Abstract
This chapter discusses the potential complications associated with the use of radiotherapy for breast cancer. These may occur in the weeks during or after radiation, such as fatigue or dermatitis; months or years after radiation, such as changes in breast cosmesis or pneumonitis; or decades after treatment such as cardiac effects or second malignancy. Radiation is well tolerated and associated with a low risk of serious complications for the large majority of women. Improvements in radiation have fortunately led to a decrease in the incidence of many of these complications in patients treated today compared with those treated in the past with outdated techniques and equipment. The incidence of complications to the lung and heart has been steadily decreasing with improvements in radiation technique, more selective use of regional node irradiation, and decreased use of concurrent chemotherapy and radiation.
Keywords
breast cancer, radiation therapy, complications, radiation dermatitis, pneumonitis, cardiac effects
This chapter discusses the potential complications associated with the use of radiotherapy. These may occur in the weeks during or after radiation or decades after treatment. Improvements in radiation have fortunately led to a decrease in the incidence of many of these complications in patients treated today compared with those treated in the past with outdated techniques and equipment.
Such possible complications, from most to least common, include fatigue; myelosuppression; radiation dermatitis; alterations in the cosmetic appearance of the breast and local soft tissue symptoms; long-term chest wall or soft tissue complications, including rib fractures and brachial plexopathy; pulmonary effects; cardiac complications; and radiation-related second malignancies. (The effects of irradiation on the risks of arm edema and complications after breast reconstructive surgery are discussed in other chapters.) The risk of complications in patients with collagen vascular disease is also discussed.
Fatigue and Myelosuppression
Fatigue is a common side effect in women treated for breast cancer in general and also specifically in association with radiation. Baseline assessments of fatigue in breast cancer patients even before radiation show that approximately 30% or more report fatigue, that is, even higher in patients receiving chemotherapy in addition to radiation. Fatigue generally increases during radiation to plateau at weeks 4 and 5 but is usually mild and resolves or returns to pretreatment baseline within a few months of completing treatment. Radiation-related fatigue is associated with decreased quality of life in some studies. However, in a randomized trial of breast conserving surgery with or without radiation, radiation was associated with increased levels of fatigue but not global differences in quality of life. Fatigue remains common in 40% to 50% of women at end of radiation and even 1 year after completion of treatment. In one longitudinal study of breast cancer survivors, persistent fatigue was reported in approximately 20% of women up to 5 to 10 years from treatment. Fatigue is more common after chemotherapy and radiation compared with either treatment alone.
In managing the patient with fatigue, it is important to rule out other potential causes such as anemia, cardiovascular disease, depression, or hypothyroidism. There is no specific medical treatment for radiation-related fatigue. In a randomized double-blind clinical trial of vitamins or placebo during radiation for breast cancer, there was no improvement in radiation-related fatigue. Physical activity including aerobic and resistance exercise has been associated with reductions in patient-reported fatigue after radiation in randomized trials. In a randomized trial, patients assigned to up to three 60-minute classes of yoga or stretching exercises per week during their 6 weeks of radiation had improved fatigue at the end of treatment and 1 to 3 months after treatment compared with patients assigned to usual care (and offered these interventions after radiation). Activity in the structure of a regular community-based exercise program for 6 months beginning during or within 3 months of treatment was shown to reduce even long-term fatigue in participants. However, two comprehensive reviews and meta-analysis of randomized and nonrandomized controlled trials have not shown statistically significant improvements in fatigue or health-related quality of life with physical exercise compared with controls. Acupuncture has been associated with improvements in cancer-related fatigue in patients with breast cancer in one prospective randomized trial.
Myelosuppression is also very common during and for a few months after radiation, with the greatest effect on circulating lymphocytes and least effect on platelets and hemoglobin levels. Breast irradiation directly treats a very small volume of total body bone marrow of the chest wall. Because myelosuppression is rarely of clinical significance, blood counts do not need to be routinely checked. Hematologic toxicities appear to be slightly greater in patients treated with chemotherapy after radiation therapy, compared with when chemotherapy is given before radiation. In randomized trials of concurrent versus sequential chemotherapy and radiation, anemia or febrile neuropenia were more frequent with concurrent therapy. For patients receiving concurrent chemotherapy, special attention should be given to signs of infection (local or systemic) or leukopenia. However, the incidence of these problems is low with concurrent radiation and cyclphosphamide-methotrexate-5-fluorouracil (CMF) chemotherapy. There is limited experience with radiation and concurrent other systemic chemotherapy, but in one trial the incidence of grade 3 to 4 neutropenia was much higher with concurrent radiation and paclitaxel but not felt to be different than expected from paclitaxel alone.
Radiation Dermatitis and Infections
Acute radiation dermatitis includes a clinical spectrum of signs and symptoms that usually develop slowly during the course of weeks, generally escalating as treatment progresses. These usually become most severe in the last week of radiation or 1 to 2 weeks after completion. Radiation dermatitis begins with generalized dryness and itching of the skin. This will commonly progress to erythema or hyperpigmentation, which may be asymptomatic or mildly painful. This erythema can be confused with acute inflammation of the breast that can also occur in the first weeks of treatment. Often a pruritic papular rash (folliculitis) may occur in the upper inner portion of the chest wall where there is overlap with previously sun-exposed regions of the neck and chest. Desquamation may progress to a moist, weeping reaction due to breakdown of the skin integrity; the latter is particularly likely to be found in the inframammary fold and toward the axillary tail. Patients with such moist desquamation may also have substantial breast discomfort or pain and swelling. Moist desquamation is associated with a reduced global quality of life. Rarely, the most severe cases may progress to full thickness skin ulceration and bleeding. The time course of recovery is generally weeks for immediate erythema or desquamation, whereas months may be required for the resolution of hyperpigmentation and swelling.
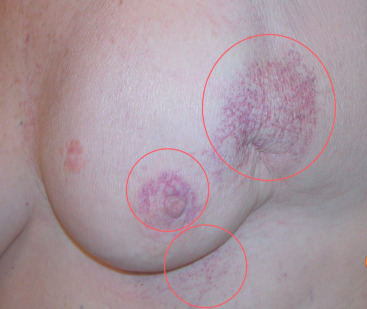
Acute dermatitis is usually scored by the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE). Grade 1 dermatitis is faint erythema or dry desquamation; grade 2 is moderate to brisk erythema; patchy moist desquamation, mostly confined to skin folds and creases; moderate edema; grade 3 is moist desquamation other than skin folds and creases; bleeding induced by minor trauma or abrasion; grade 4 is skin necrosis or ulceration of full-thickness dermis; spontaneous bleeding from involved site. The incidence of acute radiation dermatitis with breast conserving surgery and modern radiation is approximately 3% grade 0, 35% grade 1, 60% grade 2, and 2% grade 3. The definitions used in CTCAE for dermatitis include adjectives such as mild, moderate, or mostly so are subjective and may allow substantial interobserver variation. Furthermore, each grade encompasses substantial variability in the severity of symptoms experienced by patients. For example, a small 1-cm nonpainful area of moist desquamation in the inframammary fold is scored as grade 2, as would be a 10-cm painful area requiring wound care or a treatment break. Fig. 52.1 shows examples of acute radiation skin toxicity.
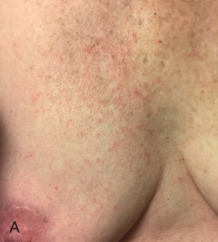

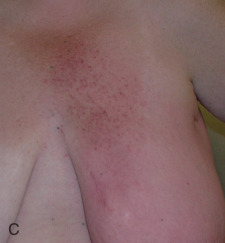
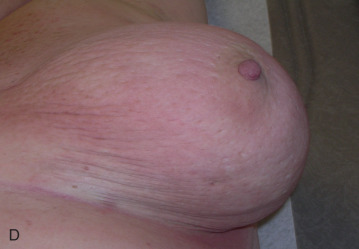
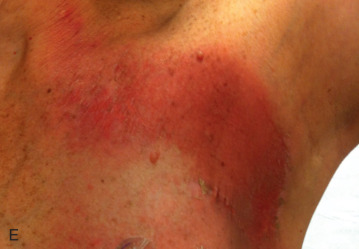
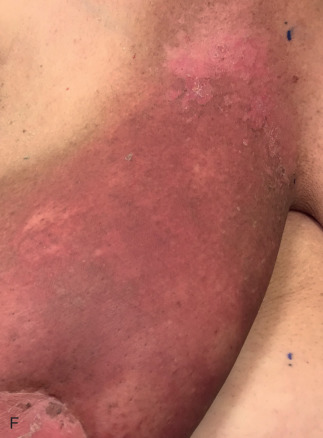
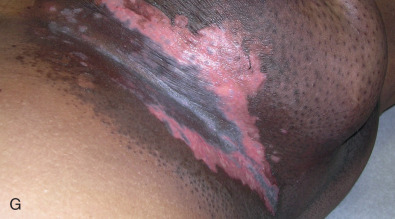
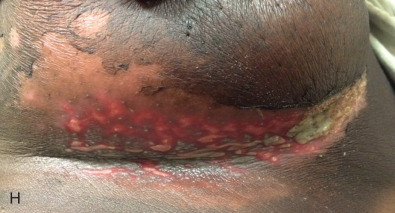
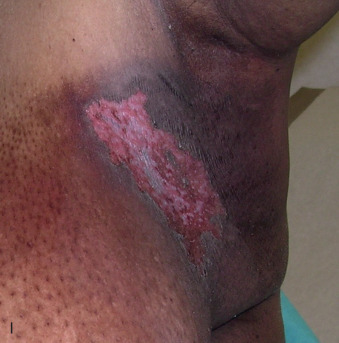
Radiation dermatitis is most directly related to increased dose inhomogeneity, which itself is a function of increasing breast size or chest wall diameter. For this reason, moist desquamation is more common in women with large breasts than those with small breasts. Intensity modulated radiation therapy (IMRT) has been associated with a decrease in the rates and severity of acute dermatitis, compared with conventional two-dimensionally compensated “wedged” tangential-field irradiation. For example, the Sunnybrook Health Sciences Centre and British Columbia Cancer Agency—Vancouver Island Centre in Canada conducted a prospective randomized trial of standard tangential whole breast radiation versus IMRT. The dosimetric characteristics and dose homogeneity of the radiation plans were significantly improved for the patients randomized to IMRT, with a reduction in the median clinically significant maximum dose within the breast tissue (105% vs. 110%) and the volume receiving greater than 105% of the prescribed dose (7.7% vs. 16.9%). Patients randomized to IMRT were less likely to develop acute moist desquamation (31.2% vs. 47.8%), particularly desquamation occurring in the inframammary fold. Improved methods of 3D conformal radiation are able to improve dose homogeneity using forward-based planning and “field-in-field” techniques compared with simple two-dimensional (2D) wedged tangents. This may make comparisons of acute dermatitis between three-dimensional (3D) conformal tangents (forward planning) and IMRT (inverse planning) less distinguishable compared with the older studies of IMRT compared with 2D tangents. Prone positioning may reduce toxicity previously seen in larger-breasted women by reducing skin folds or decreasing chest wall separation. Whole breast hypofractionation that is now standard for many postlumpectomy patients has been associated with decreased acute radiation skin toxicity compared with conventional fractionation.
Randomized controlled trials of topical skin care products used with the goal of mitigating radiation dermatitis in breast cancer patients compared with an aqueous-type cream placebo have been mostly negative or of minimal clinical significance. These include studies of Biafine (Ortho-McNeil Pharmaceuticals, Raritan, NJ), hyaluronic acid, steroids, oil-based emulsions, calendula, glycerine, or miscellaneous natural ingredients. For example, in one study of mometasone furoate, there were modest reduced average dermatitis scores but the average erythema was only faint or dull for placebo. However, up to 1 in 10 women may have avoided or at least had reduced moist desquamation. These trials are often of limited value because of their small numbers, dermatitis scoring systems used, or radiation techniques. In addition, these agents nearly uniformly have no clear mechanism for reducing radiation dermatitis, so new agents with novel biologic rationale are needed.
The treatment of radiation dermatitis depends on its severity. There are substantial variations from one center to another in the preferred approaches and products used. Grade 1 to 2 dermatitis can be treated first with any number of water-soluble skin moisturizers such as MediChoice (Owens & Minor, Richmond, VA), Lotion Soft (STERIS, St. Louis, MO), or Eucerin (Beiersdorf, Hamburg). As symptoms of dryness, skin pain, or areas of dry peeling increase, petroleum-based emollients such as Aquaphor (Beiersdorf, Hamburg) can be used as needed. However, they should not be used immediately before treatment to prevent bolus effects and increased skin dose. Steroid creams may reduce mediators of inflammation in the skin that are responsible for radiation dermatitis. Therefore a weak-strength cream such as hydrocortisone 1% in most cases will reduce papular eruptions and minor itching of the skin. Over-the-counter strength lidocaine jelly can be used alone or mixed with Aquaphor for temporary relief of skin pain as well. Moist desquamation of the skin is managed by nonstick dressings, aluminium acetate solution in water (Domeboros, Moberg Pharma North America, Cedar Knolls, NJ) or silver sulfadiazine cream (Silvadene, King Pharmaceuticals, Bristol, TN). Prophylactic oral or topical antibiotics are not needed for moist desquamation.
Infections of the breast include fungal superinfections, particularly in the inframammary folds of large-breasted women or as a complication of moist desquamation. These may occur from overuse of antibiotics. Topical antifungal creams are applied for 10 to 14 days, with oral agents reserved for refractory cases. By the end of radiation, the presence of erythema and edema and skin pain can make detection of infection more difficult. Bacterial infections are associated with a marked change or sudden onset in skin color or pain, compared with the usual gradual onset from radiation dermatitis week to week. The pain or redness may appear out of proportion to the expected appearance when present earlier in a course of treatment. The incidence of developing cellulitis or breast abscess during or within 1 year of breast radiation is 1% to 8%. In one study, delayed cellulitis continued to occur up to 5 years after treatment, with a cumulative rate of 9%. Cephalexin or ciprofloxacin are used for 10 to 14 days in cases of suspected bacterial infection. For infections refractory to antibiotics, an ultrasound may be needed to rule out an abscess that requires incision and drainage.
Cosmesis and Breast-Related Symptoms
Potential long-term effects of radiation to the breast include breast pain, edema, fibrosis, induration, and hyperpigmentation or telangiectasias of the skin. Alone or combined these can have negative effects on the overall cosmetic result. Approximately 70% to 90% of women have a good or excellent cosmetic result after breast conserving surgery and radiation ( Table 52.1 ). Traditional scoring of breast cosmesis has been a simple scale of poor, fair, good, or excellent, with excellent representing no apparent effects of the radiation. It is also important to assess cosmesis at baseline before radiation, in an attempt to distinguish what effects on cosmesis may be due to surgical changes rather than subsequent radiation changes. For example, changes in breast size can be immediate due to volume loss or delayed within months of surgery due to decrease in postsurgical edema or size of a seroma. The European Organisation for Research and Treatment of Cancer developed an objective scoring system that evaluates the treated breast for overall cosmesis and more specific differences in factors including size, shape, symmetry, color, and position of the nipple. Patient-reported cosmetic results often are better than physician-reported or other objective measurements. For example, in one large prospective randomized study, patients reported approximately 94% good or excellent cosmesis compared with only 75% by a five-person panel evaluating their images at 5 years after surgery. The Breast Cancer Treatment Outcome Scale (BCTOS) is a 22-item patient-reported measure of perceived esthetic cosmetic status, functional status and breast-specific pain that has demonstrated validity.
| Study First Author | Year | N | Good/Excellent (%) |
|---|---|---|---|
| Delouche | 1987 | 410 | 77 |
| Boyages | 1988 | 121 | 75 |
| Dewar | 1988 | 592 | 92 |
| Abner | 1991 | 170 | 90 |
| Taylor | 1995 | 458 | 87 |
| Fowble | 1996 | 471 | 90 |
| Wazer | 1997 | 509 | 85 |
| Fung | 1997 | 55 | 85 |
| Romestaing | 1997 | 1024 | 85 |
| Grills | 2003 | 1178 | 93 |
| Vass | 2005 | 142 | 75 |
| Haffty | 2006 | 52 | 77 |
| Harsolia | 2007 | 172 | 98 |
| Whelan | 2010 | 451 | 70 |
| Murphy | 2011 | 2567 | 95 |
| Hill-Kayser | 2012 | 354 | 71 |
| Hau | 2012 | 385 | 74 |
Common symptoms after breast radiation include mild breast discomfort, sensitivity to touch, or shooting pains of the breast that come and go rapidly and unexpectedly. These will generally improve over time. In a randomized prospective clinical trial performed at Princess Margaret Hospital in Toronto, breast pain and quality of life were studied in women treated with breast conserving surgery and tamoxifen with or without radiation. There was no significant difference between the arms in quality-of-life scores for physical function, pain, or breast symptoms within 12 months of treatment. In both treatment arms, the breast-reported symptoms and pain decreased over time, suggesting that the predominant factor causing them was surgery. Serious breast pain requiring medication occurs in approximately 1% of women. Initial management includes nonsteriodal antiinflammatory medication. More severe and persistent symptoms of the breast or chest wall may be treated with trials of medication such as low-dose nortriptyline, venlafaxine, or gabapentin.
Breast edema presents as heaviness, pain, or enlargement of the breast. Signs of edema include a global increase in breast size, skin thickening, or peau d’orange without other inflammatory signs. Delayed drainage from the nipple and areola may cause a distended appearance when moving from sitting to supine position. Risk factors for breast edema are large breast size, axillary node dissection, and upper extremity lymphedema. Mild breast edema may be managed by wearing a sports bra that applies passive hydrostatic pressure to the breast. Moderate to severe cases of edema may be referred to physical therapy for therapeutic massage.
Skin telangiectasias occur in 10% to 30% of women treated with postoperative radiation, and is associated with use of cobalt therapy, a breast boost, or concurrent chemotherapy. Telangiectasias commonly develop within electron boost fields or skin folds ( Fig. 52.2 ). The time course to their appearance is usually 1 to 2 years, but they may continue to evolve for many years after. Management of asymptomatic telangiectasis is observation, whereas symptomatic telangiectasis causing an unsightly appearance may be treated by sclerotherapy, pulsed dye laser, or electrodessication.
The risk of late radiation negative cosmetic effects are most related to total dose, use of a boost, dose per fraction, and dose homogeneity. In a large prospective randomized trial of dose escalation comparing 50-Gy whole breast to 50 Gy plus a 16-Gy tumor bed boost, use of the radiation boost was associated with an increased risk for severe fibrosis (1.8% in the no boost group vs. 5.2% in the boost group; p < .0001). In a different randomized trial of 50 Gy plus or minus a 10-Gy boost, a boost was associated with no difference in patient- or physician-reported cosmetic results. However, in a third randomized trial comparing 50 Gy in 25 fractions to 45 Gy in 25 fractions plus a 16-Gy boost, the physician and objective measurement of good or excellent cosmesis was approximately 80% with boost compared with 70% without boost. This suggests that the beneficial effects of reducing the whole breast dose were greater than the potential negative effects of adding the boost.
A worse long-term cosmetic outcome and complication rate has been reported with doses greater than 50 Gy to the whole breast or daily dose per fraction of 2.5 Gy or higher. However, in modern studies of whole breast hypofractionation, use of a daily dose higher than 2.5 Gy may no longer be associated with a negative cosmetic results and may actually have improved cosmetic outcomes when combined with sufficient reduction in the whole breast total dose. The Ontario Clinical Oncology Group trial randomized patients to 42.5 Gy in 16 fractions versus 50 Gy in 25 fractions without a tumor bed boost. The late cosmetic appearance was considered good or excellent in approximately 70% of women in both groups. There were similarly no reported differences in 10-year skin and subcutaneous tissue complications. The UK Standardisation of Breast Radiotherapy (START) trials consisted of two separate studies of whole breast irradiation and hypofractionation that allowed a boost. Trial A compared 50 Gy in 25 fractions, 41.6 Gy in 13 fractions, or 39 Gy in 13 fractions in 5 weeks. Trial B compared 50 Gy in 25 fractions over 5 weeks versus 40 Gy in 15 fractions over 3 weeks. The late effects of breast appearance, breast edema or hardness, or skin changes were generally equal or better with whole breast hypofractionation. There was also no significant effect of the boost in these randomized studies. However, in another study of 312 women returning questionnaires for patient-reported outcomes, a boost after whole breast hypofractionation was associated with a modest increase in pain or negative cosmetic results compared with no boost. However, this was of minimal clinical significance with patients reporting only slightly worse cosmetic assessments on the BCTOS (2.3 vs. 2.1, p = .02) with use of the boost.
Measures to improve dose homogeneity may reduce the incidence of these complications. Modern computed tomography–based planning by either field in field or forward planned 3D conformal or inverse planned IMRT can reduce dose inhomogeneity and complications such as edema or negative cosmetic effects on the breast. In a trial conducted at the Royal Marsden Hospital in England, 306 women were randomized to receive whole breast radiation after breast conserving surgery using IMRT or conventional two-dimensional tangential radiation therapy. There was improved dose homogeneity with IMRT, with 19% of the IMRT plans showing dose inhomogeneity of greater than or equal to 105% compared with 92% of the conventional plans. Photographic analysis 5 years after treatment showed that there was a change in breast appearance in 58% of patients randomized to conventional treatment, compared with 40% of those randomized to IMRT ( p = .008). There was a significant relation between the presence of regions of the breast receiving greater than 105% of the dose and any change in breast appearance. Fewer patients treated with IMRT had clinically palpable induration of the breast tissue as well. In the Cambridge Breast IMRT trial, 1145 trial patients were analyzed and 815 with a high predetermined level of dose inhomogeneity were randomized to standard wedged tangential radiation or replanned with a simple intensity modulation technique. The overall 5-year rates of moderate to good cosmesis were 88% with IMRT and 78% with controls and was significant on multivariate analysis. There was also a significantly lower incidence of skin telangiectasias with IMRT.
Concurrent chemotherapy has been associated with increased rates of breast fibrosis, telangiectasia, hyperpigmentation, breast atrophy, and a worse cosmetic result. A group at the former Joint Center for Radiation Therapy of Harvard Medical School reported that 47% of patients treated with chemotherapy had an excellent cosmetic result, compared with 71% of those treated with radiation alone, with good results in 36% and 19%, respectively. This effect was seen predominantly in those patients receiving concurrent radiation and chemotherapy, rather than sequential treatment. However, a study by the University of Pennsylvania did not see a decrease in cosmetic outcomes in relation to the use of chemotherapy, its timing with radiation, or the type of chemotherapy used. However, these studies used predominately CMF, and the results may not be applicable to patients treated with the anthracycline- and taxane-based regimens now in use.
BRCA mutation has not been associated with increased late radiation breast complications. An increase in late effects has been reported in patients with a heterozygous mutation of the gene for ataxia telangiectasia (ATM) in many but not all studies. Ho and coworkers reported the increase in late grade 2 to 4 subcutaneous side effects was observed in particular association with a 5557 G/A polymorphism of the ATM gene. There are no data on the effects of radiation complications by other breast cancer–associated incidence genes, but more data may be anticipated with the increase in clinical use of commercially available multigene panels for testing newly diagnosed breast cancer patients.
Rib Fracture
Rib fracture was associated with old series of radiation, particularly with the use of orthovoltage radiation due to its giving a higher relative dose to bone than does modern megavoltage radiation. The incidence of rib fracture after breast conserving surgery and radiation in the 1980s and 1990s was reduced to approximately 1% to 2% or less. In the UK START trial, enrolling from 1999 to 2002, the 1% to 2% of reported cases within 10 years included many with history of trauma or metastases. The confirmed cases after imaging and further investigations was 0.3% or less. Rib pain may not always be associated with abnormalities on imaging. Symptomatic rib fractures related to history of breast radiation may be treated with standard measures of pain medication and incentive spirometry. Recovery is usually within 6 to 8 weeks.
Brachial Plexopathy
Brachial plexopathy occurs in approximately 1% of patients. This complication is almost exclusively limited to patients treated with regional nodal irradiation. Patients commonly present with pain radiating down the extremity, numbness or weakness, and progressive loss of motor strength of the extremity. Such symptoms may appear years or even decades after treatment. The main differential diagnosis is tumor recurrence in the axillary apical nodes, and magnetic resonance imaging is the best study in distinguishing between these two causes. There is no standard treatment for brachial plexopathy. In one study, five cases of plexopathy were transient and resolved without treatment. A randomized double-blind phase II study of hyperbaric oxygen in these patients failed to demonstrate significant improvement in neurophysiologic tests up to 1 year after treatment.
Pulmonary Complications
Radiation therapy given to the whole breast using tangential beam arrangements will treat approximately 5% to 20% of the ipsilateral lung. Das and colleagues developed a model to estimate the irradiated volume for patients not undergoing three-dimensional CT planning: for each millimeter of central lung distance in a tangential radiation portal, the proportion of irradiated lung was 0.6% on the left side and 0.5% on the right side. In a randomized comparison of supine versus prone positioning, the prone position was associated with significantly lower lung volume in the treatment fields. The need for regional node radiation needs to be carefully weighed against the added risks for toxicity. Adding a supraclavicular or full axillary field will increase the irradiated volume by an average of 7% to 12%. The use of IMRT delivered through standard tangential beam arrangements will modestly reduce the volume of irradiated ipsilateral lung. However, some techniques of IMRT through multiple-gantry angles or moving arcs will actually increase low-dose irradiation of the lung, compared with conventional tangential radiation.
The most common complication from pulmonary irradiation is asymptomatic pulmonary scarring, which occurs most commonly in the apical portion of the lung from supraclavicular irradiation ( Fig. 52.3 ). Changes in pulmonary function such as forced vital capacity (FVC), forced expiratory volume in 1 second (FEV 1 ), and carbon monoxide diffusing capacity (DLCO) have been reported in patients undergoing pulmonary function studies before and after radiation. Studies differ on how likely such changes resolve. Kimsey and colleagues studied 34 women who underwent tangential breast irradiation with or without regional node irradiation and reported transient 5% to 10% decreases in spirometry parameters that returned to baseline by 2 years. In a study by Jaén and colleagues, pulmonary function studies showed transient modest reductions in FVC, FEV 1 , and ventilation that returned to or exceeded baseline. However, reductions in perfusion and DLCO persisted long term and only partially recovered. In a long-term study of pulmonary function after breast radiation, Erven and colleagues studied pulmonary function tests up to 10 years after treatment. An early reduction in vital capacity and forced expiratory volume at 3 to 6 months recovered nearly to baseline by 12 months, but total lung capacity and diffusion capacity of carbon monoxide did not. Small mean reductions of 5% to 10% from baseline preradiation were observed when rechecked 8 to 10 years after RT.
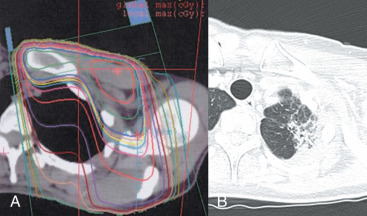
The incidence of radiation pneumonitis after breast conserving surgery and radiation is shown in Table 52.2 . Radiation pneumonitis may present as a triad of dry cough, dyspnea, and low-grade fever. The usual onset of development is within 3 to 9 months of radiation. However, a shorter interval to symptoms within 6 weeks is possible, particularly in patients treated with concurrent paclitaxel. Imaging studies should indicate an area of pulmonary inflammation that is confined to the radiation field. Pneumonitis will respond to treatment with steroids and is only life-threatening if unrecognized and treated with successive attempts at antibiotic treatment. The risk of pneumonitis is substantially higher when the internal mammary or supraclavicular nodes are treated in addition to the breast or chest wall, compared with treatment of the breast or chest wall alone. In a randomized trial reported by Choi and colleagues, 3.6% of patients had grade 1 (asymptomatic or mild symptoms [dry cough], slight radiographic appearances) and 1.2% grade 2 (moderate symptomatic fibrosis or pneumonitis [severe cough], low-grade fever, patch radiographic appearances). All cases of grade 2 were in patients treated to the internal mammary nodes (IMN). Objective measures of predicting pneumonitis by quantitative lung volume measurement is less certain. In the study by Choi and colleagues, IMN radiation was associated with higher lung doses and volumes treated, but there was no correlation with clinically significant grade 2 versus grade 1 pneumonitis. In the study by Kimsey, there were 2 cases of pneumonitis out of 8 patients in whom more than 10% of the lung volume was irradiated, compared with none out of 21 patients with less than 10% irradiated. Lind and colleagues examined 128 patients treated to the breast or chest wall and regional lymph nodes. The mean V20, or volume of lung receiving a dose of 20 Gy or higher, was 27%. The V20 was the most important predictor of pneumonitis on multivariate analysis. In the groups subsequent prospective study of 89 women, where the V20 was kept less than 30% during radiation planning, there were 4 cases of mild and 1 case of moderate pneumonitis.
| Study First Author | Year | N | Pneumonitis (%) | Comments |
|---|---|---|---|---|
| Fowble | 1996 | 491 | 0.3 | |
| Zissiadis | 1997 | 438 | 3 | |
| Fung | 1997 | 55 | 3.6 | Bilateral RT |
| Pierce | 1997 | 429 | <1 | |
| Galper | 2000 | 292 | 1.2 | |
| Lind | 2002 | 613 | 0.9 | Local RT only |
| 4.1 | Locoregional node RT | |||
| Grills | 2003 | 1178 | 0.3 | Breast only |
| 164 | 0.6 | Breast and regional node RT | ||
| Bellon | 2004 | 112 | <1 | Concurrent CMF |
| Taghian | 2005 | 41 | 15 | Paclitaxel, regional node RT |
| 1286 | 1 | |||
| Haffty | 2006 | 109 | 0 | Concurrent chemotherapy |
| Burstein | 2006 | 40 | 18 | Concurrent paclitaxel |
| Blom Goldman | 2014 | 89 | 6 (1 a ) | |
| Whelan | 2015 | 927 | 0.2 a | No IMN radiation |
| 893 | 1.2 a | IMN radiation | ||
| Choi | 2016 | 366 | 3.3 (0 a ) | No IMN radiation |
| 356 | 6.5 (2.5 a ) | IMN radiation |
The incidence of radiation pneumonitis is generally greater in patients treated with concurrent chemotherapy (see Table 52.2 ). Rates of up to 30% to 50% have been observed in patients treated with high-dose chemotherapy and regional node irradiation. However, only 1 in 112 women developed pneumonitis in a trial of concurrent CMF and reduced-dose radiation given to the breast without regional node irradiation.
Bronchiolitis obliterans organizing pneumonia (BOOP) is a rare syndrome of nonproductive cough, fever, and dyspnea with often bilateral pulmonary infiltrates. This distribution outside of radiation fields distinguishes BOOP from radiation pneumonitis. The incidence in studies from Japan has been approximately 1.5 to 2.0%. BOOP has not been described in studies reporting complications of breast conserving surgery and radiation from the United States, Canada, and Europe. This may be due to lack of recognition of the syndrome as being distinct from radiation pneumonitis, or perhaps due to a unique risk factor increasing the risk of BOOP in the Japanese population. There are no known patient- or treatment-related risk factors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








