Elio Castagnola, Małgorzata Mikulska, Claudio Viscoli
Prophylaxis and Empirical Therapy of Infection in Cancer Patients
Cancer patients probably represent the best example of how both a disease and its treatment can impair the complex immunologic network aimed at maintaining the integrity of our body and defending it against infections from both the external and the internal environment. It has been known for decades that a granulocyte count of less than 500 cells/mm3 (and especially 100 cells/mm3) is associated with an increased risk of severe bacterial and fungal infections.1,2 There is also evidence that patients with a granulocyte count between 500 and 1000 cells/mm3, especially if rapidly decreasing, are also at high risk of infectious complications because neutropenia is not a static but, rather, a dynamic concept. Indeed, a survey on fever during neutropenia in children with cancer showed the presence of severe infectious complications (e.g., bacteremia or invasive mycosis) in patients with a granulocyte count that never dropped below 500 cells/ mm3, suggesting the presence of a “gray zone” that should be carefully monitored.3 Thus, an index—D-index or c-D-index—has been proposed to evaluate the area under the curve of the neutrophil count (combining depth and duration of neutropenia), for assessing the risk of late infections, particularly invasive fungal disease (IFD), in adult patients.4 The other main factor that impacts the risk of infectious complications in these patients is mucositis, a situation that, by itself, can be the basis of severe and often polymicrobial infections, even in the absence of neutropenia. Finally, new and peculiar aspects emerge with the use of biologic response modifiers, which have become part of many chemotherapeutic regimens and pose new challenges that should be considered.
However, compared with previous editions of Principles and Practice of Infectious Diseases, the most important change in the updated version of this chapter is represented by the phenomenon of growing antibiotic bacterial resistance worldwide, which also obviously impacts the management of infections in cancer patients. Antibiotic-resistant pathogens, such as Enterobacteriaceae resistant to third-generation cephalosporins (producers of extended-spectrum β-lactamases [ESBLs]) or carbapenems, multidrug-resistant (MDR) Pseudomonas aeruginosa, methicillin-resistant Staphylococcus aureus (MRSA), methicillin-resistant Staphylococcus epidermidis (MRSE), vancomycin-intermediate Staphylococcus aureus (VISA), and vancomycin-resistant enterococci (VRE), initially confined to intensive care units (ICUs), are now spreading to other wards in many countries, with a north-south/east-west gradient of endemicity and with sporadic cases or single wards affected everywhere in the world. This is radically changing our ability to prevent and cure infections in the immunocompromised. Very few new antibiotics are on the horizon, and the challenge is to ensure that an increasing antimicrobial resistance does not reverse the gains that have been made to improve survival outcomes for patients with cancer through targeted therapies or better surgical techniques.
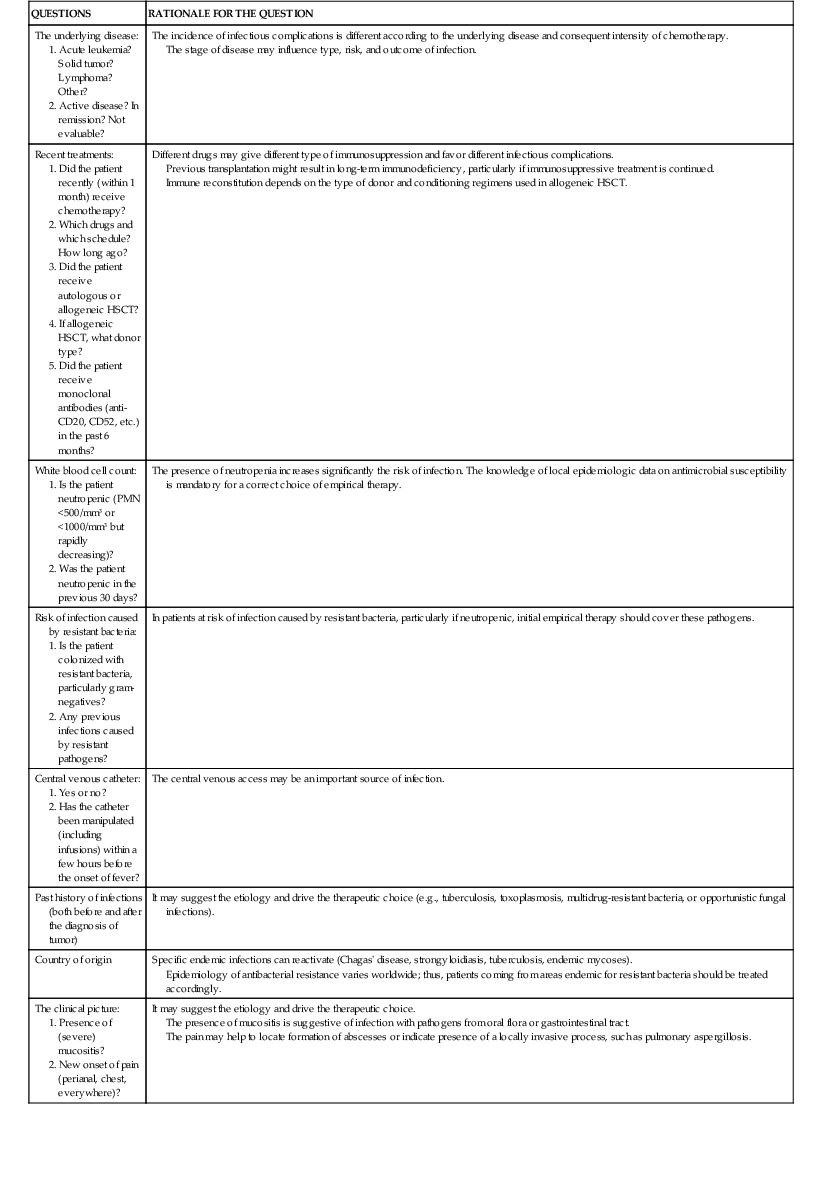
As shown in Table 310-1, the clinical approach to a cancer patient with signs and symptoms of infection is multifactorial. Before planning a rational management intervention, physicians should answer several crucial questions about the type and stage of the underlying disease and the clinical presentation, to make a thoughtful and effective intervention. Among others, factors potentially associated with the presence of highly resistant bacterial strains should currently be very carefully considered.
TABLE 310-1
What Should a Clinician Wonder and Look for When Approaching a Cancer Patient with a Suspected Infection?
| QUESTIONS | RATIONALE FOR THE QUESTION |
| The underlying disease: | The incidence of infectious complications is different according to the underlying disease and consequent intensity of chemotherapy. The stage of disease may influence type, risk, and outcome of infection. |
| Recent treatments: 1. Did the patient recently (within 1 month) receive chemotherapy? 2. Which drugs and which schedule? How long ago? 3. Did the patient receive autologous or allogeneic HSCT? 4. If allogeneic HSCT, what donor type? 5. Did the patient receive monoclonal antibodies (anti-CD20, CD52, etc.) in the past 6 months? | Different drugs may give different type of immunosuppression and favor different infectious complications. Previous transplantation might result in long-term immunodeficiency, particularly if immunosuppressive treatment is continued. Immune reconstitution depends on the type of donor and conditioning regimens used in allogeneic HSCT. |
| White blood cell count: | The presence of neutropenia increases significantly the risk of infection. The knowledge of local epidemiologic data on antimicrobial susceptibility is mandatory for a correct choice of empirical therapy. |
| Risk of infection caused by resistant bacteria: | In patients at risk of infection caused by resistant bacteria, particularly if neutropenic, initial empirical therapy should cover these pathogens. |
| Central venous catheter: | The central venous access may be an important source of infection. |
| Past history of infections (both before and after the diagnosis of tumor) | It may suggest the etiology and drive the therapeutic choice (e.g., tuberculosis, toxoplasmosis, multidrug-resistant bacteria, or opportunistic fungal infections). |
| Country of origin | Specific endemic infections can reactivate (Chagas’ disease, strongyloidiasis, tuberculosis, endemic mycoses). Epidemiology of antibacterial resistance varies worldwide; thus, patients coming from areas endemic for resistant bacteria should be treated accordingly. |
| The clinical picture: | It may suggest the etiology and drive the therapeutic choice. The presence of mucositis is suggestive of infection with pathogens from oral flora or gastrointestinal tract. The pain may help to locate formation of abscesses or indicate presence of a locally invasive process, such as pulmonary aspergillosis. |
| Administration of prophylaxis (no, yes, which drugs): | Breakthrough infections are possible, and fever during prophylaxis should be considered as failure of prophylaxis, unless proven otherwise. The occurrence of a bacterial/fungal/viral infection during specific prophylaxis may influence the choice of empirical therapy, depending on the drug used for prophylaxis. A resistant pathogen should be suspected in every case, unless the patient was clearly noncompliant and/or there is the possibility of low drug levels caused by poor absorption, increased metabolism, or drug interaction (e.g., azoles such as itraconazole, voriconazole or posaconazole). Knowledge of local epidemiology, including susceptibility pattern, is mandatory for a correct diagnostic and therapeutic management. |

HSCT, hematopoietic stem cell transplantation; PK/PD, pharmacokinetic/pharmacodynamic; PMN, polymorphonuclear neutrophils.
Infections in cancer patients have often been considered nosocomial, despite the fact that these patients are often cared for as outpatients or even on a home-care basis. In fact, a study on infectious complications in 113 adults receiving treatment for acute hematologic malignancies showed that 91% of 223 infectious episodes were actually associated with the type of care patients had received, but only 42% of the episodes were truly “nosocomial” in origin.5 For this reason, the terminology of health care–associated infections is more appropriate for describing infections in the cancer management field. In any case, even if developing in a hospital, infections in cancer patients should not necessarily be considered the result of bad clinical practice, and their rate cannot be necessarily decreased by exceptional infection control measures because most of the infectious pathogens come from an endogenous source.
In the following sections, the epidemiology and management principles of infections in cancer patients will be described. Risk factors and clinical presentations of specific infections, along with their treatment in non-neutropenic cancer patietns, will not be discussed here but dealt with in chapters focused on individual infectious agents. Similarly, infections in recipients of allogeneic hematopoietic stem cell transplant (HSCT) are discussed elsewhere (see Chapter 312), and we will only touch on these patients, especially in correlation with early infections during the preengraftment, neutropenic phase.
Epidemiology and Risk Factors for Infections in Cancer Patients
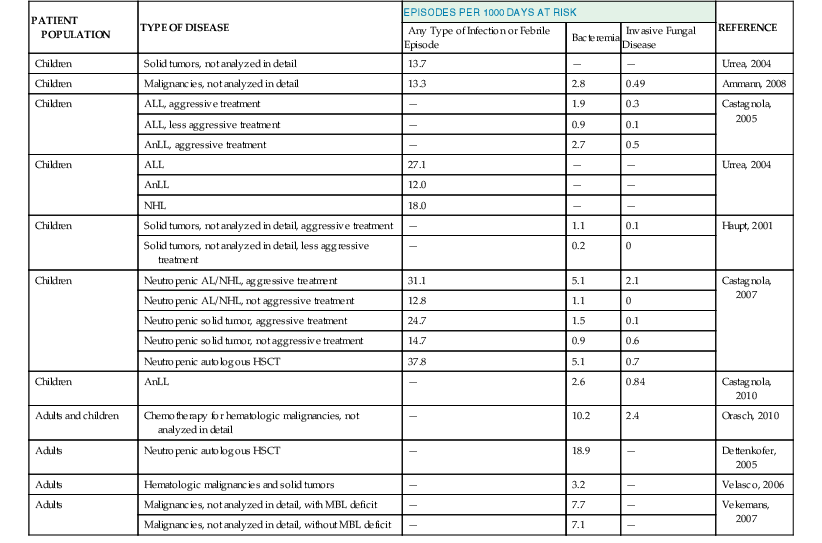
The knowledge of the incidence of fever and documented infections in cancer patients according to the type of the underlying disease and related chemotherapy is mandatory for the implementation of effective management strategies, especially prophylaxis. However, the large majority of epidemiologic data about these patients come from studies on empirical antibiotic therapy or prophylaxis, in which patients were selected according to inclusion and exclusion criteria. Thus, this approach might be inadequate to describe the actual epidemiologic situation in real life. In addition, little information is available about non-neutropenic patients.
Epidemiologic data on the incidence of infections are usually reported as percentages of events over a given number of patients or treatment courses, without adjusting for the duration of the period at risk. This is probably wrong because the duration of exposure is crucial to understand the clinical impact of a given phenomenon. It is probably more appropriate to speak of incidence rates, that is, the number of events during a given risk period (usually 1000 days). Tables 310-2 and 310-3 report the epidemiology of febrile episodes, bacteremia, and invasive mycoses in cancer patients.3–24 All these data clearly show that the incidence rate and proportion of infectious complications are mainly related to the intensity of antineoplastic chemotherapy. Additional factors are represented by the phase of chemotherapy and the status of the neoplastic disease, with higher incidence of infectious complications in patients receiving remission-induction and rescue chemotherapy, compared with maintenance or consolidation first-line treatments.6,10 The state of the underlying disease in terms of remission or relapse and progression is also an important factor for the occurrence and prognosis of infectious complications, as shown by studies in patients with invasive aspergillosis.25 Patients with acute myeloid leukemia, both adults and children, have the highest frequency of fevers, bacteremia, and invasive fungal diseases, especially during the first induction of remission and in relapsing leukemia, when the intensity of chemotherapy is higher. Lower frequencies have been observed in lymphoblastic leukemia, chronic lymphatic disorders, multiple myeloma, and non-Hodgkin’s lymphomas, whereas the lowest rates are observed in solid tumors, although this may be largely dependent on antineoplastic treatment strategies. Patients with chronic leukemia or multiple myeloma may represent a peculiar group because of the role played by the use of the new biologic response modifiers (imatinib, desatinib, rituximab, alemtuzumab, etc.). These new drugs have the potential to modify the risk profiles for infectious complications, with an increasing risk of infections also caused by previously unusual pathogens, such as Pneumocystis jirovecii and various viruses.26
TABLE 310-3
Infectious Complications in Cancer Patients
| PATIENT POPULATION | TYPE OF DISEASE | PERCENTAGES OF PATIENTS OR PERIODS WITH INFECTIOUS COMPLICATIONS* | REFERENCE | ||||
| Any Type of Infectious or Febrile Episode | Bacteremia | Invasive Fungal Disease | |||||
| Any (Proven, Probable, and Possible) | Proven and Probable | ||||||
| Yeasts | Molds | ||||||
| Adults and children | Malignancies, not analyzed in detail | 62 | 35 | — | — | — | Gafter-Gvili, 2005 |
| Adults | Malignancy or autologous HSCT, not analyzed in detail | — | 18 | 0 | — | — | Dettenkofer, 2005 |
| Adults | High-risk acute leukemia | 78 | 30 | — | — | — | Bucaneve, 2005 |
| High-risk NHL or HSCT in solid tumors | 73 | 23 | — | — | — | ||
| Adults | Acute promyelocytic leukemia | 59 | 22 | 7.3 | 3 | 0.3 | Girmenia, 2003 |
| Other AnLL | — | 37 | 4.2 | 4 | 0.2 | ||
| Children | AnLL | 94 | 25 | 4.2 | 0.6 | 1.6 | Lehrnbecker, 2004 |
| Children | ALL, aggressive treatment | — | 30 | 10 | — | — | Castagnola, 2005 |
| ALL, less aggressive treatment | — | 17 | 3 | — | — | ||
| AnLL, aggressive treatment | — | 34 | 9 | — | — | ||
| Adults | Low-risk solid tumors, not analyzed in detail, including lymphomas | 12 | 0.4 | — | — | — | Cullen, 2006 |
| Adults | AnLL | — | — | — | 6 | 11 | Caira, 2008 |
| Adults | New-onset hematologic malignancies | 27 | 10 | 4 | 0.5 | 1.3 | Pagano, 2012 |
| Adults and children | Hematologic malignancies | — | 21 | 5 | — | — | Orasch, 2010 |
| Children | AnLL: Incidence per patient Incidence per treatment course | — | 51 32 | 16 10 | — | — | Castagnola, 2010 |
| Children | Aggressive treatment for solid tumor, including autologous HSCT | — | 24 | 1.6 | 1 | 0.5 | Haupt, 2001 |
| Less aggressive treatment for solid tumors, not analyzed in detail | — | 3 | 0 | ||||
| Children | Neutropenic AL/NHL, aggressive treatment | 48 | 25 | 10 | 1.6 | 1.2 | Castagnola, 2007 |
| Neutropenic AL/NHL, not aggressive treatment | 21 | 8 | 1 | 0.8 | 0 | ||
| Neutropenic ST, aggressive treatment | 32 | 6 | 0.4 | 0.1 | 0.1 | ||
| Neutropenic ST, not aggressive treatment | 22 | 7 | 4 | 0.5 | 0.5 | ||
| Neutropenic postautologous HSCT | 58 | 14 | 2 | 0.6 | 0.6 | ||
| Children | Neutropenic with AML, receiving G-CSF | 61 | 14 | 2 | 1.8 | 0.6 | Lehrnbecher, 2007 |
| Neutropenic with AML, not receiving G-CSF | 56 | 11 | 0 | — | — | ||
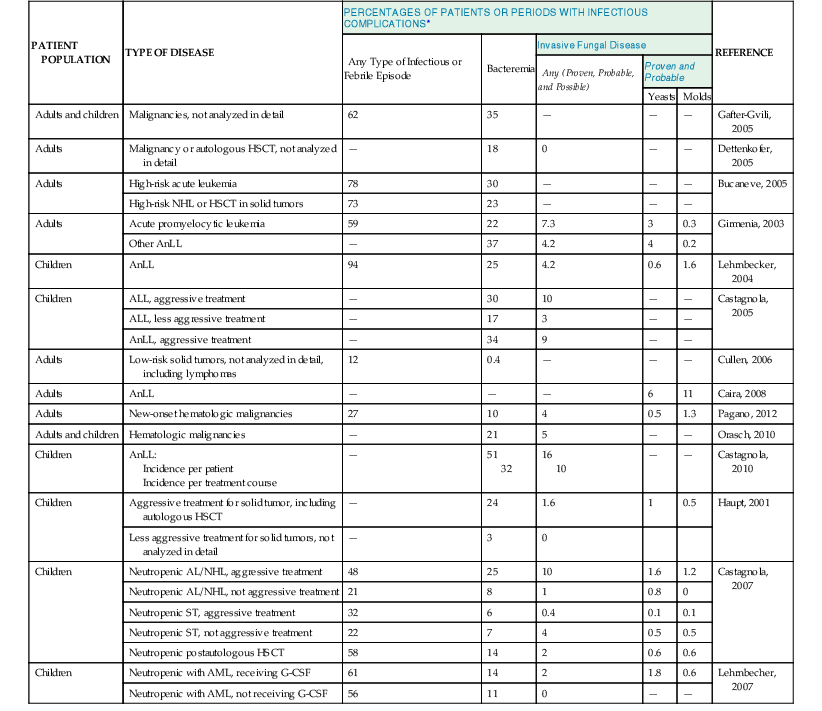
* Numbers are percentages of event over enrolled patients.
—, data not reported; AL, acute leukemia; ALL, acute lymphocytic leukemia; AML, acute myelocytic leukemia; AnLL, acute nonlymphocytic leukemia; G-CSF, granulocyte colony-stimulating factor; NHL, non-Hodgkin’s lymphoma; HSCT, hematopoietic stem cell transplantation; MBL, mannose-binding lectin; ST, solid tumor.
Neutropenia
Although the first scientist who showed, in pivotal studies, the direct relationship between infection and neutropenia was Gerald Bodey, from Houston in 1966, the first report in which the risk was reported in terms of infection rate was probably published in 1993 by Carlisle and colleagues,27 who showed that the rate of infections in neutropenic cancer patients was 46.3 episodes per 1000 days of neutropenia (DN), with rates of 12.9 for bacteremia and 2.9 for invasive mycoses. More recent data from a prospective study in children and adults with neutropenia showed a median incidence of infectious complications of 43% and a rate of 22.8 episodes per 1000 DN; bacteremia was diagnosed in 21% of the episodes and mold infections in 5%, with rates of 10.2 and 2.4 for 1000 DN, respectively.7 As shown in Table 310-2, the rate of infections is higher after high-intensity chemotherapies and lower after maintenance treatment. Although the epidemiology of infections has been studied more extensively in children, in general, data from adults report higher rates of infectious complications compared with pediatric populations. The majority of primary febrile episodes usually occur soon (a few days) after the onset of neutropenia.
Mucositis
As already mentioned, in addition to neutropenia, the severity of mucosal barrier injury may have an impact on infection rates. Indeed, it has been demonstrated that in patients receiving HSCT, the fever is strictly related to the severity of mucosal barrier injury, independently of the severity and duration of myelosuppression. Mucositis is one of the most important factors predisposing to bloodstream infections, both caused by bacteria and by Candida. The damage of mucosal barrier allows colonizing pathogens to enter the bloodstream, where, in the absence of granulocytes, severe infection can rapidly develop, even in case of a low bacterial load. Mucositis, with or without neutropenia, might also be responsible for severe oral and intestinal infections. The pathogenesis and the role of mucositis after chemotherapy is described in detail elsewhere (see Chapter 309).
Central Venous Catheters
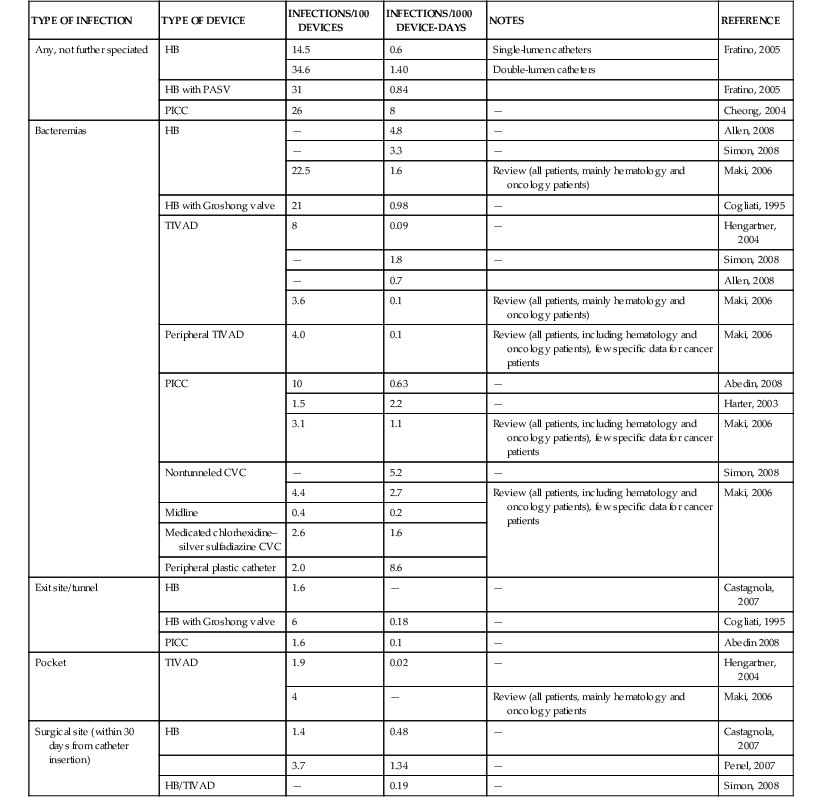
The presence of a central venous catheter (CVC) is another well-known factor facilitating infection in cancer patients and influencing the etiology of bacteremia. Table 310-4 reports the incidence of CVC-related complications in cancer patients.20,28–35,36–38 Bacteremia represents the most frequent condition, whereas exit site, tunnel, and other types of CVC-related infections are less frequently reported. These complications are more frequent in partially implanted than in totally implanted catheters and also in double-lumen compared with single-lumen devices. It is noteworthy that in patients with totally implanted CVCs, infectious complications are more frequent in younger patients with hematologic malignancies than in other categories.37 The number of CVC manipulations, which is mainly related with the intensity of antineoplastic chemotherapy and the severity of clinical conditions, represents the most important risk factor for the development of these infections.
A detailed description of complex problem of CVC-related infections is beyond the scope of this chapter and can be found elsewhere (see Chapter 302). The incidence of CVC-related infections should be kept at the minimum by several educational and organizational measures, such as repeated teaching sessions for patients, parents, and staff, or establishing teams dedicated to the insertion and maintenance of intravascular devices. Technical measures that have been suggested include the use of chlorhexidine-impregnated dressings or sponges and the antiseptic/antimicrobial coating of intravascular catheters, for example, with chlorhexidine and silver sulfadiazine. The benefit of their routine use depends on their cost, the observed rate and outcome of CVC infections, and, in case of antibiotic-impregnated devices, on the risk of inducing antimicrobial resistance.
Genetic Factors
The existence of genetic factors that are able to increase or decrease the susceptibility to infection in immunocompromised patients underlines an apparently trivial but important aspect, that is, all cancer patients are not the same, and every single patient might deserve an individualized approach. For example, in nonleukemic patients receiving less intensive chemotherapy, decreased levels of mannose-binding protein were associated with an increased risk of infection (49.9 vs. 29.6/1000 days at risk, P = .01). This effect, less evident in the context of prolonged neutropenia, was not confirmed in all the studies. However, in a recent study of 269 children with cancer, mannose-binding lectin deficiency influenced both the incidence and the severity of febrile neutropenia.39 Polymorphisms of Toll-like receptors and other components of innate immunity have been associated with an increased risk of invasive aspergillosis, both in cancer patients (including recipients of HSCT) and in other immunocompromised patients.40 The future will tell us whether genetic polymorphisms, alone or in combination, have an actual impact and might dictate prophylactic or therapeutic approaches.
Biologic Agents and Other New Drugs
As already mentioned, in recent years, monoclonal antibodies and other pharmaceutical compounds, specifically engineered for targeting cells or cytokines involved in the pathogenesis of specific diseases, have been introduced in the armamentarium of antineoplastic chemotherapy. These include biologic response modifiers and protein kinase inhibitors. Alemtuzumab is a monoclonal antibody against the CD52 receptor that is used in the treatment of acute or chronic lymphocytic leukemia or non-Hodgkin’s lymphoma. This monoclonal antibody has a clear role in increasing the infection risk because it is associated with long-lasting and profound lymphopenia. Bacteremias, invasive fungal diseases (including pneumocystosis), several viral diseases, and tuberculosis have been all described in association with this drug. A low CD4+ T-lymphocyte count (with a possible cutoff of 200 CD4+/mm3) has been indicated as one of the most important factors related to the development of infectious complications. Obviously, infections seem to be more common (with more severe clinical pictures) in patients previously treated with other antineoplastic protocols. Rituximab is an anti-CD20 (B-cell) antibody that causes a prolonged (2 to 6 months median, but sometimes more) suppression of immunoglobulin production. It has been associated with reactivation or acute exacerbation of viral hepatitis (both hepatitis B virus [HBV] and C virus [HCV]) and, more rarely, with disseminated parvovirus infections, enteroviral meningitis, progressive multifocal leukoencephalopathy, babesiosis, and pneumocystosis.41 Bacterial and fungal diseases have also been described, usually when rituximab was administered in combination with other chemotherapeutic agents. For other anti-CD20 monoclonal antibodies, such as ibritumomab, tositumomab, ofatumumab, and ocrelizumab, less data on infectious complications exist. Such monoclonal antibodies were reported to cause severe myelosuppression, with a spectrum of infectious complications somewhat similar to those associated with classic cytostatic drugs. It is noteworthy that patients receiving antilymphocytic monoclonal antibodies (anti-CD52 and/or anti-CD20) for relapsing diseases (i.e., after previous prolonged chemotherapy cycles) present more frequent and severe complications compared with those who receive front-line therapies. Bevacizumab is another monoclonal antibody that targets vascular endothelial growth factor and is used in colon, kidney, brain, or lung cancer. Febrile neutropenia and bacterial infections have been reported in approximately 10% of patients, usually when the drug was used in combination with other chemotherapeutic agents. Cetuximab (approved for colon, head, and neck cancer) and panitumumab (approved for colon cancer) both have the epidermal growth factor receptor as their main target of activity and cause important dermatologic toxicity, such as rash, skin drying and fissuring, or paronychial inflammation, with infectious complications in up to 30% of patients, including sepsis caused by S. aureus. Trastuzumab reacts with the human epidermal growth factor receptor 2 (HER2) and is administered, usually in combination with standard chemotherapies, for treatment of HER2-positive breast or gastric cancers. Infections associated with this compound are generally mild, though not infrequent, and they may exacerbate chemotherapy-induced neutropenia. Small molecules with protein kinase activity targeting oncogenic tyrosine kinase BCR-ABL (breakpoint cluster region–Abelson murine leukemia), such as imatinib, desatinib, and nilotinib, are used in patients with both acute and chronic leukemia and solid tumors, even for very long periods of time. Infectious complications seem to be similar to those observed with other immunosuppressive drugs affecting mechanisms of cell-mediated immunity, such as pneumocystosis and viral diseases, including reactivation of hepatitis. Because of the absence of major myelosuppression, there is no apparent increase in the rates of bacterial or fungal infections.26 Finally, there are kinase inhibitors, such as sunitinib and sorafenib, that are used before nephrectomy for renal cell carcinoma and for hepatocellular carcinoma. These drugs apparently do not increase the risk of surgical infections, although one of them (sunitinib) has been associated with necrotizing fasciitis, respiratory infections, and sepsis.
Several problems impair our ability to understand the exact role of these new compounds on the risk of infection in cancer patients. Even in randomized, placebo-controlled trials and in large open-label studies, it is difficult to establish the rate of infectious complications. The main reason is that these trials were powered to measure efficacy but not safety, and if the effect is very rare, it might go undetected. Second, there are many confounding factors because new drugs are usually used together with old or classic therapies, making it difficult, if not impossible, to evaluate their respective role. In addition, the trials did not use the same definitions of infectious complications or simply did not pay enough attention to diagnosing them. Sadly, in some cases, there was a tendency toward minimization and covering. Finally, in some cases, there was not enough attention to forecast the risk of infection. This is the case of eculizumab used for paroxysmal nocturnal hemoglobinuria, which targets the C5 complement component. As widely known among infectious disease physicians dealing with infections in immunocompromised hosts, the inherited deficiency of the C5 complement component is associated with repeated episodes of meningococcal disease. Thus, this risk might have been forecast.
Oncologic Surgery
Bacteremia, usually associated with surgical site infection and deep organ abscess, is not uncommon in urologic, gynecologic, and abdominal surgery in cancer patients, but it is difficult to say with certainty if this happens significantly more often among oncologic versus non-oncologic patients.42,43 Several studies reported the rates of postsurgery infections in different cancer populations. For example, among patients with peritoneal carcinomatosis undergoing peritonectomy and intraperitoneal hyperthermic chemotherapy, the proportion of infectious complications was rather high, varying from 24% to 36%,42–44 with more than two infectious episodes per patient. The rate of infectious complications is lower in other oncologic surgeries. In breast cancer, surgical site infection is a complication in 4% to 8% of cases, depending whether breast reconstruction is performed in one or two steps and whether surgery follows previous chemotherapy cycles.45,46 In case of malignant biliary obstruction, early infectious complications after percutaneous biliary stent insertion were present in 6.5% of patients.47 Similar incidence was reported in patients undergoing surgery for hepatocellular or metastatic carcinoma (3% to 11%), and this incidence was apparently lower than in surgery for nonmalignant conditions such as hepatolithiasis (24%).48 The rate of infectious complications after hepatectomy for hepatocarcinoma was associated with surgical risk factors such as bile leakage and blood loss.49 A similar incidence of surgical site infections was reported after elective colon and rectal surgery (9% and 18%, respectively),50 and after orthopedic surgery (9.5%).51 Of interest, in the latter study, the use of an implant or allograft did not represent a risk factor for infectious complications.51 Finally, postoperative respiratory infections have been reported in nearly 4% of patients undergoing surgery for lung cancer, and they occurred more frequently in the presence of advanced age, impaired respiratory function, advanced pathologic stage, and induction chemotherapy.
In conclusion, although not many data are available on infectious complications after surgery in solid tumors, it seems that surgical and ICU-related factors are more important than previous antineoplastic chemotherapy in determining the risk of infection. However, it must be noted that in patients with solid tumors, surgery, together with long hospital stay and use of third-generation cephalosporins and glycopeptides, has been associated with an increased risk of infections caused by MDR pathogens.52
Etiology
Surveillance studies on pathogens causing infections in cancer patients are of the utmost importance for the implementation of management strategies. Large-scale studies are obviously crucial because they can provide information about worldwide trends, but single-center surveillance reports are just as important because every center may have peculiarities related to the type of patient, type of care, and local historical antibiotic policies. Most of the available information concerns bacterial and fungal pathogens isolated in bloodstream, whereas the role of deep-seated infections is less known, as well as the impact of viral infections.
Bacterial Infections
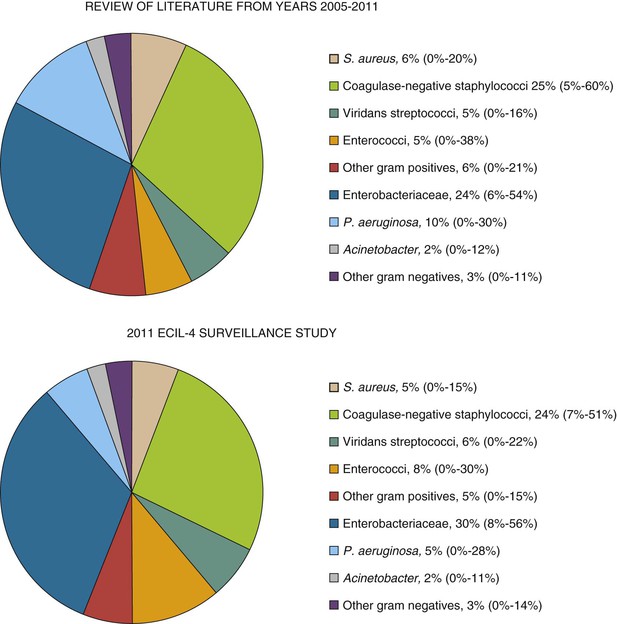
In the last 30 years, gram-positive bacteria have been the most frequent pathogens in bloodstream infections in cancer patients. However, more recently, an increase in the frequency of bacteremias caused by gram-negative rods has been reported in many centers worldwide, with gram-negative pathogens becoming either predominant or at least as frequent as gram-positive pathogens. This trend has been observed also in the results from a recent literature review and European surveillance study performed in 2011 in 39 hematology centers from 18 countries for the Fourth European Conference of Infections in Leukemia (ECIL-4).52a As shown in Figure 310-1, gram-negative pathogens are almost as frequent as gram-positive ones, with the gram-positive versus gram-negative ratio in bloodstream infections at 60% versus 40% and 55% versus 45% in the literature review and ECIL-4 surveillance, respectively. The detailed etiology was similar (see Fig. 310-1) in the literature review and surveillance study, with slightly increased rates of enterococci and Enterobacteriaceae and a decreased rate of P. aeruginosa in the ECIL-4 surveillance study. These changes in etiology seemed to be accompanied by an important and alarming increase in the proportion of resistant pathogens, such as ESBL-producing Enterobacteriaceae, VRE, or, the most worrisome, carbapenem-resistant gram-negative pathogens, both P. aeruginosa and Enterobacteriaceae—mostly Klebsiella pneumoniae. Last but not least, in leukemia patients, most of staphylococci are resistant to methicillin, whereas most of gram-negative pathogens are fluoroquinolone resistant. Of note, the rates of resistance were generally higher in southern and eastern Europe than in northern and western Europe.
Anaerobic bacteria are isolated in less than 1% of positive blood cultures in cancer patients, but the proportion may increase to 3% among those undergoing abdominal surgery.42 Anaerobes are usually isolated in polymicrobial bacteremias, especially together with gram-negative rods,53 with a rate that seems to be higher than that observed in nononcologic patients undergoing similar surgery (0.597/1000 vs. 0.033/1000 hospital days, respectively). Of interest, in non-neutropenic febrile cancer patients, gram-negative pathogens are the most frequently isolated, followed by gram-positive pathogens, yeasts, and filamentous fungi, probably in relation to severe gastrointestinal mucositis.54 CVC-related bacteremias are generally caused by gram-positive cocci (especially coagulase-negative staphylococci) that are isolated in more than 50% of the episodes, compared with the rate of 25% to 40% for gram-negative rods. The source of infection is likely to be partially different between gram-positive and negative CVC-related bacteremias. In both cases, contamination of skin or hub caused by incorrect catheter management is pivotal, although many gram-positive cocci come directly from the patient’s skin flora. Infusate contamination is a rare but possible event, and in this case, gram-negative rods, such as Klebsiella, Enterobacter, Citrobacter, Achromobacter, Serratia, and Pseudomonas (other than P. aeruginosa), are more likely involved. Polymicrobial infections are not rare (8% to 49% of the episodes), with a predominance of gram-negative bacteria, whereas fungi (mainly Candida spp.) are usually isolated in no more than 10% of CVC-related bloodstream infections.20,29–35,38
Bacterial gastroenteritis caused by classic enteric pathogens (Salmonella and Shigella) is a rare event in patients with acute leukemia, involving less than 1% of acute enteritis after chemotherapy.55 On the contrary, Clostridium difficile is not unusual in cancer patients, with an incidence that is twofold higher than in the noncancer population.56 Helicobacter pylori has also been described as possible cause of gastrointestinal (GI) disease in cancer patients.
Mycoplasma pneumoniae and Chlamydia pneumoniae have been rarely described as a cause of pneumonia in cancer patients, but it is possible that their incidence is underreported. Similarly, tuberculosis is probably underestimated and underdiagnosed in cancer populations, although there are data showing that the rate approximates 90 cases per 100,000 persons (i.e., ninefold higher than in the general population in developed countries).57 Patients from high-endemicity countries or belonging to racial and ethnic minorities account for most of the cases. On the contrary, infections caused by nontubercular mycobacteria are rare. Of note, disseminated tuberculosis caused by Mycobacterium bovis may occur in patients receiving Calmette-Guérin bacillus immunotherapy for bladder cancer.
Fungal Infections
The etiology of fungal infections in cancer patients is shown in Table 310-3. Aspergillus spp. and Candida spp. are the most common fungal pathogens, with the former now being seen more frequently than the latter. Other fungal pathogens include P. jirovecii, cryptococci, and molds, such as Mucorales or Fusarium.
Among yeasts, Candida is the most frequently isolated organism, usually in bloodstream infections. There is an increasing proportion of non-albicans strains, at least partly in correlation with the extensive use of prophylactic fluconazole, to which some non-albicans strains are resistant or less susceptible. Already, in a 1999 European Organisation for Research and Treatment of Cancer (EORTC) study, C. albicans was responsible for only 35% of candidemias in patients with hematologic malignancies and for 70% of episodes in those with solid tumors.58 Similarly, recent EORTC data from 297 patients with fungemia, presented at the 2012 European Congress of Clinical Microbiology and Infectious Diseases, reported an overall incidence of 2.3%, with C. albicans responsible for only 40% of monomicrobial infections.58a Of note, only 38% of fungemias occurred during neutropenia.
In general, yeasts belonging to the Candida parapsilosis complex are usually associated with CVC contamination, whereas the other Candida species are supposed to come from the GI tract after selection and translocation.
Among molds, Aspergillus represents the most frequently isolated or suspected organism. The majority of episodes are caused by Aspergillus fumigatus, although some centers report a predominance of infections caused by Aspergillus flavus and Aspergillus terreus.23,59 Aspergillus species are ubiquitous molds whose primary ecologic niche is represented by decomposing vegetable material, including potted plants, soil, flowers, and carpets. In healthy individuals, Aspergillus conidia are trapped in the upper respiratory tract, and only a small proportion of them enter the lower airways, where Aspergillus may become an allergen. In immunocompromised patients, especially those with hematologic malignancies or after allogeneic transplantation, spores can germinate and cause an invasive disease. Thus, invasive aspergillosis in patients with malignancy or receiving HSCT is an endemic disease, which is usually community acquired and endogenous, although epidemic outbreaks of exogenous infection associated with massive environmental exposures (in and outside the hospital) can occur. The incidence of invasive aspergillosis depends on the patient’s age (lower in those younger than 10 years), the underlying malignancy, and its treatment, being the highest in patients with prolonged neutropenia, followed by those receiving high doses of steroid therapy. In a multicenter Italian study, the incidence of aspergillosis among hematologic patients varied from 7.9% in acute nonlymphoblastic leukemia; 4.3% in acute lymphoblastic leukemia; 2.3% in chronic myelogenous leukemia; to less than 1% in chronic lymphocytic leukemia, Hodgkin’s disease, non–Hodgkin’s lymphoma, and multiple myeloma.23 A recently described risk group is represented by patients with chronic lymphoproliferative disorders, probably resulting from more intensive treatment protocols.59 The incidence of invasive aspergillosis after autologous HSCT is low (0.3% to 2%), and it occurs during neutropenia preceding the engraftment.23,60–62
P. jirovecii is a well-known cause of pneumonia in cancer patients not receiving specific prophylaxis, especially in association with high-dose and prolonged steroid therapy. Attack rates vary from 6.5% to 43% in acute lymphoblastic leukemia, 4% to 25% in rhabdomyosarcomas, and nearly 1% in Hodgkin’s disease and primary or metastatic central nervous system tumors.63
In some centers, there have been increasing reports of mucormycosis; however, it is unclear whether this represents a general trend, is influenced by local factors, or that these infections are simply diagnosed more often because of an increased clinical awareness. Other fungi, such as Cryptococcus, Fusarium, Blastoschizomyces, Trichosporon, and Scedosporium, have been reported sporadically. Finally, infections or reactivations of dimorphic fungi, such as Histoplasma or Coccidioides, are possible in patients who live or used to live in endemic areas.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree