Abstract
This chapter outlines the anatomy, blood, and nerve supply of the mammary gland and its embryologic development and hormonal influences through the various stages of life as well as the axilla, chest wall, and related metastatic sites.
Keywords
anatomy, breast, axilla, chest wall, metastatic sites
Paired mammary glands, or breasts, are a distinguishing feature of mammals. These glands evolve as milk-producing organs to provide nourishment to the offspring, in a relatively immature and dependent state with embryonic development in utero. The organ develops from the primordially derived breast tissue, which anatomically matures as a modified sweat gland. The act of nursing the young provides physiologic benefits to the mother by aiding in postpartum uterine involution and to the newborn with nutritional feeding and the transfer of passive immunity. The nursing of the offspring is also of significance in the emotional bonding between the mother and her infant.
With embryologic development, there is growth and differentiation of the breasts in both sexes (for a review, see Morehead ). Embryologically, the nascent paired glands develop along parallel lines, the “milk lines” ( Fig. 2.1 ), extending between the limb buds of the developmental axilla to the future inguinal region. Among the various mammalian species, the number of paired glands varies greatly and is related to the number of fetuses in each litter. In humans and most other primates, only one gland develops ipsilaterally on each side in the pectoral region. An extra breast ( polymastia ) or nipple ( polythelia ) may occur as a heritable condition with frequency of 1% in the female population. These relatively rare conditions also may occur in the male gender. When present, the supernumerary breast or nipple usually forms appendages along the milk lines; about one-third of the affected individuals have multiple extra (supernumerary) breasts or nipples.
With hormonal steroidal influence of the female gender, the breasts undergo extensive postnatal development, which is correlated with age and regulated by ovarian hormones that influence reproductive function. By about 20 years of age, the female breast has reached its developmental maturity, and by age 40, it begins atrophic changes, even before formal presentation of menopause. During each menstrual cycle, structural changes occur in the breast under the influence of fluctuations of ovarian hormone levels. During pregnancy and lactation, striking changes occur not only in the functional activity of the breast but also in the volume of glandular tissue. The actual secretion and production of milk are induced and enhanced by prolactin from the pituitary and by somatomammotropin from the placenta. With the changes in the hormonal environment that occur at menopause, the glandular component of the breast regresses, or involutes, and is replaced by fat and connective tissue stroma (see Fig. 2.1 ).
Gross Anatomic Structure: Surface Anatomy
Form and Size
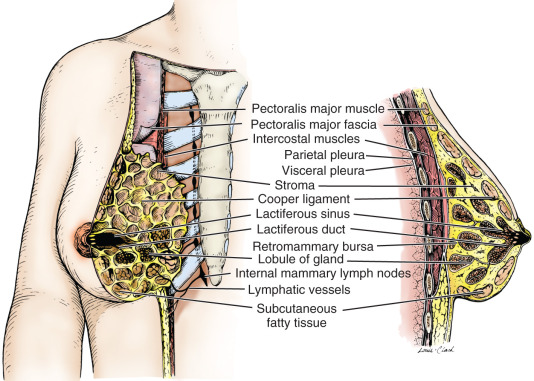
The mammary gland is located within the superficial fascia of the anterior thoracic wall. It consists of 15 to 20 lobes of glandular tissue of the tubuloalveolar type. Longitudinal fibrous connective stroma forms a latticed framework that supports the lobes; adipose tissue fills the space between the lobes. In the premenopausal female, structure and stroma are dense; the organ remains mobile on the chest wall residing on the mammary bursae. Subcutaneous connective tissue surrounds the gland and extends as septa between the lobes and lobules, providing support for the glandular elements; however, the septae does not form a distinctive capsule around any component of the breast. The deep layer of the superficial fascia lies upon the posterior (deep) surface of the breast and resides upon the pectoral (deep) fascia of the thoracic wall. A distinct space, the retromammary bursa, can be identified surgically on the posterior aspect of the breast between the deep layer of the superficial fascia and the deep investing fascia of the pectoralis major, and contiguous muscles of the thoracic wall ( Fig. 2.2 ). As noted, the retromammary bursa contributes to the mobility of the breast on the thoracic wall. Fibrous thickenings of the connective tissue interdigitate between the parenchymal tissue of the breast. This connective tissue extends from the deep layer of the superficial fascia (hypodermis) and attaches to the dermis of the skin. These suspensory structures, called Cooper ligaments, distinctively insert perpendicular to the delicate superficial fascial layers of the cutis reticularis of the dermis, or corium, permitting remarkable mobility of the breast, while providing central support.
At maturity, the glandular portion of the breast has a unique and distinctive protuberant conical form. The base of the cone is roughly circular, measuring 10 to 12 cm in diameter and 5 to 7 cm in thickness. Commonly, breast tissue extends into the axilla as the axillary tail (tail of Spence). There is tremendous variation in the size of the breast with frequent asymmetry in any individual. A typical nonlactating breast weighs between 150 and 225 g, whereas the lactating breast may exceed 500 g. In a study of breast volume in 55 women, Smith and colleagues reported that the mean volume of the right breast was 275.46 mL (SD = 172.65, median = 217.7, minimum = 94.6, maximum = 889.3) and the left breast was 291.69 mL (SD = 168.23, median = 224, minimum = 106.9, maximum = 893.9).
The breast of the nulliparous female has a typical hemispheric configuration with distinct flattening above the nipple. The multiparous breast, which has experienced the hormonal stimulation associated with pregnancy and lactation, is usually larger, more dense, and more pendulous. As noted, during pregnancy and lactation, the breast increases dramatically in size, and with weight of milk assumes a more pendulous configuration. With increasing age, the postmenopausal breast usually decreases in volume, becoming somewhat flattened and gravitationally pendulous; thereafter, the organ is less dense, second to replacement of parenchyma with fat.
Extent and Location
The mature female breast extends inferiorly from the level of the second or third rib to the inframammary fold, which is at about the level of the sixth or seventh rib, and laterally from the lateral border of the sternum to the anterior or midaxillary line. The deep or posterior surface of the breast rests on portions of the deep investing fasciae of the pectoralis major, serratus anterior, and external abdominal oblique muscles and the uppermost superior extent of the rectus sheath. The axillary tail (tail of Spence) of the breast extends into the anterior axillary fold. The upper half of the breast, and particularly the upper outer quadrant, contains more glandular tissue than does the remainder of the breast. This anatomic fact accounts for the higher frequency of breast cancer in this quadrant.
Microscopic Anatomic Structure
Nipple and Areola
The epidermis of the nipple and areola is highly pigmented and somewhat wrinkled. It is covered by keratinized, stratified squamous epithelium. The deep surface of the epidermis is invaded by unusually long dermal papillae that allow capillaries to bring blood perfusion to its surface, giving the nipple-areola a pinkish color in young, fair-skinned individuals. At puberty, the pigmentation of the nipple and areola increases, and the nipple becomes more prominent. With the gravid state, the areola enlarges, and the degree of pigmentation increases. Deep to the areola and nipple, bundles of smooth muscle fibers are arranged radially and circumferentially in the dense connective tissue and longitudinally along the lactiferous ducts that extend up into the nipple. These muscle fibers are responsible for the erection of nipple that occurs in response to various stimuli (for a review of the anatomy of the nipple and areola, see Giacometti and Montagna ). The areola contains sebaceous glands, sweat glands, and accessory areolar glands (of Montgomery), which are intermediate in their structure between true mammary glands and sweat glands. The accessory areolar glands produce small elevations on the surface of the areola. The sebaceous glands (which usually lack associated hairs) and sweat glands are located along the margin of the areola. Whereas the apex of the nipple contains numerous free sensory nerve cell endings and Meissner corpuscles in the dermal papillae, the areola contains fewer of these structures. In a review of the innervation of the nipple and areola, Montagna and Macpherson reported observing fewer nerve endings than described by other investigators. They reported that most of the sensory endings were at the apex of the nipple. Neuronal plexuses are also present around hair follicles in the skin peripheral to the areola, and palpatory pacinian corpuscles (vibratory pressure sensation and touch) may be present in the dermis and in the glandular tissue. The rich sensory innervation of the breast, particularly the nipple and areola, is of great functional significance. The suckling infant initiates a reflex chain of neural and neurohumoral events, resulting in the release of milk and maintenance of glandular differentiation that is essential for continued lactation.
Inactive Mammary Gland
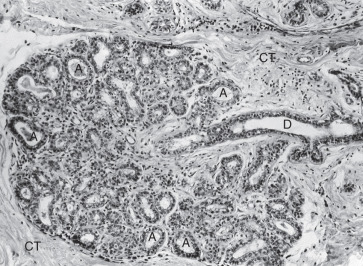
The adult mammary gland is composed of 15 to 20 irregular lobes of branched tubuloalveolar glands. The lobes, separated by fibrous bands of connective tissue, radiate from the mammary papilla, or nipple, and are further subdivided into numerous lobules. Those fibrous bands that connect with the dermis are the suspensory ligaments of Cooper. Abundant adipose tissue is present in the dense connective tissue of the interlobular spaces. The intralobular connective tissue is much less dense and contains little fat.
Each lobe of the mammary gland ends in a lactiferous duct (2–4 mm in diameter) that opens through a constricted orifice (0.4–0.7 mm in diameter) onto the nipple (see Fig. 2.2 ). Beneath the areola, each duct has a dilated portion, the lactiferous sinus. Near their openings, the lactiferous ducts are lined with stratified squamous epithelium. The epithelial lining of the duct shows a gradual transition to two layers of cuboidal cells in the lactiferous sinus and then becomes a single layer of columnar or cuboidal cells through the remainder of the duct system. Myoepithelioid cells of ectodermal origin are located within the epithelium between the surface epithelial cells and the basal lamina. These cells, arranged in a basketlike network, are present in the secretory portion of the gland but are more apparent in the larger ducts. They contain myofibrils and are strikingly similar to smooth muscle cells in their cytology.
Under light microscopy, epithelial cells are characteristically seen to be attached to an underlying layer called the basement membrane. With electron microscopy, the substructure of the basement membrane can be identified. The inner layer of the basement membrane is called the basal lamina. In the breast, the parenchymal cells of the tubuloalveolar glands, as well as the epithelial and myoepithelial cells of the ducts, rest on a basement membrane or basal lamina. The integrity of this supporting layer is of significance in evaluating biopsy specimens of breast tissue. Changes in the basement membrane have important implications in immune surveillance, transformation, differentiation, and metastasis.
Morphologically, the secretory portion of the normal mammary gland varies greatly with age and during pregnancy and lactation ( Fig. 2.3 ). In the inactive gland, the glandular component is sparse and consists chiefly of duct elements ( Fig. 2.4 ). Most investigators believe that the secretory units in the inactive breast are not organized as alveoli and consist only of ductules. During the menstrual cycle, the inactive breast undergoes slight cyclical changes. Early in the cycle, the ductules appear as cords with little or no lumen. Under estrogen stimulation, at about the time of ovulation, secretory cells increase in height, lumina appear as small amounts of secretions accumulate, and fluids and lipid accumulate in the connective tissue. Then, in the absence of continued hormonal stimulation, the gland regresses to a more inactive state through the remainder of the cycle.
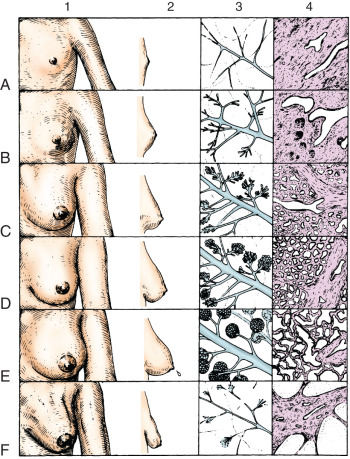
Active Mammary Glands: Pregnancy and Lactation
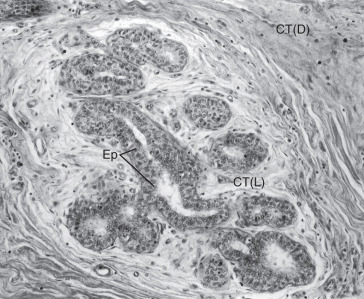
During pregnancy, in preparation for lactation, the mammary glands undergo dramatic proliferation and development. These changes in the glandular tissue are accompanied by relative decreases in the amount of connective and adipose tissue. Plasma cells, lymphocytes, and eosinophils infiltrate the fibrous component of the connective tissue as the breast develops in response to hormonal stimulation. The development of the glandular tissue is not uniform, and variation in the degree of development may occur within a single lobule. The cells vary in shape from low columnar to flattened. As the cells proliferate by mitotic division, the ductules branch and alveoli begin to develop. In the later stages of pregnancy, alveolar development becomes more prominent ( Fig. 2.5 ). Near the end of pregnancy, the actual proliferation of cells declines, and subsequent enlargement of the breast occurs through hypertrophy of the alveolar cells and accumulation of their secretory product in the lumina of the ductules.
The secretory cells contain abundant endoplasmic reticulum, a moderate number of large mitochondria, a supranuclear Golgi complex, and a number of dense lysosomes. Depending on the secretory state of the cell, large lipid droplets and secretory granules may be present in the apical cytoplasm. Two distinct products produced by the cells are released by different mechanisms. The protein component of the milk is synthesized in the granular endoplasmic reticulum, packaged in membrane-limited secretory granules for transport in the Golgi apparatus, and released from the cell by fusion of the granule’s limiting membrane with the plasma membrane. This type of secretion is known as merocrine secretion. The lipid, or fatty, component of the milk arises as free lipid droplets in the cytoplasm. The lipid coalesces into large droplets that pass to the apical region of the cell and project into the lumen of the acinus before their release. As they are released from the cell, the droplets are invested with an envelope of plasma membrane. A thin layer of cytoplasm is trapped between the lipid droplet and plasma membrane as lipid is being released. It should be emphasized that only a very small amount of cytoplasm is lost during this secretory process, classically known as apocrine secretion.
The milk released during the first few days after childbirth is known as colostrum. It has low lipid content but is believed to contain considerable quantities of antibodies that provide the newborn with some degree of passive immunity. The lymphocytes and plasma cells that infiltrate the stroma of the breast during its proliferation and development are believed to be, in part, the source of the components of the colostrum. As the plasma cells and lymphocytes decrease in number, the production of colostrum stops and lipid-rich milk is produced.
Hormonal Regulation of the Mammary Gland
Physiologically enhanced production of estrogens and progesterone by the ovary at puberty induces the initial growth of the mammary gland. Subsequent to this nascent development, slight changes occur in the morphology of the glandular tissue with each ovarian, or menstrual, cycle. With pregnancy, the corpus luteum and placenta continuously produce estrogens (estrone, estradiol, estriol) and progesterone, which further stimulate proliferation and development of the mammary gland (see Fig. 2.5 ). The growth of the glands is also dependent on the presence of prolactin, produced by the adenohypophysis; somatomammotropin (lactogenic hormone), produced by the placenta; and adrenal corticoids. The level of circulating estrogens and progesterone diminishes acutely at parturition with the degeneration of the corpus luteum and loss of the placenta. The secretion of milk is then brought about by increased production of prolactin and adrenal cortical steroids. A neurohormonal reflex regulates the high level of prolactin production and release. The act of suckling by the infant initiates impulses from receptors in the nipple; these impulses provide feedback regulation for cells in the hypothalamus. The impulses also cause the release of oxytocin in the neurohypophysis. The oxytocin stimulates the myoepithelial cells of the mammary glands, causing them to contract and eject milk. In the absence of the suckling reflex stimulus, secretion of milk ceases and the lobular glands regress and return to an inactive state. After menopause, the gland atrophies, or involutes. As the release of ovarian hormones is diminished, the secretory cells of the alveoli degenerate and disappear, but some of the ducts remain. The connective tissue also demonstrates degenerative changes that are marked by a decrease in the number of stromal cells and collagen fibers.
Thoracic Wall
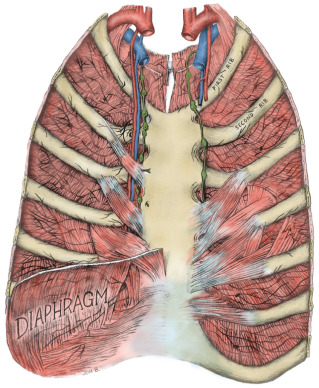
The thoracic wall is composed of both skeletal and muscular components. The skeletal components include the 12 thoracic vertebrae, the 12 ribs and their costal cartilages, and the sternum. The spaces between the ribs, the intercostal spaces, are filled with the external, internal, and innermost intercostal muscles and the associated intercostal vessels and nerves ( Fig. 2.6 ). Some anatomists refer to the innermost layer as the intima of the internal intercostal muscle. The terminology chosen is of no particular consequence; the relationship that should be appreciated is that the intercostal veins, arteries, and nerves pass in the plane that separates the internal intercostal muscle from the innermost (or intimal) layer. The endothoracic fascia, a thin fibrous layer of connective tissue forming a fascial plane continuous with the most internal component of the investing fascia of the intercostal muscles and the adjacent layer of the periosteum, marks the internal limit of the thoracic wall. The parietal pleura rests on the endothoracic fascia.
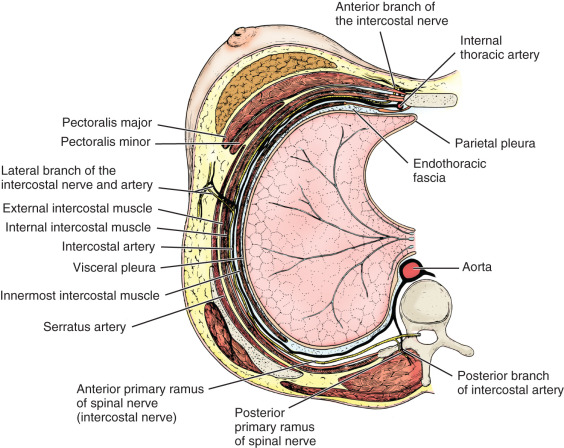
It is important to recognize that the muscles and skeletal girdles of the upper extremities almost completely cover the thoracic wall anteriorly, laterally, and posteriorly. For the surgeon concerned with the breast, knowledge of the anatomy of the axilla and pectoral region is essential.
The 11 pairs of external intercostal muscles whose fibers run downward and forward form the most superficial layer (see subsequent section on the innervation of the breast and Fig. 2.12 later). The muscle begins posteriorly at the tubercles of the ribs and extends anteriorly to the costochondral junction. Between the costal cartilages, the muscle is replaced by the external intercostal membrane. The fibers of the 11 pairs of internal intercostal muscles run downward and posteriorly. The muscle fibers of this layer reach the sternum anteriorly. Posteriorly, the muscle ends at the angle of the ribs and then the layer continues as the internal intercostal membrane. The innermost intercostal muscles (intercostales intimi) form the most internal layer and have fibers that are oriented more vertically but almost in parallel with the internal intercostal muscle fibers. The muscle fibers of this layer occupy approximately the middle half of the intercostal space. This is the least well developed of the three layers. It can best be distinguished by the fact that its fibers are separated from the internal intercostals by the intercostal vessels and nerves.
The subcostalis and transversus thoracis muscles are located on the internal surface of the thoracic wall. They occur in the same plane as the innermost intercostal muscles and are considered anterior and posterior extensions of this layer. The subcostal muscles are located posteriorly and have the same orientation as the innermost intercostal muscles. They are distinct because they pass to the second or third rib below (i.e., they pass over at least two intercostal spaces). Anteriorly, the transversus thoracis muscles form a layer that arises from the lower internal surface of the sternum and extends upward and laterally to insert on the costal cartilages of the second to sixth ribs ( Fig. 2.7 ). These fibers pass deep to the internal thoracic artery and accompanying veins.
All of these muscles are innervated by the intercostal nerves associated with them. These nerves also give branches to the overlying skin. In a similar fashion, the intercostal vessels supply intercostal muscles and give branches to the overlying tissues. The intercostal nerves are direct continuations of the ventral primary rami of the upper 11 thoracic spinal nerves. As the nerves pass anteriorly, they give branches to supply the intercostal muscles. In addition, each nerve gives a relatively large lateral cutaneous branch, which exits the intercostal space along the midaxillary line near the attachment sites of the serratus anterior muscle on the ribs. The lateral cutaneous nerves then give branches that extend anteriorly and posteriorly. As the intercostal nerve continues anteriorly, it gives additional branches to the intercostal muscles. Just lateral to the border of the sternum the upper five intercostal nerves pierce the internal intercostal muscle and the external intercostal membrane to end superficially as the anterior cutaneous nerves of the chest. These nerves give rise to medial and lateral branches that supply the overlying skin. The lower six intercostal nerves continue past the costal margin into the anterior abdominal wall and are therefore identified as thoracoabdominal nerves.
The intercostal arteries originate in two groups: the anterior and posterior intercostal arteries. The posterior intercostal arteries, except for the first two spaces, arise from the thoracic aorta. The posterior intercostals for the first two spaces arise from the superior intercostal arteries, which on the left and right sides branch from the costocervical trunk. The anterior intercostals are usually small paired arteries that extend laterally to the region of the costochondral junction. The anterior intercostal arteries of the upper five intercostal spaces arise from the internal thoracic (or mammary) artery; those of the lower six intercostal spaces arise from the musculophrenic artery. The anterior and posterior intercostal veins demonstrate a similar distribution. Anteriorly, they drain into the musculophrenic and internal thoracic veins. Posteriorly, the intercostal veins drain into the azygos and hemiazygos systems of veins.
The superficial muscles of the pectoral region include the pectoralis major and minor muscles and the subclavius muscle. The pectoralis major muscle is a fan-shaped muscle with two divisions. The clavicular division (or head) originates from the clavicle and is easily distinguished from the larger costosternal division that originates from the sternum and costal cartilages of the second through sixth ribs. The fibers of the two divisions converge laterally and insert into the crest of the greater tubercle of the humerus along the lateral lip of the bicipital groove. The cephalic vein serves as a convenient landmark defining the separation of the upper lateral border of the pectoralis major muscle from the deltoid muscle. The cephalic vein can be followed to the deltopectoral triangle, where it pierces the clavipectoral fascia and joins the axillary vein. The pectoralis major muscle acts primarily in flexion, adduction, and medial rotation of the arm at the shoulder joint. This action brings the arm across the chest. In climbing, the pectoralis major muscles, along with the latissimus dorsi muscles, function to elevate the trunk when the arms are fixed. The pectoralis major muscle is innervated by both the medial and the lateral pectoral nerves, which arise from the medial and lateral cords of the brachial plexus, respectively. Located deep to the pectoralis major muscle, the pectoralis minor muscle arises from the external surface of the second to the fifth ribs and inserts on the coracoid process of the scapula. Although its main action is to lower the shoulder, it may serve as an accessory muscle of respiration. It is innervated by the medial pectoral nerve.
The subclavius muscle arises from the first rib near its costochondral junction and extends laterally to insert into the inferior surface of the clavicle. It functions to lower the clavicle and stabilize it during movements of the shoulder girdle. It is innervated by the nerve to the subclavius muscle, which arises from the upper trunk of the brachial plexus.
Axilla
Knowledge of the anatomy of the axilla and its contents is of paramount importance to the clinician. It is also essential that the surgeon be thoroughly familiar with the organization of the deep fascia and neurovascular relationships of the axilla.
Boundaries of the Axilla
The axilla is a pyramidal compartment between the upper extremity and the thoracic walls ( Fig. 2.8 ). It is described as having four walls, an apex, and a base. The curved base is made of axillary fascia and skin. Externally, this region, the armpit, appears dome-shaped (and covered with hair after puberty). The apex is not a roof but an aperture that extends into the posterior triangle of the neck through the cervicoaxillary canal. The cervicoaxillary canal is bounded anteriorly by the clavicle, posteriorly by the scapula, and medially by the first rib. Most structures pass through the cervical axillary canal as they course between the neck and upper extremity. The anterior wall is made up of the pectoralis major and minor muscles and their associated fasciae. The posterior wall is composed primarily of the subscapularis muscle, located on the anterior surface of the scapula, and to a lesser extent by the teres major and latissimus dorsi muscles and their associated tendons. The lateral wall is a thin strip of the humerus, the bicipital groove, between the insertions of the muscles of the anterior and posterior walls. The medial wall is made up of serratus anterior muscle that covers the thoracic wall in this region (over the upper four or five ribs and their associated intercostal muscles).
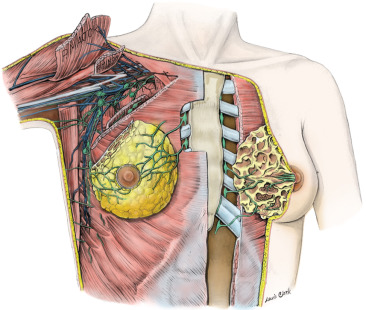
Contents of the Axilla
The axilla contains the great vessels and nerves of the upper extremity. These, along with the other contents, are surrounded by loose connective tissue. Fig. 2.8 illustrates many of the key relationships of structures within the axilla. The vessels and nerves are closely associated with each other and are enclosed within a layer of fascia, the axillary sheath. This layer of dense connective tissue extends from the neck and gradually disappears as the nerves and vessels branch.
The axillary artery may be divided into three parts within the axilla:
- 1.
The first portion, located medial to the pectoralis minor muscle, gives one branch—the supreme thoracic artery that supplies the thoracic wall over the first and second intercostal spaces.
- 2.
The second portion, located posterior to the pectoralis minor muscle, gives two branches—the thoracoacromial artery and the lateral thoracic artery. The thoracoacromial artery divides into the acromial, clavicular, deltoid, and pectoral branches. The lateral thoracic artery passes along the lateral border of the pectoralis minor on the superficial surface of the serratus anterior muscle. Pectoral branches of the thoracoacromial and lateral thoracic arteries supply both the pectoralis major and minor muscles and must be identified during surgical dissection of the axilla. The lateral thoracic artery is of particular importance in surgery of the breast as it supplies the lateral mammary branches.
- 3.
The third portion, located lateral to the pectoralis minor, gives off three branches—the anterior and posterior circumflex humeral arteries, which supply the upper arm and contribute to the collateral circulation around the humerus, and the subscapular artery. Although the latter artery does not supply the breast, it is of particular importance in the surgical dissection of the axilla. It is the largest branch within the axilla, giving rise after a short distance to its terminal branches, the subscapular circumflex and the thoracodorsal arteries, and it is closely associated with the central and subscapular lymph node groups. In the axilla, the thoracodorsal artery crosses the subscapularis and gives branches to it and to the serratus anterior and the latissimus dorsi muscles. A surgeon must use care in approaching this vessel and its branches to avoid undue bleeding that obscures the surgical field.
The axillary vein has tributaries that follow the course of the arteries just described. They are usually in the form of venae comitantes, paired veins that follow an artery. The cephalic vein passes in the groove between the deltoid and pectoralis major muscles and then joins the axillary vein after piercing the clavipectoral fascia.
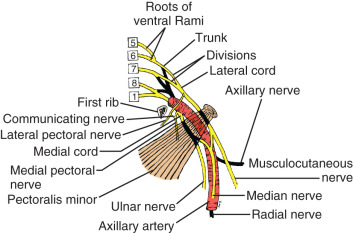
Throughout its course in the axilla, the axillary artery is associated with various parts of the brachial plexus ( Fig. 2.9 ). The cords of the brachial plexus—medial, lateral, and posterior—are named according to their relationship with the axillary artery. A majority of the branches of the brachial plexus arise in the axilla. The lateral cord gives four branches, namely, the lateral pectoral nerve, which supplies the pectoralis major; a branch that communicates with the medial pectoral nerve, which is called the ansa pectoralis ; and two terminal branches, the musculocutaneous nerve and the lateral root of the median nerve. Injury to the medial or lateral pectoral nerves, or the ansa pectoralis, which joins them, may lead to atrophy with loss of muscle mass and fat necrosis of the pectoralis major or minor muscles, depending of the level of nerve injury. The ansa pectoralis lies anterior to the axillary artery, making it vulnerable to injury during lymph node dissection in the axilla.