RABIES
ALAN C. JACKSON
Rabies is an acute viral infection of the central nervous system (CNS) that also involves the peripheral nervous system both in its pathogenesis and clinical manifestations. Rabies is a zoonotic disease affecting mammals that is usually transmitted to humans by bites from animal vectors. Rabies virus infection causes most cases of rabies, although non–rabies virus lyssaviruses have been recognized to very rarely cause disease with identical clinical and pathologic features, but not in the Americas. Physicians in geographic regions where rabies is rare must consider the diagnosis based on the clinical picture alone, because there may not be a history of an animal exposure. Rabies can nearly always be prevented after a recognized exposure with initiation of appropriate prophylactic therapy. Once rabies develops, the disease is almost invariably fatal with rare exceptions, and survival has usually been associated with the administration of rabies vaccine prior to the onset of the disease.
HISTORY
Rabies has a long and colorful history going back to antiquity. Perhaps the earliest reference to rabies was in the pre-Mosaic Eshnunna Code of Mesopotamia in about 2300 BC (1). Rabid dogs were recognized in China centuries before the birth of Christ (2). The works of Democritus, Aristotle, Hippocrates, and Celsus made reference to rabies in both humans and animals (3). In 100 AD, Celsus described human rabies and used the term hydrophobia, which is derived from the Greek words meaning fear of water. Celsus recognized that the saliva of the biting animal contained the poisonous agent, and he recommended the practice of using caustics, burning, cupping, and also sucking the wounds of individuals bitten by rabid dogs in order to prevent the subsequent development of rabies (3).
In the early nineteenth century, Zinke (4) demonstrated experimentally that the infectious agent causing rabies was transmitted in the saliva by painting saliva from a rabid dog into incisions made in healthy animals. In 1879, Galtier, who was working at a veterinary school in Lyon, France, used rabbits in his rabies experiments and noted that it was technically much less difficult and dangerous than experiments using dogs and cats (5). Subsequently, Louis Pasteur took up this experimental rabbit model of rabies. He transmitted rabies virus by inoculating CNS tissues of rabid animals into the brains of other animals and noted that sequential brain passages led to attenuation for peripheral inoculation (6). In 1885, Pasteur (7) successfully immunized a 9-year-old boy, Joseph Meister, who had been severely bitten by a rabid dog, with a series of inoculations of infected rabbit spinal cord tissues that had been partially inactivated after variable periods of desiccation. Joseph Meister never developed rabies and, subsequently, many people were immunized with nervous system vaccinations in Paris and other locations throughout the world.
In 1903, Adelchi Negri (8) described eosinophilic cytoplasmic inclusions in infected neurons, which are now called Negri bodies. Negri bodies proved to be useful for a pathologic diagnosis of rabies. In 1958, fluorescent antibody staining was applied in order to demonstrate rabies virus antigens in tissues (9), which became very useful for rabies diagnosis and also for early rabies pathogenesis studies in animals performed by Richard Johnson (10) and Frederick Murphy (11,12) and their colleagues.
RABIES VIRUS
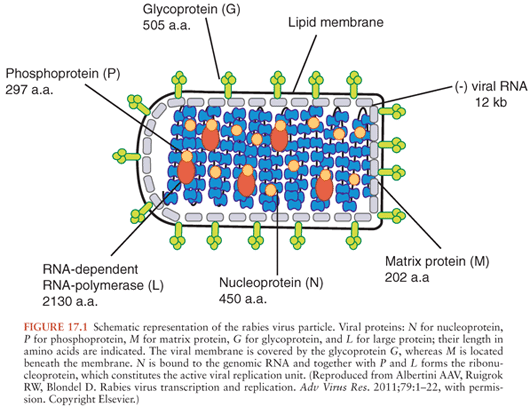
Rabies virus is in the family Rhabdoviridae and genus Lyssavirus and is a nonsegmented negative-strand (antisense) RNA virus consisting of 11,932 nucleotides that code for five viral proteins: nucleocapsid protein (N), matrix protein (M), phosphoprotein (P), glycoprotein (G), and large polymerase protein (L) (13) (Fig. 17.1). Helical genomic RNA forms a ribonucleoprotein (RNP) core associated with the N, P, and L proteins. The RNP serves as a functional template for viral transcription and replication. The G and M proteins are associated with a lipid bilayer envelope that surrounds the RNP core. The G protein forms spikelike projections on the surface of the viral envelope and serves as the major surface antigen of the virus and binds viral neutralizing antibodies and is important for immunity. Rabies virus belongs to genotype 1 of lyssaviruses, which includes wild type rabies virus strains (also called street rabies viruses) and also laboratory-adapted strains, including vaccine strains. The non–rabies virus lyssaviruses that have been recognized to rarely cause human disease, which is indistinguishable from rabies, include Mokola virus (genotype 3), Duvenhage virus (genotype 4), European bat lyssavirus 1 (genotype 5), European bat lyssavirus 2 (genotype 6), Australian bat lyssavirus (genotype 7), and Irkut virus (genotype pending) (14).
PATHOGENESIS
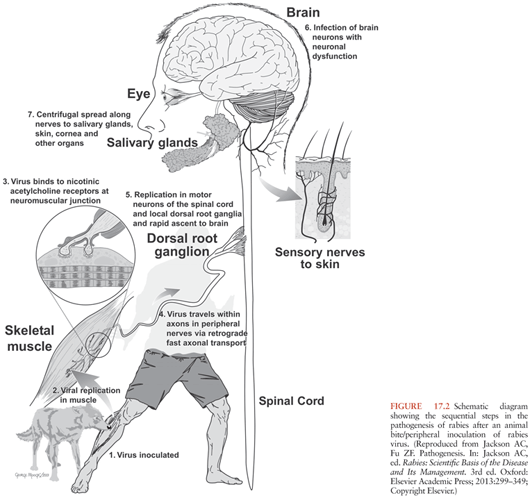
Rabies virus is usually transmitted in the saliva to humans and animals via a bite, although a scratch or abrasion with salivary contamination can also result in viral transmission. Aerosol transmission in a laboratory accident (15,16) and in a cave containing millions of bats (17) has also been documented but only occurs very rarely. Organ and tissue (corneal and vascular conduit) transplantation are also well-documented causes of transmission of rabies virus in humans, which account for a total of 16 well-documented cases (14,18,19). Much has been learned about the steps involved in rabies pathogenesis (Fig. 17.2) from experimental studies in animals. Usually, the incubation period lasts about 20 to 90 days after the time of the exposure (e.g., bite), although it may vary from a few days to over a year. Based on studies in animals, the virus is thought to remain close to the site of viral entry during most of this incubation period. After an exposure involving muscle, rabies virus is known to bind to nicotinic acetylcholine receptors (20), which are located in the postsynaptic membrane of the neuromuscular junction. The virus spreads across the synaptic cleft and then spreads centripetally toward the spinal cord in motor nerve fibers of peripheral nerves by retrograde fast axonal transport (21). Bats cause more superficial exposures with their bites involving cutaneous and subcutaneous tissues, but experimental studies have not yet examined the detailed pathways of viral spread in animal hosts. After infecting spinal cord neurons (e.g., ventral horn neurons), rabies virus spreads within axons of the CNS by fast axonal transport along neuroanatomic connections. After CNS infection is established, there is centrifugal spread of rabies virus to multiple organs along sensory and/or autonomic nerves. In rabies vectors, viral spread to the salivary glands is important, and saliva is secreted containing infectious rabies virus, which is important for transmission to new hosts via bite exposures. Viral spread also occurs to multiple organs, including the skin (important for rabies diagnosis using a skin biopsy), heart (with myocarditis in some cases), adrenal medulla, and gastrointestinal tract (22).
PATHOLOGY
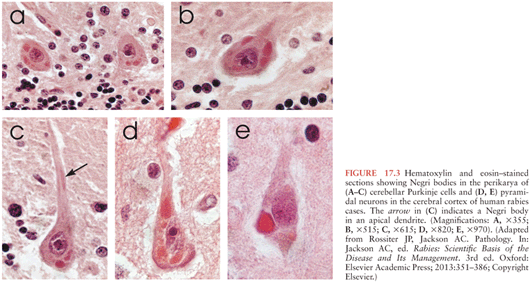
Characteristic microscopic features of rabies encephalomyelitis include mild mononuclear inflammatory changes involving the leptomeninges, perivascular regions, and the parenchyma. There are microglial nodules called Babes nodules in the parenchyma, which were described by Babes (23), that consist of activated microglia and monocytes. Degenerative neuronal changes are not usually prominent in rabies. However, neuronophagia can be observed with accumulations of activated microglia/macrophages in the process of phagocytosing degenerating or dying neurons (24). Infected neurons may contain characteristic eosinophilic inclusions called Negri bodies (Fig. 17.3), which were described by and named after Adelchi Negri (8,25). Electron microscopy has demonstrated that Negri bodies are composed of large aggregates of granulofilamentous matrix material and variable numbers of viral particles (24).
EPIDEMIOLOGY
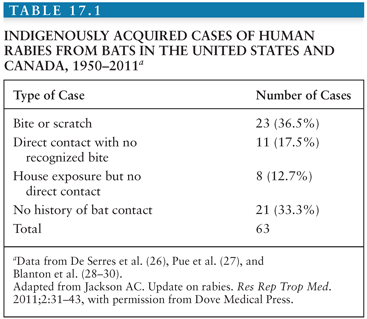
Worldwide, about 99% of human rabies cases are related to transmission from dogs related to the presence of endemic dog rabies, particularly in Asia and Africa. Although the means of controlling dog rabies are very well established, for a variety of economic, cultural, and political reasons, dog rabies persists in many countries, and people in these regions are at a continued risk of transmission via dog bites. In other countries, rabies is endemic in wildlife, and this poses the main risk for transmission of rabies virus. Rabies virus variants can be identified with molecular techniques, including monoclonal antibody characterization and reverse transcriptase polymerase chain reaction (RT-PCR) amplification with sequencing. Bats are the most important wildlife rabies vectors, and in the United States, bat rabies is present in every state except Hawaii (Fig. 17.4). Because of their small size, bat bites may not be recognized, leaving no opportunity for initiating effective preventive measures. Patients infected by bat rabies virus variants may not even be aware that they have had contact with bats (Table 17.1). A bat rabies virus variant associated with silver-haired bats and tricolored bats are most frequently associated with human rabies in the United States and Canada. A variant associated with Brazilian (Mexican) free-tailed bats is the second most common variant associated with human rabies cases in the United States. Although big brown and little brown bats are often found in houses and they are commonly found to be infected with rabies virus, they are not frequently responsible for human rabies cases.
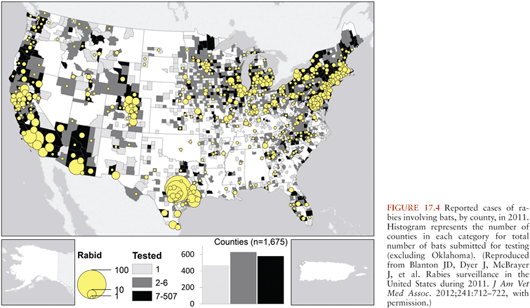
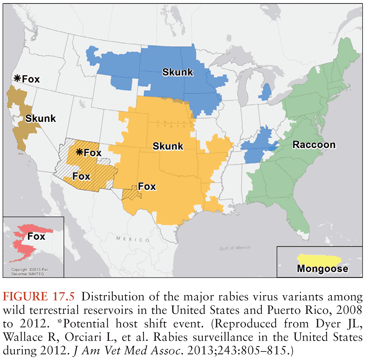
Terrestrial animals that are vectors of rabies in North America include raccoons, skunks, and foxes (30) (Fig. 17.5). Raccoon rabies is endemic along the entire eastern coast of the United States. In the 1940s, raccoon rabies was present in Florida, and over a period of decades, it gradually spread north and the first incursion occurred into Ontario, Canada in 1999. There are only two documented cases of human rabies due to a raccoon rabies virus variant (19,31). Skunk rabies is present in midwestern states, the prairie provinces of Canada, and in California. Fox rabies is now uncommon in North America because it has been well controlled, particularly in Ontario (red fox) and Texas (gray fox), and in Europe with oral vaccination programs. Companion animals, especially dogs and cats, are also at risk of developing rabies transmitted from wildlife vectors, and they may then pose a danger to humans.
CLINICAL FEATURES
Typically, the incubation period from the time of the exposure until the time of the onset of clinical disease is between 20 and 90 days but may be as short as only a few days or exceed a year. There is a well-documented report of rabies with an incubation period as long as 6 years (32). Prodromal symptoms in rabies are nonspecific and include fever, chills, malaise, fatigue, insomnia, anorexia, headache, anxiety, and irritability. They may last for up to 10 days prior to the onset of neurologic symptoms. The earliest neurologic features of rabies include paresthesias, pain, and pruritus at or close to the site of exposure, which likely reflects infection and inflammatory changes in local sensory ganglia (e.g., dorsal root ganglia or cranial sensory ganglia). By this time, the wound may have completely healed. There are two clinical forms of disease in rabies: encephalitic rabies (in 80% of cases) and paralytic rabies (in 20% of cases). The main burden of the infection in encephalitic rabies involves the brain, whereas in paralytic rabies, the main burden likely involves the spinal cord, nerve roots, and peripheral nerves. Fever occurs in both forms. In encephalitic rabies, there may be episodes of generalized arousal or hyperexcitability, which are separated by lucid periods (33). Patients may have aggressive behavior, confusion, and hallucinations. Features of autonomic dysfunction are common, and these may include hypersalivation, piloerection (gooseflesh), sweating, priapism, and cardiac arrhythmias. Hydrophobia is a characteristic clinical feature of encephalitic rabies and occurs more frequently with infections due to rabies virus variants associated with dogs than with bats. Patients may initially experience pain in the throat or have difficulty swallowing. When they attempt to swallow, they experience contractions of the diaphragm and other inspiratory muscles, typically lasting for 5 to 15 seconds. Subsequently, this may become a conditioned reflex and the sight, sound, or even mentioning water (or other liquids) may trigger the spasms. Aerophobia is the occurrence of these same spasms precipitated by a draft of air on the skin. There is progressive neurologic deterioration with worsening in the level of consciousness to coma and the development of paralysis.
In paralytic rabies, there is early prominent weakness that usually initially involves the bitten extremity and progresses to involve the other extremities and facial muscles. Sphincter involvement, pain, and sensory disturbances also occur. Hydrophobia is unusual in paralytic rabies, although weakness of bulbar and respiratory muscles also develops. Patients with paralytic rabies later develop neurologic deterioration with progression to coma, and they typically survive longer than patients with encephalitic rabies.
Medical complications are common in rabies patients treated aggressively in a critical care unit. Cardiac and respiratory complications are common. Cardiac disorders include heart failure, hypotension, a variety of arrhythmias, and cardiac arrest. Both cardiac ganglia and the myocardium may become infected with rabies virus, and in some cases, there is an associated myocarditis (34–36). Respiratory complications include hyperventilation, hypoxemia, respiratory depression with apnea, atelectasis, and aspiration pneumonia (37). Hyperthermia or hypothermia may occur, likely secondary to hypothalamic infection. Endocrine complications include inappropriate secretion of antidiuretic hormone and diabetes insipidus (37,38). Multiple organ failure commonly occurs in patients treated aggressively in critical care units.
DIFFERENTIAL DIAGNOSIS
The diagnosis of rabies may be difficult without a history of an animal exposure. Physicians may not ask about animal exposures, and the patient may not recall an exposure or may not be able to provide this information at the time of presentation. In early phases, encephalitic rabies may be misdiagnosed as a psychiatric disorder and paralytic rabies as Guillain-Barré syndrome. Rabies hysteria is a conversion disorder (somatoform disorder) that likely occurs as a psychologic response to the fear of developing rabies (39). It is characterized by a shorter incubation period than rabies, aggressive behavior (not common in humans), inability for the patient to communicate, and a long clinical course with recovery.
Other viral encephalitides may show behavioral changes with fluctuations in the level of consciousness. Hydrophobic spasms are not observed, and the presence of brainstem signs is unusual in conscious patients in most of the other viral encephalitides. Herpes simiae (B virus) encephalomyelitis, which is transmitted by monkey bites, is usually associated with a shorter incubation period and recovery may occur (40) (see Chapter 14). Tetanus has a shorter incubation period (3 to 21 days) than rabies and is characterized by sustained muscle rigidity involving paraspinal, abdominal, masseter (trismus), laryngeal, and respiratory muscles with superimposed brief recurrent muscle spasms (41) (see Chapter 37). In tetanus, consciousness is preserved, there is no cerebrospinal fluid (CSF) pleocytosis, and the prognosis is much better than in rabies. In Africa, rabies is commonly misdiagnosed as cerebral malaria (42). Anti-N-methyl-D-aspartate receptor (anti-NMDA) encephalitis occurs in young patients (especially females) and is characterized by behavioral changes, autonomic instability, hypoventilation, and seizures, and it has recently been recognized that this autoimmune disease rivals viral etiologies as a cause of encephalitis (43). Postvaccinal encephalomyelitis is an important differential diagnosis in patients immunized with a vaccine derived from neural tissues (e.g., Semple vaccine), which is currently used in only a few resource-poor countries. Patients with paralytic rabies may resemble the Guillain-Barré syndrome, and the pathologic features may also be similar (44). Local symptoms at the site of the bite, piloerection, early or persistent bladder dysfunction, and fever are all more suggestive of paralytic rabies.
INVESTIGATIONS
Routine blood tests and computed tomography (CT) head scans are typically normal in rabies. Magnetic resonance (MR) imaging may be normal or show signal abnormalities in the brain, spinal cord, and nerve roots/plexuses, but these findings are not specific for rabies and the main usefulness of MR imaging is to exclude other diagnostic possibilities (45). CSF analysis usually shows a mononuclear pleocytosis with a cell count of less than 100 cells/µL. Serum-neutralizing anti–rabies virus antibodies may develop in unvaccinated patients but may not appear for a week or more during the clinical course of disease, and some patients never develop antibodies prior to death. Neutralizing anti–rabies virus antibodies may also develop in the CSF, whereas CSF antibodies are not present in vaccinated patients who do not have rabies encephalitis. Specific laboratory tests for confirmation of a diagnosis of rabies include a full-thickness skin biopsy taken from the posterior region of the neck at the hairline. Rabies virus antigen may be detected in nerve fibers around hair follicles with direct fluorescent antibody staining. A recent advance in the laboratory diagnosis of rabies is detection of rabies virus RNA in fluids or tissues using RT-PCR amplification. Detection of rabies virus RNA in saliva is the most useful. RT-PCR can also be used on skin biopsies (46) and CSF but is much less sensitive on CSF. A negative laboratory test for rabies never excludes rabies unless performed on brain tissues, and the tests may need to be repeated for diagnostic confirmation of a rabies diagnosis. Brain tissues are only very rarely obtained by biopsy antemortem but are routinely evaluated postmortem by direct fluorescent antibody staining and by culture techniques.
PREVENTION OF RABIES
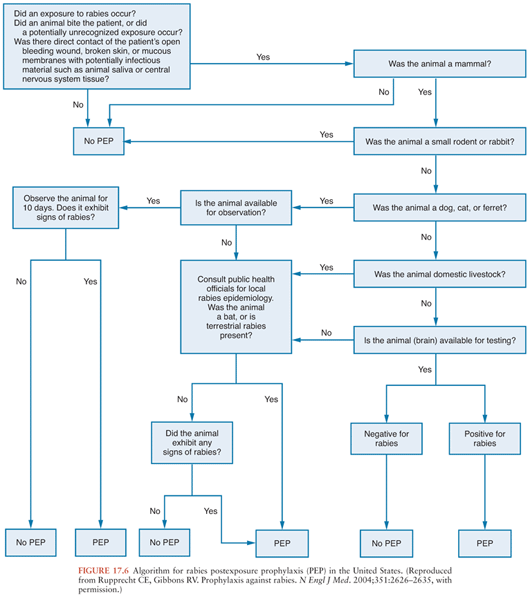
Rabies can be very effectively prevented after recognized exposures. Detailed guidelines that are periodically updated are available from the Centers for Disease Control and Prevention (47) and from the World Health Organization (48), and these documents are available on the Morbidity and Mortality Weekly Report (http://www.cdc.gov/mmwr/) and World Health Organization (http://www.who.int/en/) Web sites. Algorithms can be very useful in making decisions concerning postexposure rabies prophylaxis (Fig. 17.6). The first step is to determine whether there is a real risk of rabies virus transmission, which depends on obtaining the details of the exposure, the species of animal involved, and also on knowledge about the local epidemiologic situation. Advice from local public health officials can be very helpful in determining whether postexposure rabies prophylaxis measures should be initiated. Laboratory testing on brain tissues from an animal is needed for a definitive diagnosis of rabies, which is usually performed by an antigen detection method using the fluorescent antibody technique. If a dog, cat, or ferret remains healthy for a 10-day period after an exposure, then a confident conclusion can be made that rabies virus transmission did not occur during the exposure because the brainstem infection associated with the salivary excretion of infectious virus would have progressed to overt clinical signs within the period. Of course, unwanted animals may be tested without an observation period. Other animals must not be observed after an exposure because there is uncertainty about the period of time for clinical disease to develop, and this period may substantially exceed 10 days. If an animal escapes after an exposure, then it should be considered rabid unless information from public health officials indicates that this is unlikely. Current recommendations indicate that the physical presence of a bat may warrant postexposure prophylaxis when a person such as a small child or sleeping adult is unable to reliably report contact that could have resulted in a bite (47). However, in light of the low risks and high costs, recommendations for bedroom exposures to a bat while sleeping and without known physical contact have been questioned (49).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree