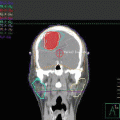
Fig. 9.1
Patient setup in a frog leg for scrotal irradiation
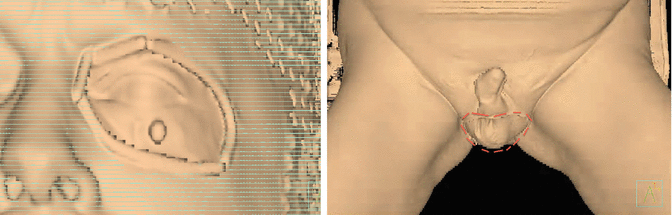
Fig. 9.2
Patient setup, skin rendering showing the in tomato colour the edge of the field, the position of the penis in a frog leg position for scrotal irradiation
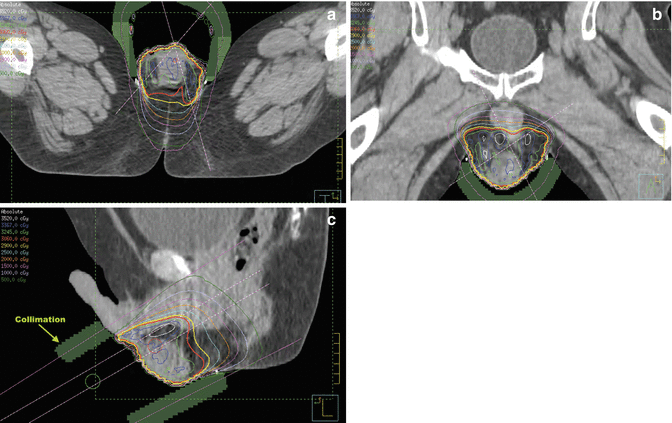
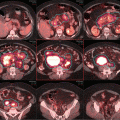
Fig. 9.3
CT planning showing the 100 % isodone line in red in (a) axial, (b) coronal, (c) sagittal views
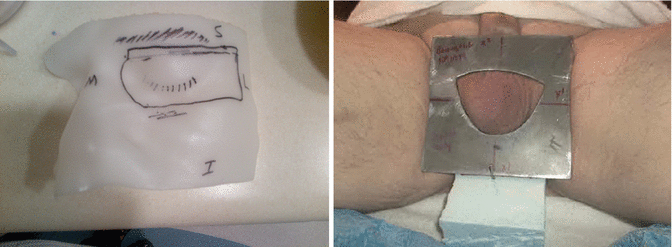
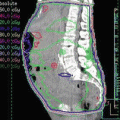
Fig. 9.4
Treatment set using the skin collimation while making sure that the whole scrotum is exposed out of the skin collimation during the daily treatment
Supportive Care and Follow-Up
The patient was seen weekly during radiotherapy, with particular attention to scrotal and perineal skin care. Patients invariably end up with grade 2/3 dermatitis, especially at the end of the radiation course or the week following; skin care with sitz baths and local moisturisers is quite often needed. A follow-up plan is to include regular clinical assessment looking for evidence of tumour recurrence or late toxicity including features of hypogonadism. Periodic measurements of serum testosterone and gonadotropins will be performed and replacement therapy instituted if clinically appropriate with appropriate endocrinology consultation.
Discussion and Rationale
Pathology
The majority of PT-DLBCL cases are of activated B-cell type [5, 15–20]. This predominance of ABC type may partially account for the historically poor outcomes from PTL [21, 22]. Although co-expression of MYC and Bcl-2 protein may contribute to the inferior survival of ABC-type nodal DLBCL, cytogenetic “double-hit” cases with concurrent rearrangements of MYC and BCL2 and/or BCL6 in PTL are infrequent [23–25]. MYD88 mutations are also more frequent in PTL than nodal DLBCL [26, 27]. Other histologies that present as PTL include mantle cell lymphoma, extra-nodal NK-cell lymphoma, peripheral T-cell lymphoma, extra-nodal marginal zone lymphoma, ALK1-negative anaplastic large cell lymphoma and paediatric follicular lymphoma [18, 28–39]. The biological basis for the propensity of PTL for extra-nodal relapse is unknown, but may include the development of an immune escape phenotype origin in an immune privileged site behind the blood-testis barrier and overexpression of the chemokine receptor CXCR4 [40–47]. Chapuy et al.’s recent observed copy gains in 9p24.1/CD274 (PD-L1) were present in >40 % of a cohort of 43 PTL cases and were associated with overexpression of PD1 ligands [48].
Rationale for the Initial Evaluation, Staging and Prognosis
Initial imaging of a scrotal swelling should include ultrasonography, which may demonstrate focal or diffuse areas of hypo-echogenicity with hypervascularity in an enlarged testis [49, 50]. Magnetic resonance imaging (MRI) allows simultaneous evaluation of the testis, para-testicular spaces and spermatic cord. Findings in testicular lymphoma include T2 hypointensity and strong heterogeneous gadolinium enhancement [51]. Inguinal orchiectomy is mandatory for the diagnosis as well as for optimal local disease control. Expert hemato-pathological review is essential with the performance of appropriate immunohistochemistry, as distinguishing some cases of PTL from seminoma can be difficult [52].
Recommended staging includes positron emission tomography (PET)-CT and bone marrow biopsy. It is essential to perform baseline MRI of the brain and lumbar puncture for CSF analysis by both cytology and flow cytometry, given the propensity of PTL to disseminate to the CNS [53]. The skin should be thoroughly examined as it is a common site of extra-nodal recurrence and cutaneous “DLBCL, leg type” has been concurrently reported with PTL [54]. HIV serology should be performed.
Staging is based on the Ann Arbor system [55]; 60–70 % of patients have stage I–II at presentation [2, 4, 12, 56]. Patients with isolated bilateral involvement of the testes have similar prognosis to stage I/II disease, and it is reasonable to consider such cases as having stage I disease [57, 58].
For the majority of patients with PTL who present with limited stage disease, the IPI is typically <2 and therefore has limited prognostic utility [59]. A number of adverse prognostic markers have been reported including older age, advanced stage, B symptoms, poor performance status, more than one extra-nodal site of involvement, tumour size >10 cm, raised LDH or β-2 microglobulin, low albumin, left testis involvement and infiltration of adjacent tissues [1, 2, 4, 6, 11, 12, 60–65]. A Dutch series found evidence of transformed extra-nodal marginal zone lymphoma associated with smaller tumour size, less frequently elevated LDH, absence of B symptoms, more frequent stage IE disease and lower IPI than “pure” DLBCL, with a non-significant trend towards improved survival [32].
PTL is characterised by a pattern of continuing relapses for more than 10–15 years after initial treatment. Relapse typically involves sanctuary sites such as the contralateral testis and CNS, but may occur at multiple extra-nodal sites including the lung, soft tissue, adrenals, liver and bone marrow [4, 6, 11, 12].
Rationale for Systemic Therapy
The outcomes of patients treated with orchiectomy and/or radiation alone are poor [2, 3, 66]. Available data on the efficacy of systemic therapy are drawn from either non-randomised phase II studies or retrospective series and suggest an inferior prognosis to nodal DLBCL, with no plateau in PFS and OS curves in retrospective studies [5, 6, 9, 11, 12, 56, 63]. There is evidence that the prognosis of PTL has improved over time as treatment strategies have evolved [2, 11]. The SEER registry analysis reported a median OS of 1.8 years for patients diagnosed in 1980–1985 but with the median not yet reached for those diagnosed 20 years later [2]. Three-weekly CHOP was the most commonly used regimen for PTL prior to the introduction of rituximab, achieving 5-year OS of 30–52 % [11, 67]. Small series evaluating the addition of bleomycin, increasing dose density or intensified chemotherapy regimens such as hyperfractionated cyclophosphamide, doxorubicin, vincristine and dexamethasone (Hyper-CVAD) have not been shown to improve outcomes and are challenging to deliver in older patients [10, 63, 68].
A small retrospective analysis from the British Columbia Cancer Agency (BCCA) demonstrated no improvement in 5-year rates of progression or OS with the addition of rituximab; however the use of rituximab was associated with improvements in both time to progression (p = 0.006) and overall survival (OS) (p = 0.009) on multivariate analysis [64].
The reported results of limited systemic therapy have been mixed. Connors et al. reported excellent results with three cycles of CHOP or a 6-week regimen termed ACOB (cyclophosphamide, doxorubicin, vincristine, bleomycin and prednisolone) [68]. However, other series including a large IELSG analysis and a single-centre study from Australia suggest that patients who receive <6 cycles of chemotherapy have a poorer outcome; therefore limited chemotherapy is not recommended for PTL [6, 12].
Few prospective clinical trials in PTL have been conducted. The GOELAMS protocol was based on three cycles of VCAP (vindesine, cyclophosphamide, doxorubicin and prednisolone) and IT chemotherapy (vindesine, cyclophosphamide, epirubicin, prednisolone and bleomycin for patients aged over 60). Radiotherapy was given to regional lymph nodes as well as to whole brain [69]. With a median follow-up of 73.5 months, the DFS and OS were 70 % and 65 %, respectively, with one CNS relapse.
Aviles et al. reported a single-arm study using 6 cycles of R-CEOP (rituximab, cyclophosphamide 1500 mg/m2, epirubicin 120 mg/m2, vincristine and prednisolone) dosed at 14-day intervals. Scrotal/contralateral testis irradiation (30 Gy) was given to patients achieving CR to chemotherapy. CNS prophylaxis comprised 4 cycles of high-dose intravenous methotrexate (6 g/m2) with leucovorin rescue [70]. Of the 38 patients enrolled, 86 % achieved CR and the actuarial 5-year EFS and OS were 70 % and 66 %, respectively. No CNS relapses were reported in this study.
Finally, the International Extranodal Lymphoma Study Group (IELSG) has reported a phase II multicentre study of 53 patients with stage I/II DLBCL-type PTL. Treatment was 3-weekly R-CHOP with IT methotrexate, followed by loco-regional irradiation [59]. Ninety-eight percent achieved CR, and after a median follow-up of 65 months, the 5-year PFS and OS were 74 % and 85 %, respectively. The 5-year actuarial incidence of CNS relapse was 6 %. The excellent results in this study established R-CHOP Q21 with IT methotrexate and loco-regional radiation as the reference treatment for patients with limited stage PTL, including those with bilateral testicular involvement.
Novel therapeutic strategies for DLBCL may have applicability to PTL. The addition of lenalidomide [71] or the tyrosine kinase inhibitor ibrutinib [72] to R-CHOP [73–76] appears to be well tolerated and effective. Lenalidomide has been detected in the semen of male patients and has been reported as having efficacy for myeloma invading the testis [77, 78]. Pomalidomide, another immunomodulatory drug, appears to be synergistic with rituximab [79] and has excellent CNS penetration [80]. Other agents of interest for DLBCL include the CXCR4 inhibitor plerixafor and small molecule inhibitors of the NF-kB, STAT3 and NF-kB signalling pathways [81]. The recent finding of PD1 overexpression in many cases of PTL makes this an appealing therapeutic target [48]. Currently, 3-weekly R-CHOP (or an equivalent anthracycline-based regimen) with intrathecal methotrexate and loco-regional RT is the international standard of care for stage I–II PTL.
CNS Prophylaxis
The reported incidence of CNS involvement in PTL varies widely but has been as high as 44 % [4, 6, 11, 56, 82]. The IELSG retrospective study reported a 10-year actuarial risk of CNS involvement of 34 % that appears to be substantially greater than nodal DLBCL [12, 83]. The addition of rituximab to anthracycline-based chemotherapy has not had an appreciable impact on the rate of CNS relapse risk [83, 84].
IT chemotherapy has been used in many retrospective series; however methodological limitations make it difficult to draw firm conclusions about its efficacy [4–6, 11]. In two prospective clinical trials that used IT chemotherapy alone, the reported CNS relapse rate was 6 %, whereas a study that used both IT and systemic methotrexate reported no CNS relapses among 38 patients [59, 69, 70]. In all three studies, the crude incidence of CNS relapse was significantly less than for historic controls. Parenchymal CNS relapse is more common than isolated leptomeningeal relapse [12, 85]. As the penetration of IT methotrexate into brain parenchyma is limited, there is conceptual appeal to the use of high-dose systemic methotrexate for CNS prophylaxis, as it achieves higher drug levels in brain parenchyma [86, 87]. This concept is supported both by the results of the Aviles study noted above and the apparent benefit of high-dose systemic methotrexate as CNS prophylaxis for nodal DLBCL [88–91]. The merits of this approach are reflected in treatment guidelines, and the ongoing IELSG-30 prospective protocol incorporates IV methotrexate (1.5 g/m2) in addition to IT liposomal cytarabine (ClinicalTrials.gov identifier: NCT00945724) [92]. Although prophylactic cranial irradiation (PCI) has been used to reduce the incidence of CNS relapse (as in the GOELAMS study), and may be effective, the use of PCI is not routinely recommended due to the risks of late neurocognitive toxicity [93].
Discussion of Prophylactic Testicular Irradiation
Rationale
It is recognised that patients treated by orchidectomy and anthracycline-based chemotherapy have an appreciable risk of relapse in the contralateral testis [1, 3, 4, 9, 12, 57, 59, 65, 67, 69, 94, 95]. This presumably reflects poor penetration of commonly used chemotherapy agents into the intact testis. There are no randomised trial data regarding the use of prophylactic testicular irradiation, nor regarding the optimal dose or technique. Available data are derived from retrospective studies and a small number of non-randomised prospective trials. Mazloom et al. reported the MD Anderson Cancer Center experience and found that 3/35 (9 %) patients who did not receive testicular irradiation relapsed in the contralateral testis, compared to 4 % in patients who receive a median testicular radiation dose of 30.6 Gy [11]. Individual series have reported rates of contralateral testicular relapse ranging from 0 to 35 % [96]. In the largest retrospective multicentre series, reported by the IELSG, testicular relapse was a component of 43 of 195 treatment failures, with a 15-year actuarial incidence of contralateral testicular relapse of 42 % in the absence of scrotal irradiation [12]. A number of individual studies have reported a low incidence of testicular relapse following prophylactic scrotal irradiation, typically ranging from 0 to 10 %, though reportedly as high as 20 % in one series, which may reflect uncertainty about the quality of radiotherapy delivery in older series [96, 97]. Prophylactic scrotal radiation in the IELSG retrospective study was associated with a statistically significantly lower incidence of testicular relapse from 42 % to fewer than 10 % (p = 0.011). Importantly, this local control advantage was associated with an improvement in both 5-year PFS (70 % v 36 %, p = 0.00001) and OS (66 % v 38 %, p = 0.00001), which remained statistically significant on multivariate analysis [12]. Other studies have also suggested an association between the use of adjuvant RT and improved survival, although it is uncertain to what extent this reflects patient selection for adjuvant irradiation [2, 98].
Prophylactic testicular irradiation was used in three of the four reported prospective studies of PTL. In two phase II studies reported by Aviles et al., all patients received prophylactic testicular irradiation and no testicular relapses were reported [10, 70]. Similarly in the more recently reported IELSG-10 prospective study, adjuvant testicular radiation (median dose 30 Gy, range 24–40 Gy) was used routinely with no testicular relapses observed [59]. Based on the preponderance of data from both retrospective and prospective trials, prophylactic testicular irradiation is considered standard of care for patients with stage I–II PTL.
Adjuvant scrotal radiation may have a greater impact in patients with limited stage disease, as the competing risks of systemic relapse appear to increase with advancing initial stage. In the IELSG retrospective series, contralateral testicular relapse as a component of the first site of failure occurred in 28 %, 14 % and 16 % of patients with stages I, II and III–IV, respectively. Corresponding figures for sole initial site of failure were 10 %, 2 % and 2 %, respectively [12]. Although the absolute benefit from prophylactic testicular irradiation may be smaller for more advanced stage disease, given the relatively low morbidity of this treatment, it is reasonable to consider it for all patients having potentially curative chemotherapy. Despite the demonstrated efficacy of scrotal irradiation, these findings have not yet been fully translated into routine practice, as the SEER data suggest that only 30–40 % of patients appear to receive RT, without an apparent increase in utilisation over time [2].
Testicular Radiotherapy Volume
Testicular Radiotherapy Dose
Scrotal irradiation doses reported in the literature have ranged from 18 Gy to over 50 Gy, but are most commonly reported in the range of 25–30 Gy [96]. The prospective studies reported by Aviles and the more recent publication of the IELSG prospective intergroup study both used testicular doses in the range of 25–30 with a high degree of efficacy [10, 59, 70]. As there is some evidence from the IELSG retrospective analysis that doses less than 30 Gy were associated with a lower survival rate, 30 Gy may be considered a reasonable standard of care [12]. Radiotherapy should be fully fractionated to minimise acute scrotal skin morbidity.
Radiotherapy Technique
Several radiotherapy techniques have been described for irradiation of the scrotum, and as with other aspects of radiotherapy for PTL, they have not been compared in prospective clinical trials. In fact, many reported series either do not describe the techniques used or provide inadequate information on techniques used both for testicular and nodal irradiation. Some older series describe techniques that may have provided inadequate or marginal coverage of the testis [96]. A group of Dutch investigators have reported an extensive review of radiotherapy techniques for PTL, including a survey of techniques used in Dutch centres and a phantom study to compare PTV and critical organ dosimetry using various techniques. The three techniques compared were a direct anterior electron beam, a direct anterior photon beam and a wedge pair photon beam [96]. They found that the most commonly used technique in Holland was an anterior electron beam used in 10 of 16 eligible centres surveyed. The most homogeneous dosimetric coverage of the PTV was achieved using either of the photon techniques, but this came at the cost of higher doses to normal tissues such as anus and rectum. Although the doses to normal tissues were within conventional tolerances, it can be argued, particularly in younger patients, that photon techniques are best avoided to minimise exposure of pelvic and perineal structures in order to minimise the risk of second malignancies. A key message is that the method used in any centre needs to be evaluated carefully from a physics and dosimetric perspective to ensure adequate coverage.
The use of bolus over the scrotal surface is not always clearly described in reports of testicular irradiation for PTL. It should certainly be included in rare cases of overt scrotal skin infiltration to avoid underdosing potentially involved skin. It has been reported that invasion of the tunica albuginea is observed in approximately half of cases of PTL at pathological examination [62, 96]. Given that the skin is only a few mm thick, techniques that spare skin may also lead to underdosing of the tunica and potentially increase the risk of in-field failure.
Patient setup will depend on the technique used. Most surveyed Dutch centres used clinical palpation both to define the CTV and for verification during treatment [96]. Given the variation in scrotal anatomy due to fluctuation in temperature or patient anxiety, clinical localisation may in fact be superior to imaging-based localisation, although CT planning and CBCT verification have been used in some centres and potentially offer better documentation of dosimetry and selection of electron energy [96].
Considerations for the Use of Nodal Irradiation
For patients with stage I PTL, radiation is generally confined to the scrotum, and there is no clear role for adjuvant irradiation to apparently uninvolved para-aortic-pelvic nodes. In studies in which scrotal RT only was used for stage I disease, the reported incidence of isolated nodal relapse is low [99].
It is not possible to draw firm conclusions regarding the role of RT to involved regional lymph nodes in patients treated with R-CHOP for stage II disease, because of small numbers of patients reported in the literature with stage II disease treated with or without nodal radiation. Even in the IELSG retrospective study with over 300 cases, it was not possible to make meaningful observations on the role of nodal irradiation in stage II disease [12]. In the IELSG-10 study, of 13 stage II patients, nine received nodal RT, and the single relapse occurred in one of these patients, within the radiotherapy field [59]. The four patients not treated with RT were all in remission at the time of reporting.
The broader literature on the role of adjuvant radiotherapy for DLBCL suggests a benefit for patients with localised and/or bulky disease even in the era of R-CHOP [100–103]. It is uncertain whether the principles of consolidative nodal radiotherapy derived from more common presentations of DLBCL can be translated to the setting of testicular lymphoma, as the pattern of recurrence in PTL is unusual and is dominated by widespread extra-nodal relapse. Despite these caveats, for patients with stage II disease who present with involvement of the pelvic and para-aortic lymph nodes, it is common for adjuvant RT to be delivered to initially involved nodes following chemotherapy, and this may be considered a reasonable standard of care [59]. For patients who present with stage III to IV, the benefit of regional nodal irradiation is even less certain, and radiotherapy would not be used routinely. A possible exception would be cases where there is a dominant sight of nodal disease that is bulky (>7 or 10 cm) or responds slowly or incompletely to initial chemotherapy, particularly in the elderly patient who is unsuitable for aggressive salvage therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree