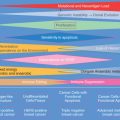
Abstract
Gynecomastia is benign enlargement of the male breast due to proliferation of the glandular component. This common clinical condition, which may be unilateral or bilateral, presents as an incidental finding on routine physical examination, a painless unilateral or bilateral breast enlargement, or a painful and tender mass beneath the areolar region. This chapter focuses on the prevalence, clinical presentation, physiology, histopathology, pathophysiology, diagnosis, and treatment of gynecomastia. Finally, certain specific clinical situations of gynecomastia are reviewed to provide easily understood summaries of the complexities of this topic.
Keywords
gynecomastia, estrogen/androgen, aromatase, subcutaneous mastectomy
Gynecomastia is benign enlargement of the male breast due to proliferation of the glandular component. This common clinical condition, which may be unilateral or bilateral, presents as an incidental finding on routine physical examination, a painless unilateral or bilateral breast enlargement, or a painful and tender mass beneath the areolar region. This chapter focuses on the prevalence, clinical presentation, physiology, histopathology, pathophysiology, diagnosis, and treatment of gynecomastia. Finally, certain specific clinical situations of gynecomastia are reviewed to provide easily understood summaries of the complexities of this topic.
Prevalence
There are three distinct peaks in the age distribution of physiologic gynecomastia. The first peak is during the neonatal period when palpable breast tissue transiently develops in 60% to 90% of newborns because of the transplacental passage of estrogens. The second peak is during puberty, with prevalence increasing at approximately 10 years of age and peaking between 13 and 14 years of age, followed by a decline during the later teenage years. Pubertal gynecomastia is estimated to occur within 15 months after an increase in testicular size, the first sign of puberty. The third peak is found in the adult population, with prevalence increasing at approximately 50 years of age and continuing into the eighth decade of life.
Most patients seeking consultation for gynecomastia have idiopathic gynecomastia (approximately 25%) or acute/persistent gynecomastia due to puberty (25%), drugs (10%–20%), cirrhosis/malnutrition (8%), or primary hypogonadism (8%). A lesser number have testicular tumors (3%), secondary hypogonadism (2%), hyperthyroidism (1.5%), or renal disease (1%).
Clinical Presentation
The patient presents with a swelling of the breast, often unilateral, which is commonly tender. The patient may be concerned about the tenderness, the cosmetic appearance or the possibility of malignancy. Examination reveals a firm “donut” of retroareolar tissue, which is mobile.
There is usually a clear demarcation of the firm breast tissue from the softer adjacent fat. Gynecomastia may be differentiated from pseudogynecomastia or lipomastia (fatty breasts), in which the subareolar tissue is of the same consistency as the adjacent subcutaneous adipose tissue. The hallmark of gynecomastia is concentricity. If an eccentric mass is found, an alternate diagnosis should be considered, and mammography and biopsy should be performed.
Physiology
Development of the Male Breast
Male breast development in the fetus occurs in an analogous fashion to female breast development. By the ninth week of gestation, a recognizable nipple bud has formed from basal cells in the pectoral region. By the end of the third month, squamous epithelium invades the nipple bud and ducts develop, which connect to the nipple at the skin’s surface. These become canalized and form lactiferous ducts. The blind ends of these ducts bud to form alveolar structures.
Neonatal Gynecomastia
Palpable enlargement of the male breast in the neonate is normal and occurs as a result of the action of prolactin, placental estrogens, and progesterone on the neonatal breast parenchyma. At birth, with decline in these hormones, this breast enlargement usually regresses within a few weeks; however, it has been observed to persist for longer periods.
Puberty
The breast tissues of both sexes appear histologically identical at birth and remain relatively quiescent during childhood, undergoing further differentiation at the time of puberty. In the majority of males, transient proliferation of the ducts and surrounding mesenchymal tissue takes place during the period of rapid sexual maturation, followed by involution and ultimately atrophy of the ducts. In contrast, in females, the breast ductal and periductal tissues continue to enlarge and develop terminal acini, processes that require both estrogen and progesterone.
Because estrogens stimulate breast tissue and androgens antagonize these effects, gynecomastia has long been considered the result of an imbalance between these hormones. The transition from the prepubertal to the postpubertal state is accompanied by a 30-fold increase in the concentration of testosterone, with only a threefold increase in estrogen levels. Therefore, a relative imbalance between serum estrogen and androgen levels can exist during a portion of the pubertal process and may result in gynecomastia. In an analysis of tissues from 30 males with gynecomastia, estrogen, progesterone, and androgen receptors were observed in 100%.
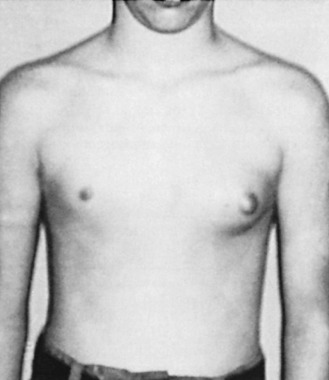
Pubertal Gynecomastia
Up to 70% of all boys will develop some degree of pubertal gynecomastia. Transient proliferation of the ducts and surrounding mesenchymal tissue takes place during this period of rapid sexual maturation, followed by involution and ultimately atrophy of the ducts. Gynecomastia is evident in as many as 69% of schoolboys in the United States. For many of these boys, the enlarged breast(s) is asymmetric, tender, and psychologically disturbing ( Fig. 7.1 ). However, by age 20, only a small percentage of these boys have a remaining palpable abnormality.
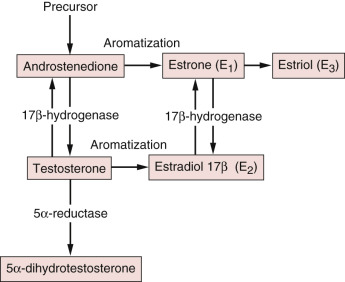
Normal Circulating Male Estrogen Concentrations
The adult testes secrete approximately 15% of the estradiol and less than 5% of the estrone in the circulation, whereas extragonadal tissues produce 85% of the estradiol and more than 95% of the estrone through the aromatization of precursors. The principal precursor of estradiol is testosterone, 95% of which is derived from the testes ( Fig. 7.2 ). Androstenedione, an androgen secreted primarily by the adrenal gland, serves as a precursor of estrone formation. The important extraglandular sites of aromatization are adipose tissue, liver, and muscle. In addition, a substantial degree of interconversion between estrone and estradiol takes place through the action of the widely distributed enzyme 17-ketosteroid reductase, which also catalyzes the conversion of androstenedione to testosterone.
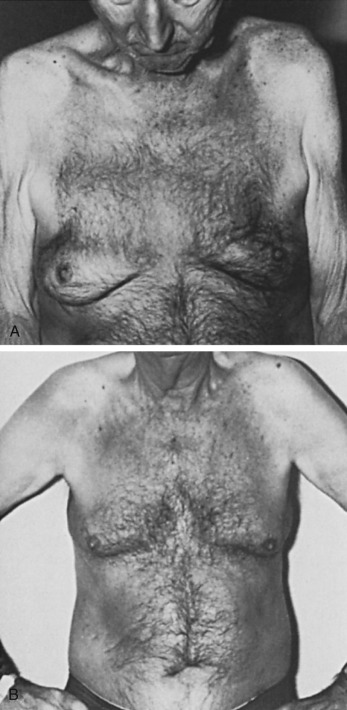
Senile Gynecomastia
Senile gynecomastia occurs in 32% to 65% of adult males ( Fig. 7.3A and B ), its prevalence correlates with the body fat content, and it does not require clinical evaluation unless symptomatic or of recent, rapid onset. Plasma testosterone concentration values begin to fall at approximately 70 years of age. In addition, there is concurrent elevation in the plasma sex hormone-binding globulin (SHBG), which causes a further fall in the free or unbound testosterone concentrations. A simultaneous increase in plasma luteinizing hormone (LH) may cause a concurrent increase in the rate of conversion of androgen to estrogen in peripheral tissues. Hence, relative hyperestrinism is evident with a decrease in the plasma androgen-to-estrogen ratio.
Histopathology
The histologic pattern of gynecomastia progresses from an early active phase (florid) to an inactive phase (fibrous), no matter what the cause. Grossly, there is a relatively sharp margin between breast tissue and surrounding subcutaneous fat. The few ductal structures of the male breast enlarge, elongate, and branch along with the encasing connective tissue. This combined increase in glandular and stromal elements provides for a regular distribution of each element throughout the enlarged breast. Often, there is an increase in cell number relative to the basement membrane. A recent study shows a consistent three-layered composition of ducts in gynecomastia, including a myoepithelial, intermediate (hormone receptor positive) luminal and inner (hormone receptor negative) luminal cell layer.
The loose connective tissue that regularly outlines the ducts as a central feature of gynecomastia is prominent only in the earliest stage of the disease. The fibroblasts within this loose tissue are relatively large but lack atypical features and are not clustered, even though they appear more frequently immediately adjacent to the basement membrane of the ducts.
Fibrous gynecomastia describes the later stage of gynecomastia and histologically has a dense collagenous stroma that contains relatively few fibroblasts. This dense collagenous tissue is applied closely to delicate basement membrane regions surrounding sparse epithelial elements in which hyperplasia is usually absent. The loose pattern of periductal stroma that characterizes the florid stage of gynecomastia is lacking. The stromal fibrosis is unlikely to respond to medical therapy. Researchers used immunohistochemical techniques to show that the majority (89%) of gynecomastia specimens are estrogen receptor positive. The investigators were unable to demonstrate an association between histopathologic staging of gynecomastia or hormonal parameters and estrogen receptor status.
Virtually any benign alteration found in the female breast (in particular, fibroadenoma and sclerosing adenosis) may be found in the male breast, although such changes are rare. Differentiating carcinoma of the male breast from gynecomastia may be difficult clinically, but the problem is easily resolved with histologic or cytologic examination. There are reports of concurrent gynecomastia and breast cancer ; ductal carcinoma in situ (DCIS) is reported to occur in the male breast, most often the solid and comedo types, although complex cribriform patterns are also demonstrated.
Whether the unusual cases of atypical hyperplasia and carcinoma in the male breast are preceded by gynecomastia is unknown. DNA analysis in patients with breast carcinoma, fibroadenoma, and gynecomastia has demonstrated HER2/neu amplification in 26.8% of breast carcinoma specimens but not in patients with fibroadenoma or gynecomastia. In addition, recent immunohistochemical studies have shown that inner luminal cells of gynecomastia differ in biomarkers from male DCIS, indicating that gynecomastia does not seem to be an obligate precursor of male breast cancer.
Pathophysiology
As previously stated, most patients who present with possible gynecomastia have idiopathic gynecomastia or acute/persistent gynecomastia due to puberty, drugs, cirrhosis/malnutrition, or primary hypogonadism. A few have testicular tumors, secondary hypogonadism, hyperthyroidism, or renal disease. Box 7.1 presents a comprehensive summary of the conditions commonly associated with gynecomastia. Gynecomastia can occur as a result of a relative or absolute excess of estrogens or a relative or absolute decrease in androgens.
Estrogen Excess
Testicular neoplasms
Adrenal cortex neoplasms
Ectopic HCG production
Hermaphroditism
Hyperthyroidism
Liver cirrhosis
Recovery from starvation
Androgen Deficiency
Primary testicular failure
Secondary testicular failure
Androgen resistance syndromes
Increased aromatase activity
Chronic renal failure
Drug Related
See Box 7.2
HCG, Human chorionic gonadotropin.
Estrogen Excess
Testicular Tumors
Testicular tumors can lead to increased blood estrogen levels by (1) estrogen overproduction, (2) androgen overproduction with aromatization in the periphery to estrogens, and (3) ectopic secretion of gonadotropins that stimulate otherwise normal Leydig cells.
Leydig Cell Neoplasms.
Leydig cell neoplasms are relatively uncommon, constituting approximately 2% to 3% of all testicular neoplasms. Such neoplasms are found in children as young as 2 years of age and in adults as old as 82 years of age. The average age at diagnosis is between 20 and 60 years. Leydig cell tumors account for up to 39% of non–germ cell tumors of the testes and 12% of the testicular neoplasms of children. Leydig cell tumors of the testes are most often unilateral. Sexual precocity is usually observed in children with these tumors and is accompanied by an increase in muscle mass and stature with advanced bone age in most patients. In children, Leydig cell tumors are almost uniformly benign.
In adults, physical changes are less frequent, with endocrine signs noted in approximately 30% of adults with these tumors ; painful gynecomastia and decreased libido are the most common manifestations. Symptoms may precede the onset of a palpable testicular mass, particularly with Leydig cell hyperplasia. Approximately 25% of the Leydig cell tumors in adult men secrete predominantly estrogen. For some patients, gynecomastia may be observed despite normal serum estrogen and testosterone levels. In these patients, gynecomastia may occur after in situ conversion of androstenedione to estrone in breast parenchyma, leading to increased tissue estrogen values without increasing serum levels.
Malignant transformation of Leydig cell tumors occurs in approximately 10% of patients, predominantly in adults and older men. Gynecomastia associated with Leydig cell tumors is more often seen when these tumors are benign, especially when 17-ketosteroid values are normal. Malignant Leydig cell tumors usually demonstrate abnormal estrogen or androgen levels and are associated more frequently with elevated estrogens and 17-ketosteroid levels without gynecomastia.
For patients with gynecomastia and increased circulating estrogen levels, pituitary suppression of LH release may initiate atrophy of the contralateral testis. A prolonged plasma estradiol response to human chorionic gonadotropin (HCG) is a useful, although nonspecific, adjunct in the diagnosis of Leydig cell tumors.
Sertoli Cell Tumors.
Sertoli cell tumors comprise less than 1% of all testicular tumors and occur at all ages, but one third occur in children younger than 13 years of age, usually in boys younger than 6 months of age. The tumors usually do not produce endocrine effects in children.
Gynecomastia is seen in 26% to 33% of individuals with benign Sertoli cell tumors, and it rapidly regresses after orchiectomy. In most cases, there was no elevation of estrogen or testosterone serum concentrations. Of five reported patients with malignant Sertoli cell tumors and gynecomastia, two had elevated gonadotropin levels.
Multifocal Sertoli cell tumors in boys have been associated with the autosomal dominant syndrome of Peutz-Jeghers syndrome. The increased risk of gonadal tumors for females with Peutz-Jeghers syndrome was recognized at an earlier date.
The majority of Sertoli cell tumors are benign, but as many as 10% can be malignant. Males with distant metastatic disease have been reported.
Germ Cell Tumors.
Germ cell tumors are the most common cancers in males between 15 and 35 years of age. They are divided into seminomatous and nonseminomatous subtypes. The theory of a common origin of germ cell tumors of the testes from embryonal carcinoma cells is supported by ultrastructural studies and experimental production of teratoma from embryonal carcinoma explants. Estrogen effects in men with germ cell neoplasms occur secondary to increased aromatization of testosterone and androstenedione into estrogens in peripheral sites. Androstenedione, which has low androgenicity and is readily aromatized to estrone peripherally, may be produced in increased concentrations by some tumors, with the result of enhanced estrogen production.
Men with germ cell tumors who exhibit gynecomastia have a higher mortality than those without gynecomastia. After orchiectomy and chemotherapy, a 75% reduction in the number of men with gynecomastia is observed.
Use of testicular ultrasound for detection and localization of early testicular masses in males with gynecomastia is essential. Any young adult male with unexplained gynecomastia, loss of libido, or impotence should have diagnostic testicular ultrasound for evaluation of occult tumors.
Adrenal Cortex Neoplasms
Feminizing adrenocortical tumors are malignant tumors, which either secrete estrogen or massive amounts of adrenal androgens that are aromatized to estrogen in other tissues. Peak incidence is in young and middle aged males.
Childhood adrenal tumors are rare (0.04% of tumors). Adrenal neoplasms should be suspected in any child with premature or inappropriate signs of virilization or feminization, especially if accompanied by evidence of hyperadrenocorticism or gynecomastia. Evidence of premature development of secondary sexual characteristics, such as enlarged penis, axillary hair, and pubic hair, may be seen in children with gynecomastia associated with tumors of the adrenal gland.
An estrogen-producing adrenal tumor in an adult male was first reported in 1919. In men, adrenal carcinomas that result only in feminization are very uncommon. However, neoplasms producing mixed syndromes, such as feminization and Cushing disease, are almost always malignant. Successful treatment of the tumor might result in regression of gynecomastia. In unresectable tumors, estrogen blockade with tamoxifen or raloxifene may provide some effect.
Ectopic Human Chorionic Gonadotropin Production
Carcinoma of the Lung.
This disease may initiate an increase in serum chorionic gonadotropin values with simultaneous escalation in estrogen secretion. Gonadotropins were identified in the urine of four male patients who died of bronchogenic carcinoma. In three patients, gonadotropins were also present in tissue samples from the primary lung tumor. The appearance of gynecomastia in an adult male smoker should arouse suspicion of an underlying carcinoma of the lung. It has been proposed that the detection of HCG may be an aid in the diagnosis of bronchogenic carcinoma.
Hepatocellular Carcinoma.
This can also initiate gynecomastia via elevated serum HCG. Normal hepatic parenchyma and primary hepatic neoplasms carry estrogen receptors. Hepatocellular carcinoma carries androgen receptors, whereas normal liver parenchyma cells do not.
Hermaphroditism
True Hermaphroditism.
True hermaphroditism occurs when an ovary and a testis or a gonad with mixed histologic features (ovotestis) is present. Four categories are recognized: (1) bilateral, with testicular and ovarian tissue (ovotestis) anatomically present on each side; (2) unilateral, with an ovotestis on one side and a normal ovary or testis on the contralateral side; (3) lateral, with a testis is evident on one side and an ovary on the opposite side; and (4) indeterminate, in which the clinical syndrome is expressed but the location and type of gonadal tissue is uncertain.
Significant gynecomastia is evident at puberty in approximately 75% of individuals with true hermaphroditism. Approximately 50% of these individuals menstruate. For the phenotypic male with true hermaphroditism, menstruation presents as cyclic hematuria. Excess estradiol secretion relative to androgen production by the ovotestis is common. Gonadal secretion of estradiol is observed in phenotypic men with feminization (gynecomastia and menstruation).
Pseudohermaphroditism.
17-Ketosteroid reductase deficiency results in male pseudohermaphroditism with a marked overproduction of androstenedione and estrone as well as a decreased production of testosterone and estradiol. A late-onset form of testicular 17-ketosteroid reductase deficiency can cause gynecomastia and hypogonadism in men.
Altered Androgen-to-Estrogen Ratio
Hyperthyroidism
Gynecomastia may develop in 20% to 40% of hyperthyroidism. In most cases the gynecomastia is bilateral and resolves after restoration of euthyroid state. The diffuse toxic goiter of Graves disease is most commonly associated with gynecomastia.
Both elevated estrogen and progesterone serum concentrations can be identified in men with hyperthyroidism. These elevations decrease with reestablishment of the euthyroid state. Gynecomastia may also be due to elevated estrogens that results from a stimulatory effect of thyroxin on peripheral aromatase.
Liver Cirrhosis
The evaluation of estrogen, testosterone, androstenedione, and cortisol concentrations, and the percentage of binding of these steroids to plasma proteins, demonstrated that alterations were most marked in patients with cirrhosis. They were also evident to a lesser degree in patients with fatty metamorphosis of the liver and in normal aging patients. Patients with cirrhosis showed an increase in estrone, a smaller increase in estradiol, a decrease in testosterone, and a rise in LH concentration. Cortisol concentration remained unchanged, whereas ratios of estradiol to testosterone and estrone to testosterone were augmented in patients with cirrhosis and were higher than those in healthy young subjects. The combination of elevated estrone and estradiol and reduced testosterone, which is strongly bound by increased SHBG, appeared to be responsible for gynecomastia and hypogonadism in chronic liver diseases. Other investigators have observed that plasma progesterone concentration is increased in 72% of men with nonalcoholic cirrhosis and gynecomastia compared with healthy male controls. However, this increase was not observed in men with alcoholic fatty change and alcoholic cirrhosis.
Gynecomastia is observed in approximately 40% of men with cirrhosis. As stated, total plasma testosterone concentrations are lower than normal. However, there is a far greater decline in the non–protein bound (biologically active) plasma testosterone. This decrease appears to result from an increased concentration of SHBG. The decreased concentration of plasma testosterone of men with cirrhosis is initiated by a reduction in testosterone synthesis by the testes; kinetic studies have confirmed that the production of testosterone is reduced by 75%. In fact, 15% of the testosterone produced in males with cirrhosis is derived from peripheral conversion of circulating androstenedione. There is disagreement about the relative roles of unbound (biologically active) plasma estradiol in men with cirrhosis and gynecomastia and the changes affecting testosterone concentrations, but the decline in non–protein bound (biologically active) plasma testosterone appears to be the most important factor.
Recovery From Starvation
Gynecomastia produced by return to diet after nutritional deprivation is well documented. Although initially seen in prisoners of war and refugees, this may also be seen in therapeutic dieting. Starvation and significant weight loss result in secondary hypogonadism; refeeding leads to transient imbalance of estrogen and androgen which leads to gynecomastia. In most cases, gynecomastia is bilateral and resolves within 1 to 2 years of resuming normal diet.
Androgen Deficiency
Primary Testicular Failure
Klinefelter Syndrome.
Klinefelter syndrome (XXY) was described more than four decades ago in adult phenotypic males with gynecomastia, hypergonadotropic hypogonadism, and azoospermia. The chromosomal pattern XXY occurs in approximately 1 in 600 live births. Klinefelter syndrome represents the most common variant of male hypogonadism. However, the full spectrum of clinical findings, gynecomastia, eunuchoidism, and macroorchidism does not emerge until the midteens and may never be fully expressed. By midpuberty, affected individuals are uniformly hypergonadotropic and testicular growth ceases. After 15 years of age, serum testosterone concentrations remain in the low-normal range, but serum estradiol values are increased, irrespective of the presence or absence of gynecomastia. Although testicular biopsy during childhood reveals a reduced number of spermatogonia, tubular fibrosis and hyalinization of seminiferous tubules are not observed until midpuberty. Biochemical findings in the adult male include reduced levels of serum testosterone with high-normal or enhanced values of serum estradiol.
It is estimated that carcinoma of the breast is 20 to 66.5 times more frequent in men with Klinefelter syndrome than in the normal male population. Bilateral carcinoma of the breast has also been reported.
Hereditary Defects of Androgen Biosynthesis.
Multiple hereditary defects have been identified that result in defective androgen biosynthesis with incomplete virilization of the male embryo. The enzymes responsible for these failures in biosynthesis include 20,22-desmolase, 17,20-desmolase, 3β-hydroxysteroid dehydrogenase, 17α-hydroxylase, 17β-hydroxysteroid dehydrogenase, and 17-oxosteroid reductase. Each enzyme represents a critical pathway for the conversion of cholesterol to testosterone. As a consequence of the variability in the blockade of these enzymatic biochemical reactions, affected individuals have a profound escalation in gonadotropin secretion after negative feedback. For individuals with complete or partial deficiencies of 17β-hydroxysteroid dehydrogenase, feminization with gynecomastia develops in the early teens. Gynecomastia is also a common occurrence in male patients with 11-β-hydroxylase deficiency. Deficiency of 17-oxosteroid reductase causes elevation in estrone and androstenedione, which is then further aromatized to estradiol.
Secondary Testicular Failure
Gynecomastia is common after testicular injury resulting from trauma, viral orchitis (mumps), or bacterial infections (e.g., tuberculosis, leprosy). The common pathogenic mechanism is functional revascularization of one or both testes. Mumps represents the most common cause of viral orchitis, although echovirus, lymphocytic choriomeningitis virus, and group B arboviruses, among others, have all been implicated in secondary testicular failure.
The use of chemotherapy and local radiation therapy as cancer treatment in children and adolescents can cause testicular failure. The testes of boys in early puberty are particularly susceptible to injury. Furthermore, radiation therapy to the hypothalamic-pituitary axis in children with brain tumors can result in gonadotropin deficiency or hyperprolactinemia, which, in turn, can result in gonadal insufficiency.
Androgen Resistance Syndromes
The androgen resistance syndromes are characterized by gynecomastia and varying degrees of pseudohermaphroditism. Androgens are not recognized by the peripheral tissues, including the breast and pituitary. Androgen resistance at the pituitary results in elevated serum LH levels and increased circulating testosterone. The increased serum testosterone is then aromatized peripherally, promoting gynecomastia.
Reifenstein Syndrome.
First described in 1947, Reifenstein syndrome (XY) is characterized by hypospadias, incomplete virilization, and maturational arrest during spermatogenesis, resulting in azoospermia. Affected males have profound gynecomastia. Laboratory studies show elevated plasma LH and estradiol concentrations along with normal to high testosterone concentrations.
Kennedy Syndrome.
Individuals with Kennedy syndrome, a neurodegenerative disease, have a defective androgen receptor, caused by an expanded number of CAG (glutamine codon) repeats in exon 1 of the gene that encodes for the receptor. In affected males, gynecomastia is the combined result of decreased androgen responsiveness at the breast level and increased estrogen levels as a result of elevated androgen precursors of estradiol and estrone. A similar increase of CAG repeats has been identified in some phenotypes of Klinefelter syndrome.
Increased Aromatase Activity
Estrogen effects on the breast may be the result of either circulating estradiol levels or locally produced estrogens. Aromatase P450 catalyzes the conversion of the C19 steroids, androstenedione, testosterone, and 16-α-hydroxyandrostenedione to estrone, estradiol, and estriol. Hence, an overabundance of substrate or increased enzymatic activity can increase estrogen concentrations and promote the development of gynecomastia.
The biological effects of overexpression of the aromatase enzyme in male mouse transgenics caused increased mammary growth, histologic changes similar to gynecomastia, an increase in estrogen and progesterone receptors, and an increase in downstream growth factors such as transforming growth factor-β and β-fibroblast growth factor. Use of an aromatase inhibitor leads to loss of the mammary gland phenotype.
A familial form of gynecomastia has been discovered in which affected family members had elevated extragonadal aromatase activity. Gain-of-function mutations in chromosome 15 have been reported to cause gynecomastia through the formation of cryptic promoters that lead to overexpression of aromatase. Obesity may cause estrogen excess through increased aromatase activity in adipose tissue.
Chronic Renal Failure
Gynecomastia is common in uremic males, and 50% of males undergoing chronic hemodialysis develop gynecomastia. Plasma LH and follicle-stimulating hormone (FSH) concentrations are increased fourfold in men whose creatinine clearance rates are 4 mL per minute or less, whereas testosterone concentrations are only 30% of normal. There is histologic damage to the testes, hypospermia, and a subnormal response to HCG administration, suggesting that secondary gonadal failure is the primary cause of gynecomastia.
Drugs Associated With Gynecomastia
Although there are many medications that have case reports of gynecomastia as a side effect, a more thorough evaluation of the literature suggests that the list of medications with moderate or strong relationships is likely much lower. Krause performed a literature review in which 92 medications were reported to have association with gynecomastia. Of those 92, less than half were deemed to be causal or highly probable in relationship to gynecomastia. There does exist evidence that some antiandrogens, antiretrovirals, exogenous hormones, and certain other medications have a higher association with gynecomastia. Box 7.2 lists the medications Krause listed as frequently causing gynecomastia and/or causal. Given the numbers of medicines that appear to be implicated in the disease process, a careful review of the patient medication list is imperative. However, one must also weigh the level of evidence supporting each medicine against the likelihood that a particular drug is causing the patient’s condition.
Anastrazole
Bicalutamide
Cimetidine
Diethylstilbestrol
Dutasteride
Estrogen
Ethinylestradiol
Finasteride
Flutamide
Gonadotropin-releasing hormone
Goserelin
Indinavir
Lamivudine
Leuprorelin
Lopinavir
Metoclopramide
Nelfinavir
Nilutamide
Phenothrin
Prednisone
Ritonavir
Saquinavir
Spironolactone
Stavudine
Tamoxifen
Zidovudine
In a significant number of cases, gynecomastia is associated with drugs or chemicals that cause an increased estrogen effect on breast tissues. With some drugs or chemicals, the mechanism of action is known ( Table 7.1 ), whereas with others it is unknown ( Box 7.3 ). Known mechanisms include estrogen-like properties or binding of the estrogen receptor, stimulation of estrogen synthesis, supply of estrogen precursors for aromatases, damage to testicles, blockage of testosterone synthesis, blockage of androgen action, and displacement of estrogen from SHBG.
| Estrogen-Like, or Binds the Estrogen Receptor | Stimulates Estrogen Synthesis | Supplies Precursors for Aromatase | Direct Testicular Damage | Blocks Testosterone Synthesis | Blocks Androgen Action | Displaces Estrogen From SHBG |
|---|---|---|---|---|---|---|
| Estrogen vaginal cream | Gonadotropins | Exogenous androgens | Busulfan | Ketoconazole | Flutamide | Spironolactone |
| Estrogen-containing embalming cream | Growth hormone | Androgen precursors (androstenedione, DHEA) | Nitrosourea | Spironolactone | Bicalutamide | Ethanol |
| Delousing powder | Vincristine | Metronidazole | Finasteride | |||
| Digitalis | Ethanol | Etomidate | Cyproterone | |||
| Clomiphene | Zanoterone | |||||
| Marijuana | Cimetidine | |||||
| Ranitidine | ||||||
| Spironolactone |
Cardiac Drugs/Antihypertensives
Calcium channel blockers
ACE inhibitors
Amiodarone
Methyldopa
Reserpine
Nitrates
Psychoactive Drugs
Neuroleptics
Diazepam
Phenytoin
Tricyclic antidepressants
Haloperidol
Amphetamines
Psychoactive Drugs
Neuroleptics
Infectious Disease Drugs
Indinavir
Isoniazid
Ethionamide
Griseofulvin
HIV antivirals
Other Drugs
Theophylline
Omeprazole
Auranofin
Diethylpropion
Domperidone
Penicillamine
Sulindac
Heparin
ACE, Angiotensin-converting enzyme; HIV, human immunodeficiency virus.
Known Mechanisms
Contact with estrogen vaginal creams can elevate circulating estrogen levels. Some of the creams contain synthetic estrogens; therefore, they may not be detected by standard estrogenic qualitative assays. An estrogen-containing embalming cream has been reported to cause gynecomastia in morticians. Abuse of marijuana, a phytoestrogen, has also been associated with gynecomastia. It has been suggested that digitalis causes gynecomastia through its ability to bind to estrogen receptors. The appearance of gynecomastia has been described in body builders and athletes after the administration of aromatizable androgens; gynecomastia results from the conversion of androgens to estrogens by peripheral aromatase enzymes.
Drugs or chemicals cause decreased testosterone levels through direct testicular damage or by blocking testosterone synthesis or androgen action. Phenothrin, a component of delousing agents, possessing antiandrogenic activity, has been identified as the cause of an epidemic of gynecomastia among Haitian refugees in the early 1980s. Chemotherapeutic agents, such as alkylating agents, cause Leydig cell and germ cell damage, resulting in primary hypogonadism. Flutamide, an antiestrogen used as treatment for prostate cancer, blocks androgen action in the peripheral tissues, whereas cimetidine blocks androgen receptors. Ketoconazole inhibits steroidogenic enzymes required for testosterone synthesis. Spironolactone causes gynecomastia by several mechanisms. Like ketoconazole, it can block androgen production by inhibiting enzymes in the testosterone synthetic pathway (17-α-hydroxylase and 17-20-desmolase), but it can also block receptor binding of testosterone and dihydrotestosterone. In addition to decreasing testosterone levels and biologic effects, spironolactone also displaces estradiol from SHBG, increasing free estrogen levels. Ethanol increases the estrogen-to-androgen ratio and also induces gynecomastia by multiple mechanisms: (1) increasing circulating levels of SHBG, which decreases free testosterone levels; (2) increasing hepatic clearance of testosterone; and (3) causing testicular damage.
Unknown Mechanisms
Many drugs or chemicals are associated with gynecomastia through unknown mechanisms. They generally are listed by their category of known action: (1) cardiac agents and antihypertensives; (2) psychoactive drugs, including illegal street drugs such as amphetamines; (3) agents for infectious disease, including antivirals for human immunodeficiency virus (HIV); and (4) miscellaneous agents (see Box 7.3 ).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree