Abstract
Neoadjuvant chemotherapy (NACT) for breast cancer is the administration of systemic therapy to patients before surgery. It was initially used to downstage inoperable tumors but over time has become a useful tool to allow breast conservation surgery and a valid setting to assess the impact of new therapeutics on pathologic complete response, a surrogate end point for long-term survival. This chapter reviews the foundations of this treatment modality and the latest progress in the field. Two decades of progress in understanding the biology of breast cancer led to the classification of this disease into subtypes with distinct clinical behavior and response to treatment. The one-size-fits-all strategy was replaced by personalized treatments and a great opportunity to improve the eradication rate of this disease.
Keywords
breast cancer, neoadjuvant chemotherapy, neoadjuvant radiation therapy, pathologic complete response, intrinsic subtypes, sentinel lymph node procedure, axillary lymph node dissection
Neoadjuvant chemotherapy (NACT) for breast cancer is the administration of systemic therapy to patients before surgery. It was initially used to downstage inoperable tumors but over time has become a useful tool to allow breast conservation surgery and a valid setting to assess the impact of new therapeutics on pathologic complete response (pCR), a surrogate end point for long-term survival. This chapter reviews the foundations of this treatment modality and the latest progress in the field. Two decades of progress in understanding the biology of breast cancer led to the classification of this disease into subtypes with distinct clinical behavior and response to treatment. The one-size-fits-all strategy was replaced by personalized treatments and a great opportunity to improve the eradication rate of this disease.
Historically, NACT was offered to patients with locally advanced breast cancer to downstage the tumor and improve surgical resectability and sometimes as the only effective strategy to treat patients with inflammatory breast cancer (IBC). Later it was proposed as a treatment modality to increase breast conservation surgery in operable breast cancer and most recently as the preferred setting to test new drugs and combinations before embarking on long and costly adjuvant clinical trials.
The need for systemic therapy emerged in the mid-20th century when increasingly aggressive surgical treatments failed to cure breast cancer patients who would succumb to systemic disease. This realization paved the way to adjuvant postoperative chemotherapy (ACT), leading to significant improvements of disease-free survival (DFS) and overall survival (OS) and proving the hypothesis suggesting that breast cancer cell dissemination occurs early in the course of the disease. The benefit of ACT was confirmed in multiple clinical trials using different chemotherapy agents and regimens that enrolled tens of thousands of patients; many of these trials were included in the Oxford overview. As ACT became established, it was tempting to take the regimens that had proven efficacy in the adjuvant setting to the neoadjuvant setting. This led to a large number of clinical trials comparing ACT to NACT. Collectively, these trials proved that NACT was safe and results in equivalent long-term survivals compared with ACT ( Table 65.1 ). Another benefit of this treatment modality was the increase in the breast conserving surgery (BCS) rate without significant increase of local recurrences.
| Trial | Patient N | Tumor Size | Regimen | FU (Mo) | DFS IBTR | OS | BCS NAT vs AT |
|---|---|---|---|---|---|---|---|
| Fisher et al. 1998 NSABP B-18 , a | 1523 | All | AC × 4 | 96 | = | = | 68% vs. 60% p = .002 |
| Van der Hage et al. 2001 EORTC 10902 , a | 698 | ≥1 cm | FEC × 4 | 56 | = | = | 37% vs 21% p = NA |
| Taucher et al. 2008 ABCSG-7 | 215 | All | CMF × 3 | NA | ↑IBRT | = | NA |
| Scholl et al. 1994 S6 | 414 | 3–7 cm | FAC × 4 | 66 | = | = | 82% vs. 77% |
a These trials confirmed the safety and feasibility of this treatment modality with the increase of breast conserving surgery.
Two additional reasons for the use of NACT include the monitoring of response to chemotherapy for the individual patient that would allow its adjustment and also to test new drugs. Because in vitro chemosensitivity and resistance tests proved to be useless in routine clinical practice, it was necessary to find a rapid and cheap way to test these drugs in vivo. The benefit of the NACT setting is the possibility to reach a quick conclusion (weeks or months) about the efficacy of new drugs compared with the ACT setting where the trials span over years and require much larger financial and human resources. The emergence of pCR as a predictor of DFS and OS has prompted the US Food and Drug Administration (FDA) to issue in 2014 an important memo titled “Guidance for Industry Pathologic Complete Response in Neoadjuvant Treatment of High-Risk Early Stage Breast Cancer: Use as an Endpoint to Support Accelerated Approval.” This document provides guidance to investigators and industry about the use of pCR as an end point for accelerated approval. This is discussed in detail in the following sections. Many investigators remain skeptical about the benefit of pCR, arguing that it selects for those who have chemosensitive disease that would have responded well to ACT anyway. However, when NACT is used as in vivo chemosensitivity test, it provides a unique opportunity for personalized treatment and represents a powerful research tool.
Molecular Subtypes of Breast Cancer and Response to Neoadjuvant Chemotherapy
The first generation of neoadjuvant chemotherapy trials showed that different breast cancer subtypes responded differently to chemotherapy. Hormone receptor (HR) negativity and HER2 positivity were associated with more responsiveness, whereas HR positivity and HER2 negativity were associated with less responsiveness to chemotherapy. Other predictive markers of responsiveness included small tumor size, high ki67, high tumor grade, high tumor labeling index, and lymphovascular space invasion.
The year 2000 was a turning point in our way of looking at breast cancer. The publication of a seminal paper “Molecular Portraits of Human Breast Tumors” by Perou and colleagues opened the field of molecular and genetic evaluation of this cancer and other solid tumors. The main premise of the field is that despite phenotypical similarities, breast cancers are different in terms of genes and pathways activated in each one and in their behavior and response to therapy. Using gene expression profiling, five intrinsic subtypes were identified: the basal-like, the HER2 positive, the luminal A and B, and the normal-like. On the basis of this original work, the PAM50 and PAM50 risk of relapse (PAM50-ROR) assays were developed. The first allows the classification of breast cancers into intrinsic subtypes, and the second provides important information about their prognosis. In the next decade, other gene expression profile (GEP)-based prognostic assays were developed, including the Onco type Dx and MammaPrint. All these assays generate a score that assigns a low, medium, or high risk of recurrence (PAM50-ROR, Onco type Dx) or good-bad prognosis (MammaPrint) to each individual cancer. Attempts at using a grouping of traditional classifiers such as HR, HER2, and ki67 yielded a good approximation of the intrinsic subtypes (luminal A: HR+/HER2−/ki67 low; luminal B: HR+/HER2−/ki67 high; luminal B: HR+/HER2+; HER2+: HR−/HER2+; triple negative: HR−/HER2−) that fell short of fully recapitulating the subtypes defined by GEP.
The intrinsic subtypes (basal-like, HER2 positive, luminal A and B, and normal-like) were able to describe the biological behavior and response to treatment. Indeed, pCRs after NACT were the highest for the first two and dropped sharply in the luminal subtypes and were nonexistent in the normal-like subtype (45%, 45%, 6%, and 0%, respectively).
Eligibility for Neoadjuvant Chemotherapy or Neoadjuvant Radiation Therapy
The first reports of therapeutic effects of systemic chemotherapy appeared in the 1960s and of neoadjuvant therapy in the 1970s. Historically, preoperative or NACT was offered to patients with locally advanced or inflammatory breast cancer. Locally advanced breast cancer (LABC) is defined by tumor (T3, T4) or nodal (N2 or N3) stage (American Joint Committee on Cancer). T3 tumors are those larger than 5 cm and T4 involve the chest wall (T4a), the skin (T4b) or both (T4c). N2 stage includes matted ipsilateral axillary lymph nodes (fixed to one another or to other structures) (N2a) or clinically apparent ipsilateral internal mammary lymph nodes by imaging studies or clinical examination in the absence of ipsilateral axillary lymph node involvement (N2b). N3 stage includes metastases to ipsilateral infraclavicular (N3a), internal mammary and axillary (N3b) or to supraclavicular lymph nodes (N3c). IBC is defined clinically by the rapid onset (<6 months) of erythema and edema with palpable edges (peau d’orange) involving at least one-third of the breast (T4d) and confirmed by a breast biopsy. Although the involvement of the dermal lymphatics with invasive breast cancer is considered the pathologic hallmark of this type, it is not specific nor is it required to make this diagnosis. Lumping this form with LABC, which was frequently done in clinic trials in the past, is no longer justified according to new findings of biological and prognostic differences. In LABC and IBC, upfront surgery may not be feasible and if done may leave large macroscopic disease in the tumor bed or axilla. NACT is likely to downstage the tumor and increase the likelihood of successful and complete surgical resection. Similar reasoning applies to situations in which the patient desires BCS but the tumor size is “large” relative to the breast, and upfront surgery is likely to lead to mastectomy.
Early animal studies have suggested a possible therapeutic advantage to NACT over ACT based on the increase of labeling index in the residual tumor after removal of the primary tumor with the possibility to prevent this phenomenon if chemotherapy is given to the animals before tumor removal. This hypothesis was rejected when tested in humans. Trials comparing NACT to ACT showed that these two modalities had equal efficacy and safety (see Table 65.1 ).
Another advantage for the use of NACT in early-stage breast cancer is the assessment of in vivo sensitivity to chemotherapy because no in vitro sensitivity test could prove useful to predict chemotherapy efficacy in patients. The many concerns that were raised against NACT (e.g., the fear of delaying curative local therapy, developing resistance by the metastases, or increasing the risk of subsequent surgery or radiation therapy [RT]) were not validated by the results of clinical trials. The advent of powerful imaging modalities such as magnetic resonance imaging (MRI) has mitigated the consequences of losing initial staging information.
Evaluation of Candidates for Neoadjuvant Chemotherapy or Neoadjuvant Radiation Therapy
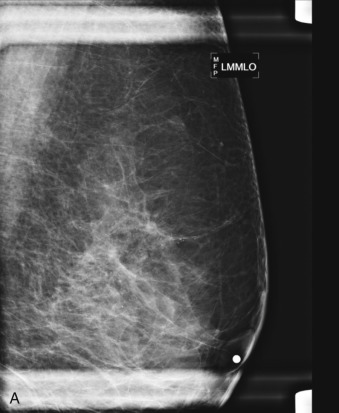
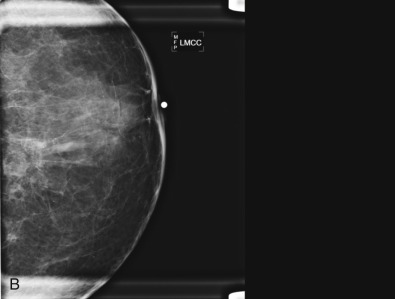
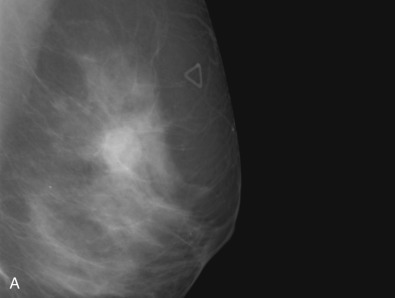
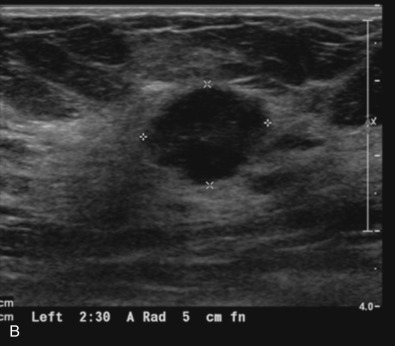
The initial evaluation of breast cancer patients helps the clinician assign a clinical stage and obtain precious information to direct the therapy ( Box 65.1 ). The tumor size is measured by a caliper (in the sitting position using a horizontal and vertical axes or the longest diameter) and the presence of chest wall or skin involvement or inflammatory signs (edema, erythema, or peau d’orange sign) are documented; palpable lymph nodes should be evaluated for adhesion to the skin or axillary structures. Mammography and ultrasound help determine the size of the cancer, the presence of multicentricity/mutifocality, diffuse microcalcifications, or synchronous contralateral breast cancer. Ultrasound can also be used for monitoring of the size of the tumor during NAT.
Primary Breast Tumor
- •
Clinical evaluation (mass size, consistency, skin and chest wall involvement, inflammatory signs, nipple discharge, etc.)
- •
Mammography/ultrasound
- •
Core biopsy (at least three), for immediate use and tumor banking
- •
Histologic examination:
- •
Tumor type and grade
- •
Estrogen and progesterone receptors
- •
HER2
- •
ki67
- •
Others
- •
Lymph Node Status
- •
Clinical evaluation (palpable or nonpalpable, matted)
- •
Mammography/ultrasound
- •
Ultrasound-guided fine-needle aspiration or core needle biopsy
- •
Sentinel lymph node biopsy, before or after but not both
Many experts consider pretreatment MRI standard of care but its role compared with mammogram and ultrasound remains controversial. Its sensitivity in detecting occult contralateral breast cancers (approaching 94%–100%) is superior to mammography, but the benefit from this high sensitivity is mitigated by low specificity (37%–97%). The increased power of modern machines (from 1- or 1.5-T to 3-T MRI) adds to the sensitivity without improving the specificity. Using diffusion-weighted MRI may increase specificity for small masses and non-masslike lesions. MRI is not recommended for routine use before NACT in small operable breast cancers because of the high false positivity, but its use is appropriate in locally advanced tumors and for response assessment and surgery planning. Two decades of experience with breast MRI allows the identification of MRI phenotypes that are predominantly associated with each biological subtype; this relationship between MRI phenotype and biological subtypes is not exclusive because all MRI phenotypes can be seen in any biological subtype. Triple-negative breast cancer (TNBC) tends to be associated with unifocal masses, whereas HER2-positive breast cancers tend to be associated with multifocal masses. Nonmass or diffuse enhancements tend to be more common with HR-positive tumors ( Figs. 65.1, 65.3, 65.5, 65.6, 65.8, 65.9, 65.11, 65.12, 65.14, and 65.15 ). The breast cancer team is interested in knowing the extent of the residual disease to help tailor surgical resection, especially if the patient is interested in breast conservation surgery. Responses to NACT and the accuracy of response assessment by MRI vary depending on the biological subtypes, with the best responses, and the most accurate assessments, being observed in TNBC and HER2-positive breast cancers compared with the HR-positive cancers, for which responses are poor and the MRI assessments are the least accurate (as was shown in the I-SPY study). Indeed, underestimation of the size of residual tumor is observed more commonly (up to two-thirds of patients) in HR-positive cancers. Positive predictive value, but not negative predictive value (NPV), of MRI is high and fairly accurate in these tumors ( Figs. 65.2, 65.4, 65.7, 65.10, 65.13 and 65.16 ).
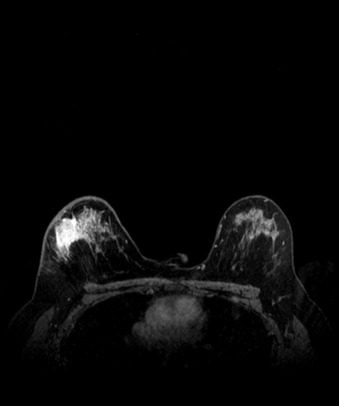
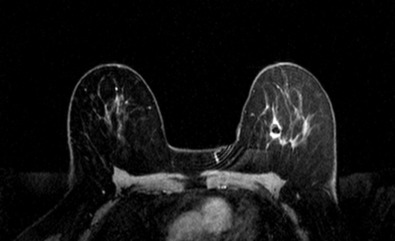
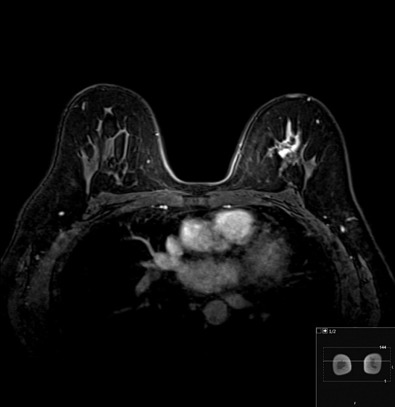
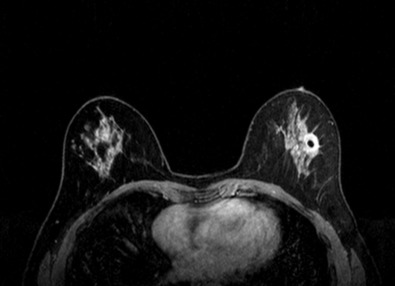
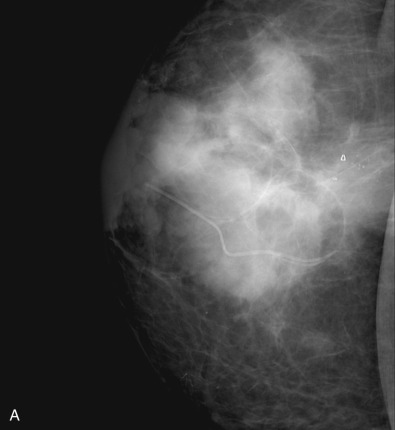
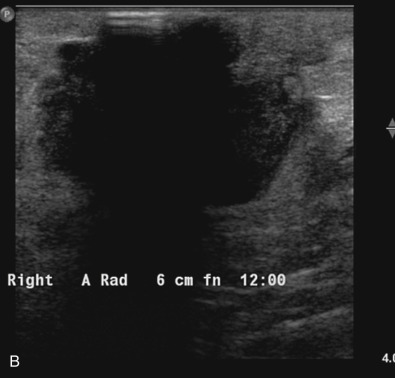
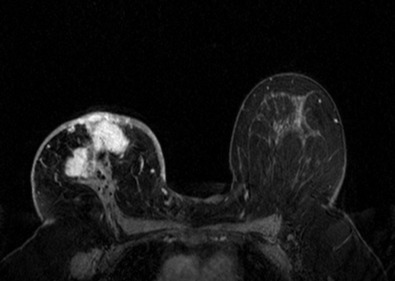
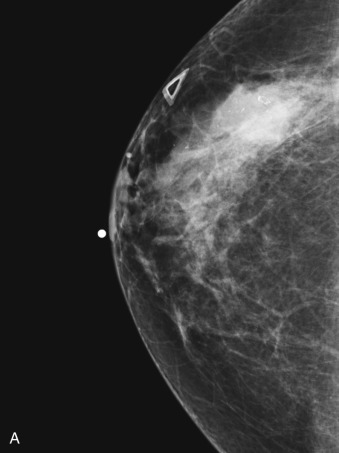
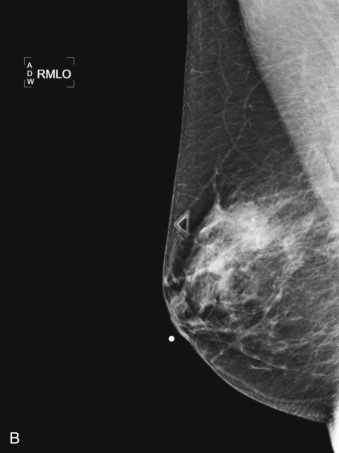
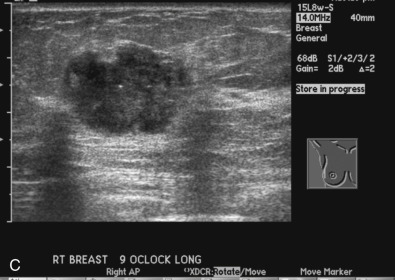
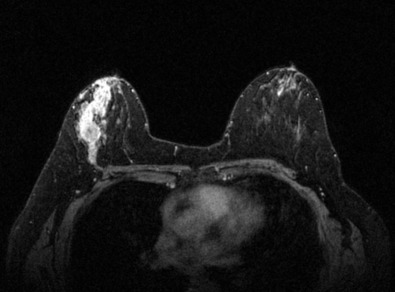
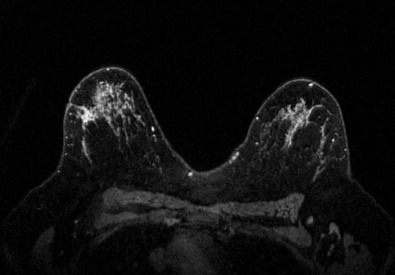
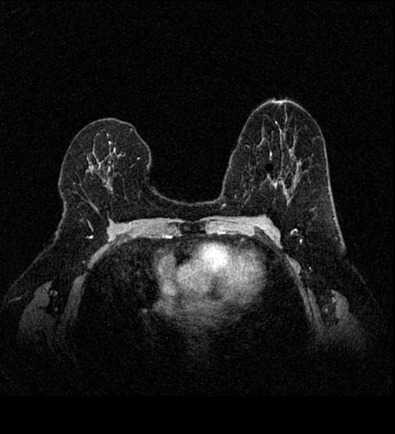
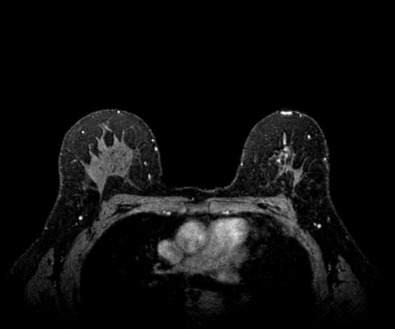
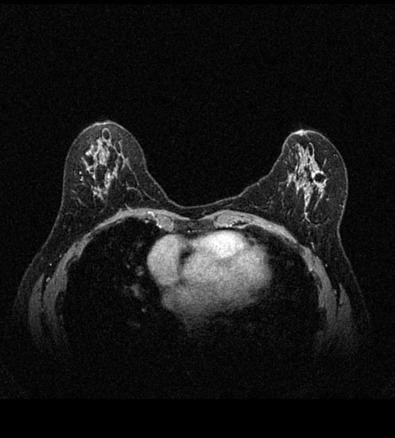
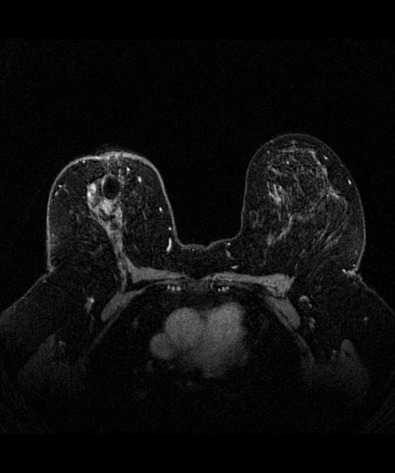
One of the major challenges of the field is to identify those patients in whom radiologic complete response can predict pCR and may allow the patients to forgo surgery. Again, assessment with MRI is the most accurate in TNBC and HER2-positive breast cancers with NPV being the highest in these subtypes (60%–91% and 62%–95%, respectively). These NPVs are still below the required cutoffs to forgo surgery because up to 40% of the patients would have false-negative MRI, and omitting surgery means that we would leave those patients with residual disease in the breast. Using diffusion-based imaging, Bufi and coworkers reported NPVs of 100% in these two subtypes. These results need to be replicated. Thus the technology is not ready for wide clinical application because its performance needs to be improved and it must be validated by prospective clinical trials.
At least three core needle biopsies from the main lesion and one from any other lesion are considered standard procedure to obtain adequate tissue for full assessment of the tumor type and HR and HER2 status determination. Radiopaque clips may be placed during the same procedure to help with a future retrieval of the tumor.
Axillary lymph node status should be assessed clinically. Patients with clinically detectable lymphadenopathy should have a fine-needle aspiration biopsy or core biopsy from their lymph nodes. Clinically, nonpalpable lymph nodes can be assessed with sentinel lymph node procedure before or after NACT (as discussed later).
Outcomes and End Points of Neoadjuvant Therapy
Whereas phase III adjuvant trials address definitive end points of relapse and survival but are slow, require large samples, and are costly, neoadjuvant clinical trials use smaller populations and can rapidly test (usually in order of months) new drugs or schedules through surrogate end points likely to predict clinical benefit. Several clinical trials have shown favorable long-term DFS and OS rates for patients who achieved pCR after NACT. The biological hypothesis behind these results is that clearance of the cancer from the breast translates into elimination of possible disseminated tumor cells or micrometastasis. Predictor of pCR after NACT are age less than 40 years, tumor less than 2 cm, ductal histology, high tumor grade, high proliferation index (assessed by ki67), negative estrogen receptor (ER) status and intrinsic subtype (basal-like or HER2-enriched).
Interestingly, the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-27 trial failed to demonstrate that doubling of pCR with the addition of docetaxel to doxorubicin and cyclophosphamide (13.7 vs. 26.1 %; p < .001) translates into improvement of DFS or OS in the experimental arm but confirmed that patients who achieved pCR (defined in this trial as absence of invasive tumor in the breast only) had a better outcome irrespective of the regimen received.
On the basis of the accumulated evidence of the prognostic value of pCR, the FDA announced consideration for accelerated approval in early breast cancer for new drugs tested in neoadjuvant setting if the product “has an effect on a surrogate end point likely to predict clinical benefit” and recommended pCR as such surrogate end point. Thus accelerated approval was granted in September 2013 to use pertuzumab as a part of neoadjuvant regimen for early-stage breast cancer expressing HER2 based on increased pCR achieved in the Neosphere and TRYPHAENA trials.
Despite the fact that pCR has been the primary end point for all recent NACT trials, the pathologic criteria to define pCR have been variable. The three most commonly used definitions of pCR are the following:
- •
ypT0 ypN0: absence of invasive cancer and in situ cancer in the breast and axillary nodes;
- •
ypT0/is ypN0: absence of invasive cancer in the breast and axillary nodes, irrespective of carcinoma in situ; and
- •
ypT0/is: absence of invasive cancer in the breast irrespective of ductal carcinoma in situ or nodal involvement.
To investigate the relationship between pCR and long-term clinical benefit, the FDA established a working group known as the Collaborative Trials in Neoadjuvant Breast Cancer (CTNeoBC) that conducted a meta-analysis of twelve international large randomized neoadjuvant clinical trials with more than 12,000 patients enrolled. Several lessons were learned from the CTNeoBC meta-analysis:
- 1.
Individuals who achieved pCR by any of the three preceding definitions have a more favorable long-term outcome for both event-free survival (EFS) (hazard ratio 0.48, 95% confidence interval [CI] 0.43–0.54) and OS (hazard ratio 0.36, 95% CI 0.31–0.42).
- 2.
Eradication of invasive cancer from both breast and lymph nodes (ypT0 ypN0 or ypT0/is ypN0) rather than in breast only (ypT0/is) was better associated with improved EFS (ypT0 ypN0: hazard ratio 0.44, 95% CI 0.39–0.51; ypT0/is ypN0: 0.48, 0.43–0.54; ypT0/is: 0.60, 0.55–0.66; and OS (ypT0 ypN0: hazard ratio 0.36, 0.30–0.44; ypT0/is ypN0: 0.36, 0.31–0.42; ypT0/is: 0.51, 0.45–0.58. On the basis of these data, the FDA recognizes a definition of pCR for consideration for US marketing approval as the absence of residual invasive carcinoma in the complete resected breast specimen and all sampled regional lymph nodes irrespective of the presence or absence of residual ductal carcinoma in situ (DCIS) (ypT0/is ypN0 or ypT0 ypN0).
- 3.
As expected, frequency of pCR was low in low-grade HR-positive tumors (hazard ratio 7.5%, 95% CI 6.3–8.7) but higher in the more aggressive subtypes: triple-negative (33.6%, 30.9–36.4), HER2-positive tumors treated with (50.3, 95% CI 45.0–55.5) or without (30.2%, 95% CI 26.0–34.5) trastuzumab, and grade 3 HR-positive/HER2-negative breast cancer (16.2%, 13.4–19.3). Improvement in long-term outcome was more significant in the aggressive cancer subtypes if pCR (defined as ypT0/is ypN0 based on findings described earlier) was achieved, with a reduction of the risk of death by 84% (95% CI 75–89), 92% (95% CI 78–97), 71% (95% CI 50–83), and 71% (95% CI 35–87), respectively. In contrast, the association between pCR and long-term outcome was weakest for HR-positive subtypes (particularly lower-grade cancers).
- 4.
At a trial level, the CTNeoBC analysis demonstrated a weak association between pCR (ypT0/is, ypN0) and both EFS and OS. A potential explanation for the inability to demonstrate a clear correlation could be that the inclusion of heterogeneous populations in the pooled analysis may have obscured the association as the absolute improvements in the pCR rates between treatment arms was low (1%–11%). In contrast, when the improvement in pCR was high (20%) as in the case of the Neoadjuvant Herceptin (NOAH) trial comparing trastuzumab plus chemotherapy to chemotherapy alone in women with HER2-positive locally advanced breast cancer, a certain correlation was found between effect on pCR and long-term benefit. These results suggest that a correlation between pCR and long-term outcome might be identified in trials conducted in more homogeneous breast cancer populations and in more aggressive subtypes in which higher pCR rates are expected.
Switching nonresponders to a non–cross-resistant regimen did not result in increased rates of pCR. Indeed, there is no evidence that clinical response should be used to tailor subsequent treatment choices based on the results of the Aberdeen and the GeparTrio trials. The Aberdeen trial randomized patients who responded to four cycles of cyclophosphamide, vincristine, doxorubicin, and prednisone (CVAP) to receive either four cycles of the same regimen or four cycles of docetaxel. Nonresponders to four cycles of CVAP were switched to docetaxel. pCR doubled in responders who were switched to docetaxel (31% vs. 15%), whereas nonresponders had a modest pCR (2%) despite the addition of docetaxel. The GeparTrio trial randomized nonresponders, defined as patients who did not achieve a decrease in size of their tumor by at least 50% after two cycles of docetaxel, doxorubicin, and cyclophosphamide (TAC), to four additional cycles of TAC or to four cycles of vinorelbine and capecitabine (NX). Switching to NX did not improve pCR.
Because of the low rates of pCR after NACT and the weaker correlation between pCR and survival end points in HR-positive breast cancers, the neoadjuvant field has moved more generally toward strategies to omit chemotherapy in these subsets of patients. It is assumed that endocrine therapy acts through induction of cell-cycle arrest and changes in surrogates of cell proliferation could be considered a potential surrogate of response to neoadjuvant endocrine therapy (NET). One candidate is the nuclear nonhistone protein ki67, widely used as a marker for proliferation. Interestingly, changes in levels of ki67 in relatively small NET were able to anticipate outcomes from large adjuvant trials that showed the superiority of aromatase inhibitors (AIs) over tamoxifen and equivalence between the three AIs in postmenopausal women. The best time to assess ki67 response is not well defined, however. In the Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) trial, in which ki67 was measured as early as 2 weeks, the superiority of anastrozole over tamoxifen was confirmed based on a greater reduction in ki67 levels either after 2 or 12 weeks ( p = .013 and p = .0006, respectively), despite the fact that the trial was negative for its primary end point (clinical response). Furthermore, in a multivariate analysis, ki67 expression at 2 weeks was significantly associated with recurrence-free survival (hazard ratio = 1.95; p = .004).
One potential limitation of this early assessment of ki67 is the development of delayed or acquired resistance. Paradoxical increases in Ki67 in the surgical specimen compared with baseline (>5%) were seen in 12.3% of luminal A and 5.8%, luminal B patients included in American College of Surgeons Oncology Group (ACOSOG) Z1031 trial. In addition, in IMPACT trial, the authors showed that 15% of patients who had early decreases in ki67 (on week two biopsy) on NET had increases in ki67 at the time of surgery (as assessed on surgical specimens), raising doubts about the optimal time points for assessment of this and other potential biomarkers. Currently, the advantage of measuring 2-week ki67 instead of pretreatment ki67 is being prospectively investigated in the large ( n = 4000) Perioperative Endocrine Therapy for Individualising Care (POETIC) window-of-opportunity trial.
Neoadjuvant Chemotherapy by Breast Cancer Subtypes
Chemotherapy is the first type of systemic therapy used neoadjuvantly. However, the introduction of endocrine and targeted therapies, based on better understanding of the disease, enriched our armamentarium in the recent years in this setting. The first chemotherapeutic agents used in this setting were the ones that had proven track records in the adjuvant setting such as cyclophosphamide, methotrexate, and fluorouracil (CMF). These were replaced by newer drugs such as anthracyclines and taxanes ( Table 65.2 ). Most neoadjuvant chemotherapy regimens in 2016 contain an anthracycline (doxorubicin or epirubicin), a taxane (docetaxel or paclitaxel), and cyclophosphamide. The addition of the vinorelbine, gemcitabine, and capecitabine in the adjuvant or neoadjuvant settings did not add more benefit. Only the addition of platinum compounds in TNBC and HER2-positive cancers may have additional efficacy that is still being explored.
| Trial | Patient N | Regimen | pCR (%) |
|---|---|---|---|
| Buzdar et al. 1999 | 174 | FAC × 4 | 16.4 (23) |
| Paclitaxel × 4 | 8.1 (14) | ||
| Smith et al. 2002 (Aberdeen) | 104 | CVAP | 15 |
| CVAP-D | 31 | ||
| Dieras et al. 2004 | 200 | AC × 4 | 10 |
| AT × 4 | 16 | ||
| Evans et al. 2005 | 363 | AC × 6 | 16 (24) |
| AD × 6 | 12 (21) | ||
| Gianni et al. 2005 | 451 | AT × 4, CMF × 4 | 23 |
| Green et al. 2005 | 258 | T Q3W × 4, FAC × 4 | 15.7 |
| T QW × 12 − FAC × 4 | 28.2 | ||
| von Minckwitz et al. 2005 (GeparDuo) | 913 | AD | 11 |
| AC-D | 22.3 | ||
| Steger et al. 2007 | 288 | ED × 3 | 7.7 |
| ED × 6 | 18.6 | ||
| Rastogi et al. 2008 (NSABP-B27) | 2411 | AC | 9.6 (13.7) |
| AC-D | 18.9 (26.1) | ||
| von Minckwitz et al. 2008 (GeparTrio) | 622 | DAC × 6 | 5.3 |
| DAC × 2 -NX × 4 | 6 | ||
| von Minckwitz et al. 2008 (GeparTrio) | 1390 | DAC × 6 | 21 v |
| DAC × 8 | 23.5 | ||
| von Minckwitz et al. 2010 (GeparQuatro) | 1590 | EC × 4, D × 4 | 22.3 |
| EC × 4, DX × 4 | 19.5 | ||
| EC × 4, D × 4, X × 4 | 22.3 |
It is now established that multiagent chemotherapy regimens are superior to single-agent regimens. Combinations of anthracyclines and taxanes are superior to each one alone and their efficacy is better when they are used sequentially rather than concurrently (see Table 65.2 ). Dose-dense chemotherapy was also tested to increase pCR in a trial randomizing patients with LABC to fluorouracil, epirubicin, and cyclophosphamide (FEC) every 3 weeks or epirubicin/cyclophosphamide (EC) every 2 weeks. There was no difference in any of the end points evaluated (pCR, DFS, OS). Five other trials were conducted with conflicting results. A meta-analysis of all randomized trials showed significant 46.7% improvement of pCR with dose dense but as expected, no impact on overall survival.
Considering the heterogeneity of breast cancer, there is no single optimal chemotherapy regimen for all subtypes of this disease. Two schedules have been used to deliver chemotherapy; the first is the sandwich schedule in which patients receive some of the chemotherapy before and some after surgery, and the total preoperative schedule in which all the treatment cycles are given before surgery; the latter is considered the preferred modality to test the efficacy of the whole regimen and assess its impact on pCR.
Subset analyses of neoadjuvant chemotherapy trials showed unambiguously the low rates of pCRs in HR-positive breast cancer. Patients with HR-positive breast cancer and those who cannot take chemotherapy may be offered neoadjuvant endocrine therapy. However, this modality (AIs and selective estrogen receptor modulators) results in much lower pCR rates compared with chemotherapy (pCR <2% and 15%–25%, respectively) and its effects are slower than the ones obtained with chemotherapy.
Targeted Therapy
Anti-HER2 Therapy
The identification of HER2 as a major driver in breast cancer and the development of a monoclonal antibody (trastuzumab) that can specifically block it ushered in a new era in targeted therapy for breast cancer. Several small trials using trastuzumab in combination with chemotherapy in the neoadjuvant setting showed magnitudes of pCR, unseen before with chemotherapy alone ( Table 65.3 ). The high pCRs were achieved with the combination of trastuzumab and various chemotherapy regimens, but the optimal combination remains to be defined. Combinations of trastuzumab with anthracyclines were considered dangerous based on cardiac toxicity observed in the metastatic setting. Trastuzumab and different anthracycline (epirubicin) or novel formulations of doxorubicin (liposomal doxorubicin, pegylated or not) were also tested. However, the lack of long-term safety data suggest that it is premature to use these combinations outside of the context of clinical trials (see Table 65.3 ). The GeparQuatro is a large neoadjuvant trial that enrolled 445 patients who received epirubicin/cyclophosphamide with trastuzumab followed by docetaxel with trastuzumab with or without capecitabine. The addition of trastuzumab led to 16% increment in pCR from 15.7% in historical control and HER2-negative cases to 31.7% in HER2-positive patients who received the drug. Buzdar and colleagues reinvestigated the role of concurrent administration of trastuzumab and anthracyclines in a phase III trial. Concurrent administration of trastuzumab (T) and epirubicin (paclitaxel(P)/T followed by FEC-T vs. FEC followed by PT) resulted in no improvement of pCR, 56.5% (95% CI 47.8–64⋅9) vs. 54.2% (95% CI 45.7–62.6), respectively and additional cardiac toxicity in the concurrent arm as assessed at 12 weeks (2.9% vs. 0.8%, respectively). The authors concluded that concurrent use of trastuzumab and anthracyclines is not warranted. The HannaH trial explored the role of a subcutaneous (SQ) formulation of trastuzumab in a large phase III trial in patients with HER2-positive, operable, locally advanced or inflammatory breast cancer. In this trial, patients were randomized to receive FEC × 4 − docetaxel × 4 with intravenous (IV; n = 299) or SQ trastuzumab (n = 297). Although the SQ arm pCR was not statistically inferior to the IV arm (40.7% vs. 45.4%, respectively), there was excess toxicity in the SQ arm.
| Trial | Patients N/Types | Regimen | Control | pCR Definition | pCR |
|---|---|---|---|---|---|
| Burstein et al. 2003 | 40 HER2-positive (2+ or 3+ by IHC) stage II or III | T (4 mg/kg × 1, then 2 mg/kg/w × 11) with P (175 mg/m 2 Q 3 wk × 4) | None | 18% | |
| Buzdar et al. 2007 | 42 (+22): Control 19 Chemo + T 23 (additional cohort 22) HER2-positive Stage II or IIIA | P Q 3 wk × 4: 225 mg/m 2 as a 24-h infusion plus T → FEC Q 3 wk × 4: F 500 mg/m 2 on day 1 and 4, C 500 mg/m 2 IV, day 1 and E 75 mg/m 2 day 1 plus T | P × 4 → FEC × 4 | ypT0/is | Control 26.3% Chemo + T 23: 65.2% All 45: 60% |
| Sikov et al. 2009 | 55 HER2-negative (37) and HER2+ (18) Stage IIA–IIIB | Carbo AUC 6 Q 4 wk P 80 mg/m 2 Q wk × 16 and T Q wk × 16 (only for HER2+) | Carbo AUC6 Q 4 wk P 80 mg/m2 Q wk × 16 for HER2− | HER− 31% HER2+ 76% | |
| Untch et al. 2010 GeparQuattro | 1509 HER + or − HER2+ 445 Stages I–III | EC (90/600 mg/m 2 ) Q 3 wk × 4 → D 100 mg/m 2 Q 3 wk × 4 (EC-T), or D 75 mg/m 2 plus X 1800 mg/m 2 Q 3 wk × 4 (EC-TX), or D 75 mg/m 2 Q 3 wk × 4 followed by X 1800 mg/m 2 days 1–14 Q 3 wk × 4 (EC-T-X). T 6 mg/kg (after loading dose of 8 mg/kg) Q 3 wk during all chemotherapy (8 or 12 cycles) | EC (90/600 mg/m 2 ) Q 3 wk × 4 → D 100 mg/m 2 Q 3 wk × 4 (EC-T), OR D 75 mg/m 2 plus X 1800 mg/m 2 Q 3 wk × 4 (EC-TX), or D 75 mg/m 2 Q 3 wk × 4 followed by × 1,800 mg/m 2 days–14 Q 3 wk × 4 (EC-T-X) | ypT0/is, ypN0 | HER2− 17.3% HER2+ 42% |
| Pierga et al. 2010 | 340 HER2+ 120 Stage II and III | EC-D (E 75 mg/m 2 , C 750 mg/m 2 Q 3 wk × 4 followed by D 100 mg/m 2 Q 3 wk × 4 T 8 mg/kg then 6 mg/kg IV Q 3 wk × 4 with D | EC-D (E 75 mg/m 2 , C 750 mg/m 2 Q 3 wk × 4 followed by D 100 mg/m 2 Q 3 wk × 4 | Chevallier classification grade 1 + 2 (= to ypT0/is, ypN0) | Control 19% Chemo + T 26% |
| Ismael et al. 2012; The HannaH study | SQ D 75 mg/m 2 Q 3 wk × 4 → F 500 mg/m 2 , E 75 mg/m 2 , and C 500 mg/m 2 Q 3 wk × 4 Plus 600 mg fixed dose subcutaneous Q 3 wk × 8 | IV D 75 mg/m 2 plus T 6 mg/kg (after loading dose of 8 mg/kg) Q 3 wk × 4 → F 500 mg/m 2 , E 75 mg/m 2 , and C 500 mg/m 2 plus T 6 mg/kg Q 3 wk × 4 | ypT0/is, ypN0 | SQ 40.7% IV 45.4% | |
| Buzdar et al. 2013 | 282 Concurrent 140 Sequential 142 HER2+ Stage II or IIIA | Concurrent P Q 1 wk × 12: 80 mg/m 2 plus T 2 mg/kg (after cycle 1 of 4 mg/kg) → FEC Q 3 wk × 4: F 500 mg/m 2 on days 1 and 4, C 500 mg/m 2 IV, day 1 and E 75 mg/m 2 day 1 plus T 2 mg/kg Q 1 wk × 12 | Sequentil FEC Q 3 wk × 4: F 500 mg/m 2 on days 1 and 4, C 500 mg/m 2 IV, day 1 and E 75 mg/m 2 day 1 → P Q wk × 12: 80 mg/m 2 plus T 2 mg/kg (after cycle 1 of 4 mg/kg) | ypT0/is, ypN0 | Concurrent 46.3% Sequential 48.3% |
| Gianni et al. 2014; NOAH | 235 | T 6 mg/kg Q 3 wk × 7, Q 4 wk × 3 (including a loading dose of 8 mg/kg) plus P150 mg/m 2 plus A 60 mg/m 2 IV Q 3 wk × 3, followed by P 175 mg/m 2 Q 3 wk × 4 followed by C 600 mg/m 2 , M 40 mg/m 2 , and F 600 mg/m 2 (on day 1, 8) Q 4 wk × 3 followed T 6 mg/kg Q 3 wk for up to 1 year | P 150 mg/m 2 plus A 60 mg/m 2 IV Q 3 wk × 3, followed by P 175 mg/m 2 Q 3 wk × 4 followed by C 600 mg/m 2 , M 40 mg/m 2 , and F 600 mg/m 2 (on days 1 and 8) Q 4 wk × 3 | ypT0/is, ypN0 | Control chemo 19.4% Chemo + T 38.4% |
| Chumsri et al. 2010 | 33 Chemo: 14 Chemo + T: 19 Stage II and III HER2+ | AC/P with T | AC/P without T | ypT0/is, ypN0 (?) | Chemo 28.6% Chemo + T 52.6% |
| Saracchini et al. 2013 | 43 (39 evaluable) Stage II and III | NPLD (60 mg/mq IV) plus C 600 mg/m 2 IV Q 3 wk × 4 followed by D (35 mg/m 2 IV) plus T (4 mg/kg loading dose IV, then 2 mg/kg IV) Q 1 wk × 16 | None | ypT0/is, ypN0 | 49% |
| Gavilá et al. 2015 | 62 Stage II and III and inflammatory BC | TLC-D99 (50 mg/m 2 ) day 1 Q 3 wk × 6, P (80 mg/m 2 ) day 1 Q 1 wk × 18 and T (4 mg/kg as initial dose on day 1 and then 2 mg/kg Q 1 wk × 18 | None | ypT0/is, ypN0 | 63% |
| Uriarte-Pinto et al. 2016 | 30 HER2+ receptor and clinical stage IIa–IIIb | TLC-D99 (50 mg/m 2 on day 1 Q 3 wk), P (80 mg/m 2 Q 1 wk × 3) and T (8 mg/kg loading dose on day 1 followed by 6 mg/kg Q 3 wk) | None | Miller and Payne classification Grade 5 = ypT0/is, ypN0 | 40% |
A new generation of large trials explored the impact of adding trastuzumab to other anti-HER2 therapies such as the tyrosine kinase inhibitor lapatinib (Tykerb) or the anti-HER2 dimerization monoclonal antibody pertuzumab on pCR. The NeoALTTO trial is a large trial that explored the role of a combination of trastuzumab and lapatinib. The trial enrolled 455 patients who were equally divided between three arms: lapatinib (oral), trastuzumab (IV), or the combination, all given for 6 weeks followed by the addition of weekly paclitaxel for 12 weeks. After 4 weeks of rest the patients were taken to surgery, and all patients resumed their assigned anti-HER2 treatment for an additional period of 34 weeks. There was no difference in pCR between the single agent arms (29.5% and 24.7% for the trastuzumab and lapatinib, respectively; p = .34), but pCR in the combination arm was significantly superior to the trastuzumab arm (51.3% vs. 29.5%; p = .0001). EFS was not different between arms (78%, 76%, and 84% for the lapatinib, trastuzumab, and the combination, respectively), nor was overall survival at 3.84 years follow-up (93%, 90%, and 95% for the lapatinib, trastuzumab, and combination, respectively). Further analysis of the results of this study showed that high HER2 protein expression correlated with increased benefit of adding lapatinib to trastuzumab, contrary to previous findings from the metastatic setting that correlated the benefit from lapatinib with p95HER2 (the truncated form of HER2).
The NeoSphere trial is a phase II neoadjuvant trial that randomized HER2-positive breast cancer patients to one of four treatment arms: trastuzumab plus docetaxel (group A), pertuzumab plus trastuzumab plus docetaxel (group B), pertuzumab plus trastuzumab (group C), and finally pertuzumab plus docetaxel (group D); all treatments were given for four cycles at 3-week intervals. After surgery, all patients received three cycles of FEC and trastuzumab every 3 weeks for a total of 1 year. pCR with the combination of trastuzumab/pertuzumab and docetaxel (group B) was 45.8%, significantly superior to the other groups (A; 29%; D: 24%; p = .0141). Ten to seventeen percent of patients on the A, B, and D arms had serious adverse events (neutropenia, febrile neutropenia, and leukopenia). Interestingly, group C, which received the biologically targeted agents without chemotherapy, had a pCR rate of 16.8% and only 4% of the patients in this group experienced serious adverse event. Five-year PFS was 81% for group A, 86% for group B, 73% for group C, and 73% for group D (hazard ratios 0.69 [95% CI 0.34–1.40] group B vs. group A; 1.25 [0.68–2.30] group C vs. group A; and 2.05 [1.07–3.93] group D vs. group B). DFS results were consistent with progression-free survival results and were 81% for group A, 84% for group B, 80% for group C, and 75% for group D. Again, the value of achieving pCR was confirmed by this study with patients who achieved pCR having longer DFS compared with patients who did not (85% vs. 76%; hazard ratio 0.54 [95% CI 0.29–1.00]).
In the TRYPHENA study, a multicenter, open-label phase II study, 225 HER2-positive breast cancer patients were randomized to two anthracycline-containing regimens: FEC/HP × 3 followed by docetaxel/HP for three cycles (arm A) or FEC for three cycles followed by docetaxel/HP for three cycles (arm B) or to a non–anthracycline-containing regimen: docetaxel/carboplatin and HP for six cycles (arm C). pCR (ypT0/is) was seen in 61.6% (arm A), 57.3% (arm B), and 66.2% (arm C) of patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








