability of pathologic multifocality and multicentricity. Undermining of skin, with thin skin flaps, is strongly discouraged because this technique results in an unfavorable cosmetic result after radiation. Conserving the subcutaneous fat prevents skin retraction and indentation concavity ( Fig. 32.9 ). No special effort is made to include pectoral fascia in the specimen unless the lesion is attached to or approximates this anatomic boundary.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








