Abstract
The advent of screening mammography and its widespread application has resulted in more patients being diagnosed with small invasive breast cancers. Most of these early breast cancer patients can be treated with breast conservation surgery and sentinel lymphadenectomy followed by radiotherapy. Preoperative planning includes a tissue diagnosis of cancer before proceeding to surgery and assessment of whether the patient can receive radiotherapy. Surgical incisions used in breast conservation surgery should be placed such as to maximize cosmesis but not compromise a mastectomy, if one is needed. Surgeons should play an active role in inking the margins and assessing margin status intraoperatively. Shaved margins aid in obtaining negative margins. Sentinel lymphadenectomy for axillary evaluation has become the primary tool in axillary evaluation in patients with early breast cancers. Percutaneous ablation has been shown to adequately treat primary and metastatic disease in multiple organs, including the liver, kidneys, lungs, brain, and prostate. Questions regarding its applicability to breast cancer treatment have arisen over the past 2 decades. Although no large, randomized trials have been performed to assess the feasibility of replacing lumpectomy with percutaneous ablation in breast conserving therapy for early-stage breast cancer, a growing body of literature supports the safety and efficacy of percutaneous ablation and stresses the importance of undertaking such a trial.
Keywords
breast conservation therapy, lumpectomy, radiation, oncoplastic, ablation, radiofrequency, cryoablation, laser, electroporation, HIFU, ultrasound
It is imperative that early breast cancer be defined before surgical treatment is discussed. The National Cancer Institute defines early breast cancer as breast cancer that has not spread beyond the breast or the axillary lymph nodes. This includes ductal carcinoma in situ and stage I, stage IIA, stage IIB, and stage IIIA breast cancers. ( www.cancer.gov ). However, others have defined early breast cancer as noninvasive and invasive breast cancers smaller than 20 mm. For the purposes of this chapter, early invasive breast cancer is defined as invasive breast cancers up to 20 mm in size. The surgical management of lobular or ductal in situ cancers is discussed elsewhere.
The approach to surgical treatment of breast cancer has changed dramatically over the past century and in particular the past few decades. The halstedian view of local growth with lymphatic predominance dictated extensive surgeries to treat breast cancer. This philosophy was challenged by the Fisherian thesis that breast cancer was systemic in origin and therefore details and extent of surgical treatment for the primary cancer and the regional lymph nodes did not govern survival. This hypothesis of breast cancer growth has now been replaced by the spectrum biological theory described by Hellman. The spectrum model of breast cancer emphasizes that not all breast cancers behave alike and the importance of progressive tumor growth with increasing likelihood for metastasis with tumor size. According to the spectrum hypothesis, there are some breast cancers that metastasize early in their development. These are the patients who, despite participation in a breast cancer screening program and having small breast cancers, die of metastatic disease. At the other end of the spectrum, there are some breast cancers that, despite large size, never develop metastatic disease. These are the small subset of patients with large breast primaries who do well with aggressive local treatment alone. However, the vast majority of breast cancers fall in the middle. Initially, these tumors are localized within the breast. With time, these tumors progressively increase in size and become more likely to develop metastatic disease. These patients derive the most benefit from breast cancer screening programs. Because the vast majority of patients fall within this category of size influencing likelihood of metastasis, it is not surprising that small breast cancers have the best prognosis.
Screening for breast cancer with mammograms has resulted in a dramatic change in breast cancer presentation and mortality. Although there continues to be some debate regarding benefit of breast cancer screening using mammograms, overall there is consensus that mammographic screening results in a decrease in breast cancer mortality of approximately 20% in women 40 to 74 years of age who have been invited to participate in screening. The benefits of mammographic screening are best exemplified by results from the Swedish mammographic trials. In these trials, the rate of breast cancer mortality in patients invited to participate in mammographic screening was compared with the rate in those who were not. Overall, there was a reduction of breast cancer mortality of 44% in the seven Swedish counties exposed to mammographic screening. The use of mammography for breast cancer screening and benefits of particular guidelines have been a matter of much debate for decades, especially since the US Preventive Services Task Force (USPSTF) and the American Cancer Society (ACS) were published in the late 2000s for average-risk women. USPSTF recommends biennial screening mammography starting age 50 until age 74 with individualized recommendations based on personal risk for women in their 40s. The ACS recommends annual mammography for women starting at age 45 until age 54, continuing with biennial study as long as overall life expectancy is of 10 years or longer. This is valid for average risk women, meaning a lower than 15% lifetime risk of breast cancer.
The resultant implementation of mammograms to screen for breast cancer has altered breast cancer presentation. The state of Rhode Island is an example of a population that is well screened for breast cancer because screening is universally covered, and the effect on breast cancer presentation, treatment, and outcome over a long time period has been reported. Approximately 80% of Rhode Island women participate in breast cancer screening, which is defined as having had a mammogram within the previous 2 years. The Rhode Island Cancer Registry data regarding invasive breast cancer presentation and mortality in 17,522 female residents diagnosed between 1987 and 2008, inclusive, were analyzed for demographic and pathologic factors. Data were analyzed by four time periods: 1987 to 1992, 1993 to 1998, 1999 to 2003, and 2004 to 2008. Statistically significant improvements occurred over the four successive time periods, in mean cancer size (23.7, 20.9, 19.6, and 19.3 mm, p < .0001), pathologic grade (grade I: 12, 15, 19, and 17%; grade III 57, 41, 36, and 35%, p < .0001), breast conserving surgery (38, 56, 67, and 71 %, p < 0.0001) and mortality (37.3, 31.4, 25.1, and 22.6 per 100,000/year, p < 0.0001).These results confirm that breast cancer screening within a population results in smaller invasive breast cancers and greater proportion of lower-grade lesions. Detection of smaller tumors by mammography increased the rate of breast conservation surgery from less than 30% before 1990 to more than 70% in most recent years in Rhode Island.
The vast majority of early breast cancers are detected by screening mammograms. Mammographic features of malignancy include a satellite or oval mass, with or without microcalcifications, and microcalcifications that can be clustered, pleomorphic, or “casting” in appearance. Breast cancer biology and prognosis may be influenced by mammographic appearance. Tabar and colleagues evaluated mammographic tumor appearance with breast cancer outcome in patients from the Swedish Breast Cancer Screening Trials. They reported that breast cancers could be classified into five categories based on mammographic features: stellate mass without calcifications, oval mass without calcifications, powdery calcifications with or without a mass, crushed stonelike calcifications with or without a mass, and casting-type calcifications. Casting-type microcalcifications were identified in only 7% of patients, but these patients were three times more likely to have lymph node involvement. It has also been reported that patients who present with casting-type microcalcifications were more likely to have poor prognostic features such as positive axillary lymph nodes, hormone receptor negativity, and HER2/neu amplification, as well as a worse outcome independent of tumor size.
Preoperative Evaluation
Preoperative needle biopsy and breast imaging should be performed before breast cancer surgical treatment. Despite clinical suspicion of a malignancy, either by clinical examination or imaging test, it is important that a preoperative biopsy be obtained. Masses can be biopsied by fine-needle aspiration or core needle biopsy. Core needle biopsy is preferable because information on invasion, hormone receptor status, tumor grade, and, in some cases, lymphovascular invasion can be evaluated and is considered the standard of care and a quality measure for breast cancer treatment. Core needle biopsies can be guided by palpation for palpable masses or by stereotactic or sonographic guidance for mammographic or ultrasound abnormalities, respectively. In contemporary management, upward of 85% of breast cancers should be diagnosed preoperatively. A cancer diagnosis before initial surgical treatment leads to more definitive local excision, a greater proportion with negative tumor margins, and thus a decreased need for reexcision, and the ability to evaluate the axilla simultaneously. Patients can also be appropriately counseled before surgery regarding choices such as breast conservation versus mastectomy and neoadjuvant therapy, if appropriate.
However, there are some instances in which a preoperative cancer diagnosis may not be feasible. A stereotactic biopsy for suspicious or indeterminate microcalcifications may not be technically feasible because of proximity to the skin or chest wall. These patients should proceed directly to needle localization and excision. Additionally, approximately 25% of patients with a preoperative needle biopsy may be upstaged to malignancy after surgical excision. A radiologic diagnosis of radial scar mandates surgical excision because of the difficulty in differentiating a radial scar from a low-grade lesion on core needle biopsy. Finally, patients with clotting abnormalities should probably proceed directly to surgery instead of a preoperative needle biopsy.
Although many patients are diagnosed based on imaging abnormalities, there are still a few patients who present with a palpable mass. All patients with breast cancer should have, at minimum, a mammogram. A mammogram permits evaluation of the breast for disease outside the affected quadrant and may provide an estimate of tumor size. Disease outside the affected quadrant precludes the use of breast conservation and may be helpful in planning surgical treatment.
The role of magnetic resonance imaging (MRI) in the patient newly diagnosed with breast cancer is still being debated. Preoperative breast MRI may be used to evaluate the ipsilateral or contralateral breast. Additional tumor can be identified in the ipsilateral breast in 13% to 31% of patients resulting in a wider excision in 3% to 14% or conversion to mastectomy in up to 25%. These results have suggested that candidates for breast conservation surgery should have a preoperative breast MRI. However, it is unclear whether the occult disease identified on breast MRI is clinically important and would not be adequately treated with whole breast radiotherapy. Long-term local recurrence for patients treated with breast conservation is approximately 10%, which is significantly lower than the rate of additional tumor identified on breast MRI. A small retrospective study reported a higher local recurrence rate of 6.8% in women who had conventional imaging compared with 1.2% in those who had received a preoperative MRI ( p < .01). However, these rates of local recurrence are quite low and different from the series reported by Solin and colleagues where preoperative MRI at the time of diagnosis was not associated with any improvement in outcome. There are even fewer data regarding the role of breast MRI to detect occult disease in the contralateral breast. As demonstrated by Wang and colleagues, the detection of synchronous contralateral breast cancer seems to be increased by the use of preoperative MRI. In this Surveillance, Epidemiology, and End Results (SEER)-Medicare database study, this observation was not offset by a similar decrease of subsequent contralateral cancer occurrence among older women with stages I and II breast cancer, leading to the authors’ conclusion that breast MRI might lead to overdiagnosis. The largest study addressing this issue comes from the American College of Radiology Imaging Network, in which occult contralateral disease was identified in 3.1% of participants. The mean diameter of the invasive tumors was 1 cm and was not influenced by menopausal status, dense breast tissue, or tumor histology. A previously published meta-analysis, including 18 studies on MRI and detection of contralateral breast cancer, also shows that there is an increase in detection of contralateral breast cancer without clear evidence of benefit because this could be leading to overdiagnosis. At present, there is no convincing evidence to suggest that the use of preoperative MRI improves local control in women, but it may permit better definition of extent of disease and decrease the need for reexcision to obtain negative surgical margins. In the Choosing Wisely Initiate of the American Society of Breast Surgeons, one of the five tests or interventions that physicians and patients should question is the routine ordering of MRI. In an individual person data meta-analysis preoperative MRI for staging the cancerous breast did not reduce the risk of local or distant recurrence. MRI is especially useful when occult breast cancer presents via axillary lymphadenopathy or in those with BRCA mutations, but there is no evidence that it lessens reexcision rate, local recurrence, or mortality. In addition, MRI adds to false-positive biopsy rate, extra procedures, and costs, and often delays surgery or ends with increased patient anxiety and questionably a higher mastectomy rate.
Surgical Options for Early Breast Cancer
Surgical management of early breast cancers can be divided into breast conservation surgery or mastectomy and axillary sampling. Breast conservation surgery includes radiotherapy. Practice guidelines for breast conservation therapy in patients with invasive breast cancer have been published and provide a good review. There have been six randomized trials addressing the outcome of breast cancer patients treated with breast conservation surgery or mastectomy, and a full discussion is contained in Chapter 50 . As summarized in Table 45.1 , there is no difference in overall survival whether mastectomy or breast conservation is selected. In most of the trials, there was no significant difference in local recurrence in the treated breast compared with chest wall recurrences after mastectomy, and for the most part, these local breast recurrences can be addressed with mastectomy. Although patient choice may dictate surgical treatment, there are some patients for whom a mastectomy should be recommended. Contraindications for breast conserving therapy for breast cancer include prior history of whole breast radiotherapy, active collagen vascular disease precluding radiotherapy, inability to obtain tumor-free margins despite multiple surgical excisions, first and second trimester of pregnancy, cancers originating in two separate quadrants, and diffuse microcalcifications occupying more than one quadrant. These patients are best served with mastectomy. Histology, location of tumor, patient age, and tumor size (except relative to breast size) are not contraindications to breast conserving therapy.
| Study | No. of Patients | Time Period | Tumor Size (cm) | Follow-up | Surgery | Local Recurrence Rate | Breast Cancer Survival | Overall Survival |
|---|---|---|---|---|---|---|---|---|
| “QUART”: Milan Cancer Institute a | 701 | 1973–1980 | T ≤ 2 | 20 y | QUART | 9% | 73.9% | 58.3% |
| Radical mastectomy | 2% | 75.7% | 58.8% | |||||
| NSABP B-06 b | 2163 | 1976–1984 | T ≤ 4 | 69% | L + AND | 40% | 64.4 | 46% |
| >20 y | L + RT + AND | 14% | 63.3 | 46% | ||||
| MRM | 10% | 63 | 47% | |||||
| NCI c | 247 | 1979–1987 | T ≤ 5 | 10 y | L + RT + AND | 18 | ns | 77 |
| MRM | 4 | ns | 75 | |||||
| Gustave-Roussy d | 179 | 1972–1980 | T ≤ 2 | 15 y | L + AND | 9% | 73% | |
| MRM | 14% | 65% | ||||||
| EORTC e | 868 | 1980–1986 | 10 y | L + AND | 20% | 65% | ||
| MRM | 12% | 66% | ||||||
| Danish Cancer Group f | 904 | 1983–1989 | 6 y | L + AND | 3% | 79% | ||
| MRM | 4% | 82% |
a Data from Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227-1232.
b Data from Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233-1241.
c Data from Poggi MM, Danforth DN, Sciuto LC, et al. Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy: The National Cancer Institute Randomized Trial. Cancer. 2003;98:697-702.
d Data from Arriagada R, Le MG, Rochard F, Contesso G. Conservative treatment versus mastectomy in early breast cancer: Patterns of failure with 15 years of follow-up data. Institute Gustave-Roussy Breast Cancer Group. J Clin Oncol. 1996;14:1558-1564.
e Data from van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000;92:1143-1150.
f Data from Blichert-Toft M, Rose C, Andersen JA, et al. Danish randomized trial comparing breast conservation therapy with mastectomy: six years of life-table analysis. Danish Breast Cancer Cooperative Group. J Natl Cancer Inst Monogr. 1992;(11):19-25.
Determination if a patient is a candidate for breast conservation should begin with the initial history and physical examination. Although a significant family history for breast cancer does not preclude breast conservation, these patients are at significant risk for contralateral breast cancers and may opt for genetic testing. Prior history of therapeutic radiotherapy involving the breast region excludes the use of additional radiotherapy, and these patients are best served by mastectomy. This might be questioned in the scenario where a patient received accelerated partial breast radiation and the breast cancer is diagnosed in a different breast area. A few studies, like the one published by GEC-ESTRO Breast Cancer Working Group in 2013, suggest that in a selected population of patients with ipsilateral breast tumor recurrence, a technique of accelerated partial breast radiation could be considered in patients previously treated with lumpectomy and radiation. In this particular study, multicatheter radiation has been used with excellent/good cosmetic results in 85% of the patients. Overall survival rate is equivalent to those patients submitted to mastectomy at the time of recurrence. The presence of breast implants, possibility of pregnancy, and symptoms of metastatic disease should all be included in the patient history. Physical examination is focused on evaluating the breast and regional lymphatics. Tumor size, if the lesion is palpable, is important, and ratio of tumor size to breast volume and the presence of multiple tumors should be noted. Fixation of the tumor to the skin should be evaluated. Evidence of a locally advanced cancer such as skin edema and ulceration and erythema excludes these patients from breast conservation. Because patient preference is important, the option of breast conservation or mastectomy with or without reconstruction should be discussed even if the patient is an excellent candidate for breast conservation.
Breast Conservation Surgery
The vast majority of patients with early breast cancer are candidates for breast conserving therapy. Indeed, the rate of breast conservation in well-screened populations approaches 80%. The objective of breast conservation surgery for breast cancer is to excise the primary tumor to negative surgical margins while maintaining a cosmetically acceptable breast. Surgical removal of the primary breast cancer while preserving the breast has been called partial mastectomy, wide local excision, lumpectomy, segmental mastectomy, and tumorectomy. All imply surgical removal of the tumor with a grossly negative margin. Quadrantectomy implies excising a quarter of the breast and is more extensive. A quadrantectomy is more likely to result in negative surgical margins but may compromise cosmesis. As breast cancers have decreased in size, the ability to surgically excise breast cancers with a good cosmetic result has increased.
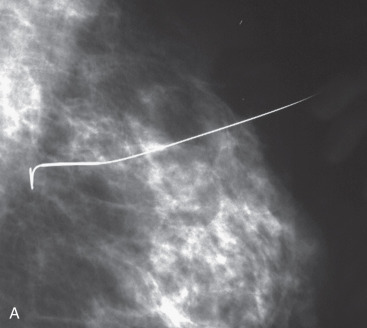
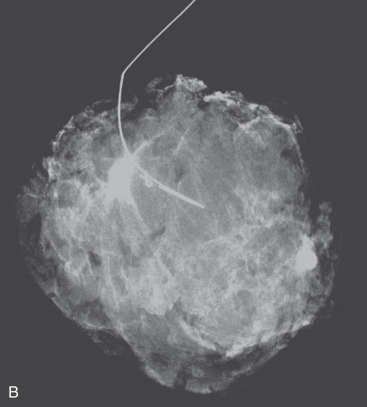
Preoperative localization is required in nonpalpable breast cancers and those that are difficult to palpate. Most nonpalpable breast cancers are localized with a guidewire (needle localization) in the mammography suite immediately before surgery. If needle localization is used, a specimen radiograph is obtained in the operating room to verify that the abnormality and the localizing clip has been included within the excised tissue ( Fig. 45.1 ). When specimen radiography is performed, two views, including an orthogonal one, is the standard. The mammographic abnormality can be localized for the pathologist either with a needle or images of the specimen performed in a grid. Although guidewire localization is most widely used for nonpalpable lesions it is difficult and painful for the patient often accompanied by vasovagal events. New approaches to localize the lesion have been described, including marking the breast cancer with a hematoma. In this technique, intraoperative ultrasound is used to localize the hematoma and guide excision. Hematoma-directed ultrasound guidance (HUG) compared with needle localization breast biopsy for excision of nonpalpable lesions has been described over a 10-year period in 455 patients. Margin positivity was significantly lower for HUG (24%) compared with needle localization breast biopsy (47%) ( p = .045).
Another technique to guide localization of nonpalpable tumors is cryo-assisted excision. This technique uses intraoperative cryoablation to create an ice ball around the primary tumor followed by ultrasound guidance during surgical excision. In a prospective randomized trial of cryo-assisted and guidewire needle localization, there was no difference in margin positivity (28% for cryo-assisted localization and 31% for guidewire localization). A major disadvantage of this approach is that freezing artifact of the primary tumor may result in difficulties with histologic examination.
Another technique with increasing data in the literature is radioactive iodine seed localization, which uses an iodine-125 seed for localization of nonpalpable lesions or previously clipped lesions that have disappeared on imaging due to complete response to neoadjuvant chemotherapy. This has been seen as equivalent to the needle localization in noninferiority studies and meta-analysis.
A pilot study has been published recently describing implantation of infrared-activated, electromagnetic wave-reflective device for localization of nonpalpable breast lesions. The reflector was implanted up to 7 days before the procedure with mammography or ultrasound guidance. Of the 50 patients studied, all had the reflector and lesion successfully removed suggesting a viable alternative to standard wire localization.
Some of the disadvantages of the wire localization compared with the localization of nonpalpable lesions with radioactive iodine seed and the electromagnetic wave reflective device include work-flow limitations because the wire needs to be placed on the day of surgery, potentially delaying surgical start times and preventing these procedures to be the first ones of the day. The HUG procedure does not have this disadvantage but must usually be performed within 5 weeks of the biopsy. Possible wire migration during patient transportation is also another disadvantage, potentially misguiding the surgeon on the optimal targeted tissue removal.
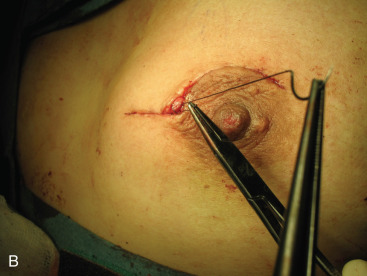
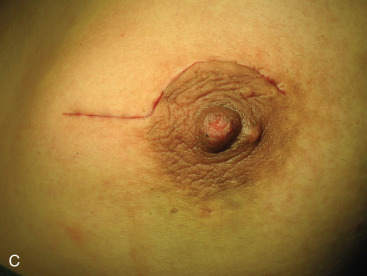
Surgical incision placement in breast conserving surgery is important for optimizing cosmetic results. Surgical incisions should be placed to optimize cosmetic results but should not compromise surgery if a mastectomy is needed. Incisions should be placed along Langer or Kraissl lines (lines of tension) because this decreases keloid formation and optimizes cosmesis. On the breast, these incisions are usually circular and are illustrated in Fig. 45.2 . Exceptions to this rule are lesions Located in the lower hemisphere of the breast up to and including nine and three o’clock lesion are best addressed with radial incisions. Curvilinear incisions are recommended if only breast tissue is being excised. A radial incision is recommended in the inferior breast if skin is to be included within the excision. When taking skin, a parallelogram is advised compared with an ellipse. In this scenario, the radial incision minimizes ptosis of the nipple. Incisions are best placed as close to the nipple-areola complex as possible because this incision can be easily included if a mastectomy is warranted.
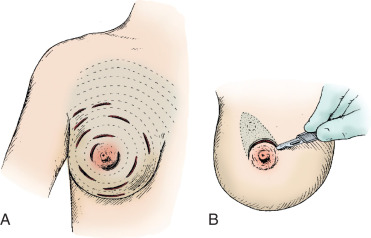
A periareolar incision to remove the breast primary has gained popularity in recent years. This incision permits access to all quadrants of the breast, can be easily accommodated if a mastectomy is needed in the future, and has an excellent cosmetic outcome because the incision is often masked by the areolar border. This incision works well if the lesion is sufficiently deep to the skin such that a 1-cm skin flap can be made. Thinner skin flaps may result in denting of the skin. It is imperative that the biopsy site be clearly marked with clips or a fiducial marker if this incision if used so that radiotherapy planning is not jeopardized. Procedures such as this, the round block technique, and others are termed oncoplastic and are covered in Chapter 40 .
The decision to include skin depends on tumor location. Mammograms and, in particular, ultrasounds can help estimate the distance between the lesion and subcutaneous tissue. If the lesion is superficial, skin should be included within the excision. Excision of skin is not required for lesions more than 1 cm deep to the subcutaneous tissue. Superficial lesions close to the areola border that require excision of skin are best approached using a boomerang incision ( Fig. 45.3 ). These lesions can sometimes be challenging; inappropriate technique can lead to nipple devascularization and deviation. A boomerang incision is a combination of a radial incision over the superficial lesion and a boomerang around the areola. This type of incision has been shown to produce excellent cosmetic results, does not compromise margin status, and can be easily included in a mastectomy incision, if needed later. Large batwing incisions are usually reserved for oncoplastic procedures and are addressed elsewhere.
There has been some debate as to whether the needle biopsy site has to be excised at the time of definitive surgery. Although seeding of the biopsy tract has been reported to be as high as 32%, recurrence at the core needle biopsy site is much lower, at approximately 1% when radiation is used. Furthermore, in a retrospective review of 530 patients with stage I and II breast cancer treated with breast conservation surgery and radiotherapy, there was no difference in local recurrence after a median follow-up of more than 70 months if the patients had a preoperative needle biopsy. It is feasible that the tumor deposits identified by washings are not clinically viable or are easily treated with postoperative radiotherapy. Indeed, a local recurrence rate at the needle biopsy site of up to 27% has been reported for patients treated with mastectomy. Therefore, it is recommended that biopsy sites be excised in breast cancer patients treated with mastectomy or partial mastectomy who do not receive postoperative radiotherapy.
The breast lesion should be excised as one specimen; morcellation of the excised tissue should be avoided. Excision as a single specimen is extremely important for tumor size determination and margin assessment. Excision with a knife is recommended because in some instances, cautery artifact at the margin may interfere with determination of margin status. Sharp smooth incisions should be used along the margins; multiple small nicks at the margin may make it difficult to determine whether a margin is involved with tumor. Once the breast tissue is removed, it should be immediately oriented, preferably with colored inks ( Fig. 45.4 ). The use of six colored inks has been shown to decrease the volume of tissue excised if a margin is found to be positive because it is possible to identify the margin involved and avoid who bed reexcision. Examination and then specimen imaging (ultrasound and/or mammography) should confirm removal of the lesion, all of the calcifications and the clip. In addition, many surgeons perform shaved margins of the cavity after tumorectomy. Chagpar and colleagues recently published the results of a randomized trial of 235 patients undergoing partial mastectomy for breast cancer with and without shaved margins. Patients undergoing shave margins had a significantly lower rate of positive margins than those in the no-shave group (19% vs. 34%, p = .01), as well as a lower rate of second surgery for margin clearance (10% vs. 21%, p = .02).

Meticulous hemostasis should be obtained after surgical excision. Although most postoperative breast hematomas do not require surgical intervention, they are painful, may increase infection, and may delay initiation of postoperative breast radiotherapy. Hematoma formation may obscure postoperative changes, resulting in unnecessary biopsy to preclude recurrent disease. Before breast closure, four clips or a fiducial marker should be placed to localize the biopsy site. These clips aid in radiotherapy planning and mark the area for future mammography. However, clips should not be placed in the biopsy site if accelerated radiotherapy by a balloon catheter is planned because these clips can puncture the balloon.
It is debatable if the biopsy site should be reapproximated before skin closure. Closure of the biopsy site may lead to a worse breast cosmesis. Conversely, leaving the biopsy site in place encourages seroma formation and potentially better cosmesis. Newer techniques that close the deep and superficial layers have also been advocated. These newer techniques include alignment of the parenchyma at right angles to the incision and reapproximation of the deep and superficial suture lines without closure of the central biopsy site. A recent report confirms that leaving the biopsy site in place does achieve a better cosmetic result. However, in this study, it was more difficult to differentiate between postoperative changes and recurrence. Drains in the breast should be avoided, and the skin should be reapproximated using a subcuticular technique. Staples should be avoided on the skin.
The objective of breast conservation surgery is to excise the tumor to negative surgical margins (tumor-free margin or negative margin) with acceptable breast cosmesis. There was much controversy regarding the definition as to what constitutes a negative surgical margin for excision of breast cancer. The definition used by the National Surgical Adjuvant Breast and Bowel Project was no tumor cells at the transected margin. Others have used 2 to 5 mm as the cutoff for a tumor-free margin, whereas others have focused on the extent of margin involvement. Approximately half of the patients with a positive margin have residual disease at mastectomy, with higher rate of residual disease in those patients with multiple close margins. If feasible and needed, multiple reexcisions do not affect local recurrence if a negative surgical margin is ultimately obtained. In 2014, the Society of Surgical Oncology and the American Society for Radiation Oncology issued a consensus guideline on margins for patient undergoing lumpectomy and whole breast radiation for stages I and II invasive breast cancer, which has also been endorsed by the American Society of Clinical Oncology. The panel of experts used a study level meta-analysis of margin width and ipsilateral breast tumor recurrence (IBTR) of 33 studies including a total of 28,162 patients. There was no clear evidence that wider margins than “no ink on tumor” reduce IBTR for patients with stage I and II disease receiving whole breast radiation. It has been stated in the study that this guideline should not be applied to patients with pure ductal carcinoma in situ (DCIS), those who received preoperative chemotherapy, or those who receive accelerated partial breast radiation or no radiation at all after breast conservation.
In 1992, the American College of Surgeons, the American College of Radiology, the College of American Pathologists, and the Society of Surgical Oncology published guidelines for breast conserving therapy that included 22 standards. Box 45.1 summarizes these standards. Although most of the guidelines related to pathology report content, three of the standards were related to the surgical specimen. These standards included labeling the breast involved (laterality), noting the affected quadrant, and orienting the breast specimen. In a follow-up study in 2002, there was excellent compliance with information regarding laterality. Approximately two-thirds of breast specimens were oriented. However, only about one-fifth of surgeons noted the affected quadrant. This has recently been updated with the development of and publication of the National Accreditation Program for Breast Centers Standards Manual ( Box 45.2 ). This accreditation process for a center has 29 standards that includes standards involving center leadership and the utilization of an interdisciplinary breast cancer conference, clinical management, research, community outreach, professional education, and quality improvement. In particular relation to surgical care is the surgical correlation with imaging/concordance; preoperative planning after needle biopsy; offering lumpectomy or mastectomy with or without reconstruction; lymph node surgery, including sentinel lymph node biopsy, initial surgical correlation, and treatment planning; as well as medical and radiation oncology consultation, education, support, rehabilitation, and survivorship programs.
Mammography (2)
- 1.
All patients should have preoperative mammography.
- 2.
Size of the mammographic abnormality should be included in the report.
Labeling of the Surgical Specimen (3)
- 1.
Laterality of the specimen
- 2.
Involved breast quadrant
- 3.
Orientation of the specimen
Pathology Report (10)
- 1.
Microscopic confirmation of disease
- 2.
Tumor size
- 3.
Histologic type
- 4.
Histologic grade
- 5.
Presence or absence of lymphatic and blood vessel invasion
- 6.
Macroscopic margin status
- 7.
Microscopic margin status
- 8.
Estrogen receptor status
- 9.
Progesterone receptor status
- 10.
Presence of in situ disease
Radiotherapy (6)
- 1.
Radiotherapy administered after breast conservation surgery
- 2.
Documentation of dosimeter or dose distribution
- 3.
Planning on a dedicated simulator
- 4.
Treatment 5 days per week
- 5.
Dose planning with use of tissue compensators
- 6.
Avoidance of breast bolus to minimize skin toxicity
Systemic Therapy (1)
- 1.
Systemic therapy for patients with positive nodes
- 1.
Imaging
- a.
Screening mammography (digital or analog)
- b.
Diagnostic mammography (additional views beyond screening mammography and workup of a clinical abnormality)
- c.
Ultrasound
- d.
Breast MRI
- a.
- 2.
Needle Biopsy
- a.
Needle biopsy—palpation-guided
- b.
Image guided—stereotactic
- c.
Image guided—ultrasound
- d.
Image guided—MRI
- a.
- 3.
Pathology
- a.
Report completeness/CAP protocols
- b.
Radiology-pathology correlation
- c.
Prognostic and predictive indicators
- d.
Gene Studies (if available)
- a.
- 4.
Interdisciplinary Conference
- a.
History and findings
- b.
Imaging studies
- c.
Pathology
- d.
Pre- and posttreatment interdisciplinary discussion
- a.
- 5.
Patient Navigation
- a.
Facilitates navigation through system for the patient
- a.
- 6.
Genetic Evaluation and Management
- a.
Genetic risk assessment
- b.
Genetic counseling
- c.
Genetic testing
- a.
- 7.
Surgical Care
- a.
Surgical correlation with imaging/concordance
- b.
Preoperative planning after biopsy for surgical care
- c.
Breast surgery: lumpectomy or mastectomy
- d.
Lymph node surgery: sentinel node/axillary dissection
- e.
Post initial surgical correlation/treatment planning
- a.
- 8.
Plastic Surgery Consultation/Treatment
- a.
Tissue expander/implants
- b.
TRAM/latissimus dorsi
- c.
DIEP flap/free flaps (if available)
- a.
- 9.
Nursing
- a.
Nurses with specialized knowledge and skills in diseases of the breast
- a.
- 10.
Medical Oncology Consultation/Treatment
- a.
Hormone therapy
- b.
Chemotherapy
- c.
Biologics
- d.
Chemoprevention
- a.
- 11.
Radiation Oncology Consultation/Treatment
- a.
Whole breast irradiation with or without boost
- b.
Regional nodal irradiation
- c.
Partial breast irradiation treatment or protocols
- d.
Palliative radiation for bone or systemic metastasis
- e.
Stereotactic radiation for isolated or limited brain metastasis
- a.
- 12.
Data Management
- a.
Data collection and submission
- a.
- 13.
Research
- a.
Cooperative trials
- b.
Institutional original research (not part of national trials)
- c.
Industry sponsored trials
- a.
- 14.
Education, Support, and Rehabilitation
- a.
Education along continuum of care (pretreatment, during, posttreatment)
- b.
Psychosocial support: i. individual support; ii. family support; iii. support groups
- c.
Symptom management
- d.
Physical therapy (for example, lymphedema risk reduction practices, and management, shoulder ROM)
- a.
- 15.
Outreach and Education
- a.
Community at-large education (including low-income/medically underserved)
- b.
Patient education
- c.
Physician education
- a.
- 16.
Quality Improvement
- a.
Continuous quality improvement through annual studies
- a.
- 17.
Survivorship Program
- a.
Follow-up surveillance
- b.
Rehabilitation
- c.
Health promotion/risk reduction
- a.
CAP, College of American Pathologists; DIEP, deep inferior epigastric perforator; MRI, magnetic resonance imaging; ROM, range of motion; TRAM, transverse rectus abdominis myocutaneous (flap).
Mastectomy
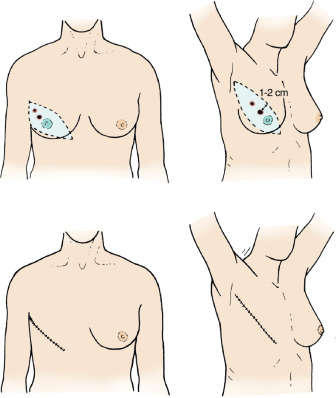
Early breast cancers can also be treated with a mastectomy. Multiple randomized trials have shown that there is no difference in overall breast cancer survival whether breast conservation or mastectomy is performed. Mastectomy, however, does result in fewer breast recurrences. A benefit of mastectomy is that for most women, postmastectomy radiation is not needed. The technical aspects of mastectomy are discussed in detail in Chapter 31 . In general, mastectomy implies the removal of breast and overlying skin and includes the nipple-areola complex. The mastectomy incision should incorporate the previous biopsy scar. The preferred mastectomy incision uses a modified Orr incision that is slightly oblique from the transverse line and cephalad toward the axilla ( Fig. 45.5 ). This incision removes the nipple-areola complex, allows access to the axilla, and provides an acceptable cosmetic result. This type of mastectomy should be used if no plastic reconstruction of the breast is planned because it minimizes skin on the chest wall, which can become irritated by wearing breast prosthesis. Patients can have either immediate or delayed reconstruction of the breast with this type of mastectomy. The breast should be oriented with sutures after removal and the posterior margin inked. If the primary breast lesion is not clinically palpable, a radiograph of the excised breast can be performed to aid in pathologic evaluation.
A variant of the classic mastectomy is the skin-sparing mastectomy. This operation is best used if plastic surgical reconstruction of the breast is planned. A skin-sparing mastectomy includes removal of the breast parenchyma with minimal removal of the overlying skin. Underlying pectoralis fascia is included with the excised breast specimen. The remaining native breast skin and inframammary fold provide for excellent cosmetic results when the breast is reconstructed with either autologous tissue or an implant. A skin-sparing mastectomy is usually performed through a circular incision around the areola with a hockey-stick extension if needed ( Fig. 45.6 ). The nipple-areola complex is usually included with a skin-sparing mastectomy.
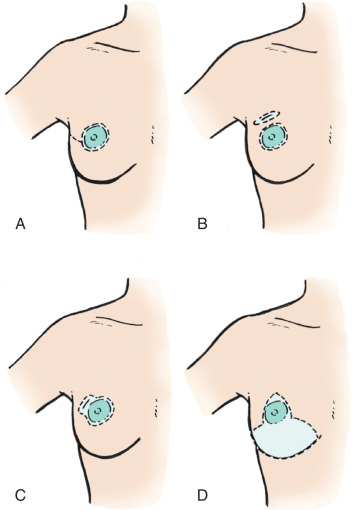
Variants of the skin-sparing mastectomy include nipple skin-sparing mastectomy, which has been advocated by some as being cosmetically superior to a skin-sparing mastectomy. However, a nipple-sparing mastectomy does leave some ductal tissue beneath the nipple, which some have suggested may lead to increase nipple areolar recurrence. However, a meta-analysis and review of the literature published in 2015 included 20 studies and a total of 2207 patients who underwent nipple-sparing mastectomy for breast cancer treatment, with majority of them being stage I or II. This study suggested likely absence of adverse oncological events when nipple-sparing mastectomies were performed. This is particularly true in carefully selected patients with early-stage breast cancer, with the authors recognizing the limitations of this being a meta-analysis of observational studies in the setting of an outcome that is unlikely to be evaluated in a randomized clinical trial setting.
Axillary Evaluation
The most important prognostic feature of an early breast cancer is nodal involvement. Nodal involvement suggests that the primary tumor has the capability to spread systemically. The likelihood of spread to the axillary lymph nodes increases with primary tumor size. Approximately 25% of breast cancer patients have positive lymph nodes at the time of diagnosis. Clinical evaluation of the axilla is notoriously inaccurate and should not be used as the primary method to stage the axilla in most patients. Surgical evaluation of the axilla is usually used for patients with breast cancer. Surgical evaluation includes sentinel lymphadenectomy, axillary node dissection, and the more recently described targeted axillary dissection.
Sentinel lymphadenectomy is a minimally invasive surgical technique that is used to stage the axilla. This surgical approach can be used to determine which patients have nodal disease and which do not. Because on average only two lymph nodes are removed, sentinel lymphadenectomy cannot accurately indicate the number of lymph nodes involved with tumor. Sentinel lymphadenectomy should be used in patients with a clinically negative axilla, a situation most frequently encountered in patients with early breast cancer.
Although the original description of sentinel lymphadenectomy used only one tracer, most centers today use a combination of dyes. The most frequent combination used is radioactive protein (technetium sulfur colloid) and blue dye ( Fig. 45.7 ). The radioactive protein can be filtered or unfiltered. Blue dyes used include isosulfan blue, methylene blue, or patent blue dye. If radioactive protein is used, it is usually injected 2 hours before surgery or up to the day before for early-morning cases. The blue dye is usually injected in the operating room followed by breast massage for approximately 5 minutes. The tracers can be injected in the tissue around the primary tumor, in the skin overlying the primary tumor or the periareolar area.
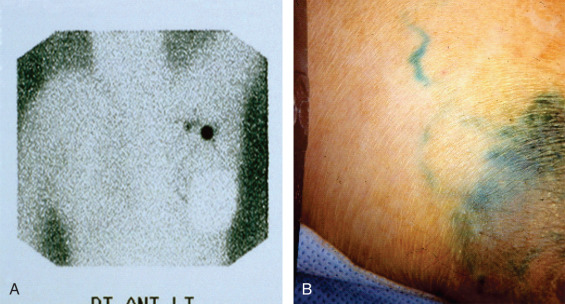
Sentinel lymphadenectomy commences with a transverse incision in the low axilla, two to three finger-breadths below the axillary apex. The sentinel lymph nodes are encountered after incision of the clavipectoral fascia. All hot (radioactive), warm, and/or blue nodes are considered sentinel lymph nodes. If radioactive protein is used, a background less than 10% of the hottest node is considered “cold.” After all sentinel lymph nodes are removed, the axilla is palpated to ensure that there are no firm nodes. All firm nodes should be removed; they may represent lymph nodes that the dye cannot drain to because of complete nodal replacement with tumor. The clavipectoral fascia should be reapproximated during closure to avoid a bulging seroma in the axilla. Patients undergoing a mastectomy can still have axillary evaluation with sentinel lymphadenectomy. A separate axillary incision is not required. Placement of a drain after sentinel lymphadenectomy is usually not required unless accompanied by a mastectomy.
Sentinel lymphadenectomy is the preferred surgical approach in women with early breast cancer. Until the introduction of sentinel lymphadenectomy, axillary node dissection was the operation most frequently used to evaluate the axilla in breast cancer patients. An axillary node dissection can provide information on nodal involvement and the number of nodes involved. Contemporary axillary dissection in breast cancer refers to the removal of level I and II lymph nodes. Axillary node dissection has significant morbidity, including lymphedema, paresthesias, and nerve damage, and an axillary node dissection in node-negative women provides no therapeutic benefit other than prognostic information. Axillary dissection is usually reserved for patients with positive sentinel lymph nodes, those with grossly positive nodes, and those with early breast cancer who cannot have a sentinel lymph node biopsy. In 2010 Giuliano and colleagues reported the American College of Surgeons Oncology Group (ACOSOG) Z0011 trial. They reported equivalent local recurrence and survival in patients with two or more positive nodes on sentinel node biopsy and whole breast radiation therapy with or without full axillary node dissection. Nondissection of the axilla with positive nodes is considered a viable choice equal to axillary node dissection. Of note is that a majority of these patients did receive radiation to the axilla. Also objective lymphedema was not different between the sentinel and axillary lymph node dissection groups in Z0011. This study and its ramification are described in detail in Chapter 42 .
Targeted axillary dissection has been more recently described and includes the performance of sentinel lymphadenectomy in combination with the localization and removal of a clipped and biopsy-proven positive lymph node(s) in patients who have undergone neoadjuvant chemotherapy. The goal is to allow a more selective removal of nodes in an attempt to prevent axillary node dissection and its possible associated morbidities. A prospective study has revealed a false-negative rate of 2% compared with approximately 10% when sentinel lymphadenectomy was performed alone. Sample size limited the statistical comparison, but research is ongoing. The Alliance trial has also suggested that the evaluation of the positive clipped lymph node should be considered when performing sentinel lymphadenectomy in post-neoadjuvant chemotherapy patients allowing for significant reduction of false negative rates in this group.
Adjuvant Radiotherapy
Treatment of early breast cancer with breast conservation usually involves radiotherapy. This is comprehensively covered in Chapter 47 , Chapter 50 , Chapter 51 . There has been much debate and many trials that have addressed the need for radiotherapy after breast conservation surgery for cancer. There have been 14 randomized clinical trials that addressed this issue. The details of these trials vary with regard to patient selection, the use of adjuvant systemic treatment, and the length of follow-up. Three studies have compared breast conserving surgery with and without radiotherapy: the Uppsala-Orebro, Ontario, and Finnish studies ( Table 45.2 ). These studies collectively showed that breast radiotherapy decreased local recurrence approximately 70% but that there was no difference in overall survival. A meta-analysis of all trials of breast conserving surgery with and without radiotherapy reported that there was a threefold increased risk of local recurrence if radiotherapy was omitted. The meta-analysis also suggested that there was a small survival benefit with breast radiotherapy.









