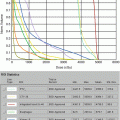
Fig. 11.1
Initial staging MRI of case 1
In the 2008 WHO criteria [3], update of the REAL classification [2], “nasal” NKTCL was defined as a disease occurring in the UADT, whereas “extranasal” NKTCL was defined as a disease occurring outside the UADT. Based on recent data, NKTCL consists of three distinct subgroups: nasal NKTCL, extranasal UADT NKTCL, and extra-UADT NKTCL [11, 12, 18]. The various clinical presentation and prognosis between the three subgroups are summarized in Table 11.1.
Table 11.1
Clinical and immunophenotypic differences between the three subgroups of extranodal nasal-type NK/T-cell lymphoma
UADT-NKTCL | |||
|---|---|---|---|
Feature | Nasal NKTCL | Extranasal UADT–NKTCL | Extra–UADT–NKTCL |
Primary site | Nasal cavity or paranasal sinus with or without extension into adjacent structures | Waldeyer’s ring (nasopharynx, tonsil, oropharynx, base of the tongue), hypopharynx, larynx, and oral cavity | The skin and soft tissue, gastrointestinal tract, testis, et al. Accounts for 10–30 % of all cases |
Phenotype | High expression of EBV (>90 %), CD3ε, CD56, and Ki-67. All cases express cytotoxic proteins. | High expression of EBV (>90 %). CD56 expression is less common than nasal variant, low proliferation index | EBV expression is relatively diverse (40–100 %). High proliferation index |
Age | Usually adults, median age of 44 years | Usually adults, median age of 38–50 years | Usually adults, median age of 50 years |
Sex | Male predominance, M/F = 2–4:1 | Male predominance, M/F = 2.6:1 | Male predominance, M/F = 1.5–2.3:1 |
Stage | Usually present with early-stage disease, majority with stage I (60–80 %); less common with stage III and IV (10–25 %). | Usually present with early-stage disease, stage I <20 %, stage II 50–60 %; more advanced-stage disease (20–30 %) | Usually present with disseminated and advanced-stage disease (>50 %) |
Performance status | Good | Good | Poor, frequently ECOG ≥ 2 |
Elevated LDH | Frequency (20–50 %) | Frequency (20–50 %) | High frequency (50–70 %) |
Lymph node involvement | Low frequency of lymph node involvement at diagnosis (<20 %) | Frequent involvement of the cervical lymph node (>50 %) | High frequency of regional lymph node involvement |
IPI | Usually low risk, IPI 0–1 > 90 % | Usually low risk, IPI 0–1 ≥ 80 % | Usually high risk, IPI 0–1 25–58 % |
Failure patterns | Extranodal organs. The skin is the most common site | Lymph nodes and extranodal organs | Extranodal organs |
Clinical course | Aggressive | Less aggressive | Highly aggressive |
Prognosis | Favorable outcome in stage I patients treated with appropriate radiotherapy, poor for stage II–IV patients | Relatively favorable outcome compared with nasal or extra-UADT variants | Extremely poor prognosis; median survival, 3–20 months |
UADT NKTCL is characterized by predominance in young male, good performance status, elevated lactate dehydrogenase (LDH) in a large proportion of patients, high frequency of early-stage disease, and low-risk group according to International Prognostic Index (IPI) [10]. Patients usually have local tumor invasion or adjacent structure involvement (40–60 %). For nasal NKTCL, the most frequently involved adjacent sites are the maxillary and ethmoid sinuses, followed by the nasopharynx [15, 26]. The major pathway of lymph node spread for nasal NKTCL is to the submandibular and cervical lymph nodes [9]. The primary pattern of failure is distant extranodal sites such as the skin, lung, and liver. The propensity for NKTCL spread to the extranodal organs, especially the skin, is related to the homing capacity of NK/T-cell.
Extranasal UADT NKTCL represents different clinical features and prognosis [11, 12, 18]. The most frequent presenting symptoms for Waldeyer’s ring NK/T-cell lymphoma (WR NKTCL) are odynophagia, dysphagia, and cervical lymphadenopathy [11]. Patients are more likely to present with regional nodal involvement (60 %) and advanced-stage diseases (20–40 %) and have a relatively favorable prognosis [11, 12, 18]. The majority of patients with extranasal UADT NKTCL or WR NKTCL present with Ann Arbor stage II to IV disease, whereas only 10–20 % patients have stage I disease. This differed from reports in which the frequency distribution (60–80 %) of stage I disease was predominant in patients with nasal NKTCL [10, 14, 27]. Several studies have demonstrated that UADT NKTCL and extra-UADT NKTCL have different clinical features and prognoses [5, 16, 28–32, 34, 35, 41]. Extra-UADT NKTCL constitutes only 10–30 % of all NKTCL cases and is characterized by poor performance status, high frequency of elevated LDH, advanced-stage disease, high-risk IPI, and extremely poor prognosis (Table 11.1).
Pathology
Extranodal nasal-type NK/T-cell lymphoma was formerly named angiocentric lymphoma in REAL classification in 1994 [1]. Other terms such as lethal midline granuloma, malignant granuloma, midline malignant reticulosis, and angiocentric immunoproliferative lesion were used before 1994.
The typical morphology of this disease includes frequent angiocentricity with zone necrosis and polymorphism of individual cells with small or medium size of tumor cells and polymorphous inflammatory infiltrate. The histological features are similar irrespective of the anatomical sites involved [3]. Repeated biopsy is usually required for pathologic diagnosis, due to necrosis and small size of specimens.
NKTCL is derived mostly from activated NK cell, and rarely from cytotoxic T lymphocyte. The typical phenotype is CD2+, CD3s−, CD3ε+, CD56+, CD20−/CD79a−, and cytotoxic molecules (T-cell intracellular antigen-1 [TIA-1], granzyme B, perforin)+. The tumor cells are infected by Epstein-Barr virus (EBV), which is detected by in situ hybridization (ISH) for EBV-encoded RNA (EBER). Some cases also show a cytotoxic T-cell phenotype that is negative for CD56 but positive for CD3ε, cytotoxic molecules (TIA, Gram B, perforin), and EBER with TCR gene rearrangement. Patients with CD56-negative NKTCL have similar clinical features as those with CD56-positive NKTCL [15, 26]. However, NK-cell or T-cell lymphoma may have different prognosis [16]. Extranodal lymphomas that are CDε + CD56−, but negative for cytotoxic molecules and EBV should be defined as peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS).
TCR rearrangement is absent for NK-cell lineage, but present for T-cell lineage. NKTCL is strongly associated with EBV infection, which is observed in more than 90 % of cases [17, 18]. Gain of 1q and 7q and loss of 6q and 7p are relatively frequent genomic aberrations [19]. Mutation of JAK3, activation of STAT3, overexpression of matrix metalloproteinase 9, and interleukin-9 have been observed in this disease [20–22]. The inactivation or mutation of p53 is common [23, 24]. High expression of Ki-67 is associated with poor survival [18, 25].
Staging Procedure
Initial Evaluation
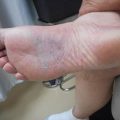
Our patient had a careful physical (including inspection of the skin) and endoscopic examination. Imaging included an MRI, CT of the head and neck, and a PET/CT (Figs. 11.1 and 11.2). Initial complete blood count, biochemistry, liver and renal function, and LDH were normal. Bone marrow biopsy was normal. Although it is rarely involved, bone marrow assessment is a crucial part of the staging [36, 37].
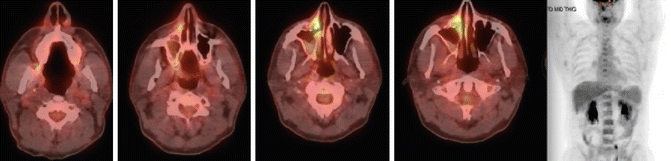

Fig. 11.2
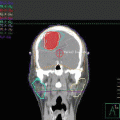
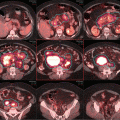
Initial staging PET-CT of case 1
To accurately determine local disease extent in both the soft tissue and bone, both MRI and CT scan of the head and neck are recommended in all cases [38–40]. Additionally, PET/CT can alter the original staging and affect treatment planning in 20–50 % of patients [38]. Moreover, a high tumor FDG uptake was associated with poor prognosis in patients with NKTCL [35, 41].
Although it is not done in our case, many institutions perform quantitative measurement of circulating EBV-DNA viral load for the diagnosis, monitoring, and predicting prognosis of the disease. High load of plasma EBV-DNA correlates with advanced-stage disease, elevated lactate dehydrogenase (LDH), initial response to therapy, and poor survival [42–44, 56].
Stage and Risk Stratification
The Ann Arbor staging system is widely used for diffuse large B-cell lymphoma. However, it is not readily applicable to NKTCL. Primary tumor invasion (PTI) or local tumor invasion is an important prognostic factor for NKTCL [45–47, 105]. A modified Ann Arbor staging system is recommended, and stage I disease is subclassified into limited stage I or extended stage I disease [9, 10]. Limited stage I disease is defined as tumor confined to a single primary site without extension of adjacent organs, as in case 1, whereas extended stage I disease extends beyond the primary site into any neighboring structure or organ, without the presence of nodal or distant dissemination. Ann Arbor stage II disease is defined as involvement of supradiaphragmatic lymph node(s).
The International Prognostic Index (IPI) and Korean Prognostic Index (KPI) are commonly used for NKTCL. The IPI model includes five independent prognostic factors: age, stage, Eastern Cooperative Oncology Group (ECOG) performance status (PS), LDH, and extranodal involvement. The KPI identifies four risk factors: B symptoms, stage, LDH, and regional lymph node [30]. The Ann Arbor stage, IPI, and KPI assign patients to four risk groups with different survivals but are not consistent to segregate patients into risk groups or predict prognosis [12, 31, 45–47, 105]. Patients with NKTCL were predominantly young males with early-stage disease and good PS, which placed the majority of patients in the low-risk groups (0–1) according to the IPI or KPI. On the other hand, the value of KPI model in early-stage patients is limited because regional lymph node involvement duplicates Ann Arbor stage II disease. Based on a recent large, multi-institutional analysis of 1383 patients, a NKTCL-specific prognostic nomogram has been developed and validated as a reliable tool to individually predict overall survival (OS) and has been shown to be superior to Ann Arbor staging, IPI, or KPI [45, 47]. The nomogram model consisted of five variables from routine clinical practice: stage, PS, age, LDH, and primary tumor invasion (PTI). PTI was defined as the presence of primary disease that extended into adjacent structures or organs or the involvement of multiple, contiguous primary sites. Furthermore, patients with early-stage NKTCL can be classified as low-risk or high-risk group based on the absence or presence of risk factors including age >60 years, elevated LDH, ECOG PS ≥ 2, stage II disease, and PTI [45, 47]. Calculation of NKTCL prognostic index should be considered as part of initial work-up. In case 1, the patient belongs to the low-risk group, with normal LDH, PS < 2, age <60, and stage I disease.
Treatment Management
Our patient presented with an early-stage, low-risk disease [45, 47]. Although radiotherapy alone would have been a viable option, this patient was treated with radiation therapy followed by chemotherapy using VIPD (etoposide, ifosfamide, cisplatin, dexamethasone) for a total of three cycles, started 2 weeks after the completion of radiation.
Current data have indicated that radiotherapy is a critical component of curative treatment for early-stage NKTCL. Radiotherapy has been effective in achieving high complete response (CR) rates and favorable long-term survival. Except for two early studies with small-field and low-dose RT [48, 49], radiotherapy alone resulted in complete response (CR) rates of 70–90 %, 5-year locoregional control (LRC) rate of approximately 90 %, and 5-year OS of 50–90 % [10, 15, 26, 45–47, 50 – 52, 55, 105]. In contrast, outcome with doxorubicin-based chemotherapy alone in early-stage NKTCL has been poor, with CR rates of 10–50 % and overall response rate (ORR) of 60–80 %, 5-year OS rates of 10–35 %, and even lower progression-free survival (PFS) [5, 27, 32, 33, 50, 51]. The low initial response and poor prognosis after chemotherapy reflect the chemoresistance, which may be associated with high expression of P-glycoprotein and p53 mutation [23, 24, 54]. In 105 patients with early-stage nasal NKTCL who received primary radiotherapy, the 5-year OS and PFS rates were 71 and 59 % for all patients, with 78 and 63 % for stage I disease, and 46 and 40 % for stage II disease [10]. Tables 11.2 and 11.3 summarize series demonstrating the critical role of radiation therapy compared to chemotherapy alone for early-stage disease.
Table 11.2
Treatment outcome of radiotherapy with or without chemotherapy compared with that of chemotherapy alone in patients with early-stage extranodal nasal-type NK/T-cell lymphoma
Authors | Time | No | Primary site | Stage | Treatment | 5-year OS | |
|---|---|---|---|---|---|---|---|
(%) | P | ||||||
You et al. | 2004 | 46 | Nasal | I–II | RT: 6 | 83.3 | 0.027 |
CT ± RT: 40 | 28.5 | ||||||
Li et al. | 2004 | 56 | Sinonasal | I–II | RT: 11 | 50 | 0.01 |
RT + CT: 27 | 59 | ||||||
CT: 18 | 15 | ||||||
Huang et al. | 2008 | 82 | Nasal | I–II | RT ± CT: 74 | 62.1 (3-years) | 0.000 |
CT alone: 8 | 12.5 (3-years) | ||||||
Kim et al. | 2008 | 280 | All sites | I | RT ± CT: NA | 90.3 | 0.022 |
CT alone: NA | 19.3 (median) | ||||||
Au et al. | 2009 | 57 | UADT | I–II | RT + CT: 34 | 57 | 0.045 |
CT alone: 23 | 30 | ||||||
Yang et al. | 2009 | 177 | UADT | I–II | RT ± CT: 140 | 53.4 | <0.01 |
CT alone: 37 | 18.3 | ||||||
Nie et al. | 2010 | 85 | Nasal | I–II | RT + CT: 17 | 54 | 0.03 |
CT + RT: 48 CT alone: 20 | 47 13 | 0.049 | |||||
Luo et al. | 2010 | 60 | Nasal | I–II | RT + CT: 37 | 56.7 | <0.05 |
CT alone: 16 | 18.8 | ||||||
Yang et al. | 2009 | 177 | UADT | I–II | RT ± CT: 140 | 53.4 | <0.01 |
CT alone: 37 | 18.3 | ||||||
Vazquez et al. | 2014 | 123 | UADT | I | RT: NA | 63.5 | <0.05 |
CT alone: NA | 47.7 | ||||||
65 | Extra-UADT | I | RT: NA | 52.3 | <0.005 | ||
Extra-UADT | CT alone: NA | 23.8 | |||||
Ahn et al. | 2012 | 20 | Skin | I | RT ± CT: 10 | About 60 | <0.005 |
CT alone: 10 | 12 m (median) | ||||||
Yang et al. | 2015 | 1273 | All sites | I–II | RT ± CT: 1103 | 67.7 | <0.001 |
RT alone: 253 | 69.6 | <0.001 | |||||
CT alone: 170 | 33.9 | ||||||
Table 11.3
Treatment outcome stratified by combined modality therapy and radiotherapy alone in patients with early-stage extranodal nasal-type NK/T-cell lymphoma
Authors | Time | No | Primary site | Stage | Treatmenta | 5-year OS | |
|---|---|---|---|---|---|---|---|
(%) | P | ||||||
Cheung et al. | 2002 | 79 | Nasal | I–II | CT + RT: 61 | 40.3 | 0.870 |
RT: 18 | 29.8 | ||||||
I | CT + RT: 47 | 36.7 | 0.892 | ||||
RT: 16 | 46.0 | ||||||
Kim et al. | 2001 | 143 | UADT | I–II | CT + RT: 39 | 35 | 0.93 |
RT: 104 | 38 | ||||||
Kim et al. | 2005 | 53 | UADT | I–II | CT + RT: 20 | 59 | 0.27 |
RT: 33 | 76 | ||||||
Li et al. | 2006 | 105 | Nasal | I–II | RT + CT: 34 | 77 | 0.518 |
CT + RT: 37 | 74 | ||||||
RT: 31 | 66 | ||||||
Ma et al. | 2008 | 64 | Nasal | I–II | RT + CT: 41 | 61.5 | 0.469 |
RT: 23 | 57.9 | ||||||
Aikema et al. | 2008 | 57 | Nasal | I | RT + CT: 20 | 61.9 | >0.05 |
RT alone: 15 | 57.1 | ||||||
Luo et al. | 2010 | 60 | Nasal | I–II | RT + CT: 20 | 56.7 | >0.05 |
RT alone: 7 | 60.2 | ||||||
Li et al. | 2011 | 214 | Nasal | I–II | RT + CT: 118 | 74.4 | 0.529 |
RT alone: 96 | 69.8 | ||||||
Li et al. | 2009 | 67 | WR | II | CMT: 54 | 79 | 0.092 |
RT alone: 13 | 57 | ||||||
Li | 2012 | 69 | UADT | I–II | CMT: 33 | 76.6 (3-years) | 0.313 |
RT alone: 36 | 88.5 (3-years) | ||||||
Aviles | 2013 | 427 | UADT | I–II | CMT: 202 | 86 | <0.01 |
RT alone: 109 | 64 | ||||||
CT alone: 116 | 45 | ||||||
Fang | 2014 | 124 | UADT | II | CMT: 84 | 71.2 | <0.001 |
CT or RT: 40 | 26.7 | ||||||
Yang et al. | 2015 | 276 | All sites | Low-risk | RT + CT: 54 | 86.9 | 0.896 |
stages I–II | CT + RT: 132 | 86.3 | 0.794 | ||||
RT alone: 90 | 88.8 | ||||||
Yang et al. | 2015 | 827 | All sites | High-risk | RT + CT: 155 | 72.2 | 0.017 |
stages I–II | CT + RT: 509 | 58.3 | 0.004 | ||||
RT alone: 163 | 59.6 | ||||||
The addition of chemotherapy to radiation therapy in the treatment of early-stage disease is controversial in Asia, with some studies showing limited benefit [10, 13, 15, 26, 46, 59, 60–63, 105], while others have demonstrated a better outcome with combined modality therapy (CMT)[11, 45, 47, 64, 65]. In the Western world, combined modality approach is generally favored.
Currently Used Combined Modality Therapy
Modern chemotherapy regimens for early-stage NKTCL (Table 11.4) including DeVIC, VIPD (etoposide, ifosfamide, cisplatin, dexamethasone), and GDP (gemcitabine, dexamethasone and cisplatin), in combination with radiotherapy, have all been shown to be associated with improved outcome compared to historical control of radiotherapy alone [67–72]. Less common regimens used include sandwich or sequential LVP (L-asparaginase, vincristine, prednisone), GELOX (gemcitabine, oxaliplatin, L-asparaginase) or CHOP-L (cyclophosphamide, doxorubicin, vincristine, prednisone, L-asparaginase), and radiotherapy ([66, 73, 74]) with limited follow-up and experience.
Table 11.4




Treatment outcome of concurrent or sequential new regimen chemotherapy and radiotherapy in patients with stage I and II extranodal nasal-type NK/T-cell lymphoma
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree