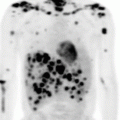
Fig. 2.1
Pre-chemotherapy-staging PET/CT scan of patient with early-stage diffuse large B-cell lymphoma
Prognostic indices have been developed for aggressive lymphomas. This patient had no risk factors according to the International Prognostic Index (IPI) (risk factors: Ann Arbor stage III–IV, >1 extranodal site, high LDH, age >60 years, performance status ≥2 (ECOG)) [18], according to the age-adjusted IPI (risk factors: Ann Arbor stage III–IV, high LDH, performance status ≥2 (ECOG)) [18], and according to the modified IPI for early-stage disease (risk factors : age >60 years, stage II disease, high LDH, performance status ≥2 (ECOG)) [19]. Bulky disease is usually defined as 7.5 cm. Therefore, this patient had localized non-bulky disease, stage IA, with no risk factors.
Treatment Management
The patient was treated with three cycles of R-CHOP (rituximab, cyclophosphamide, adriamycin, vincristine, prednisone) given every 3 weeks. After this, the patient was treated with radiation therapy (RT) to the initially involved lymphoma volume (involved site radiotherapy (ISRT) [20]) to a dose of 30 Gy given in 15 fractions with five fractions per week. Apart from some pain on swallowing, which was treated with paracetamol, he had no acute side effects. PET/CT scanning performed 2 months after the end of RT showed complete metabolic response. On CT a small residual node measuring 1.2 × 0.7 cm could be seen in the right upper neck. The patient has since been seen for regular follow-up. There has been no sign of recurrence for 2½ years.
Brief systemic treatment with immunochemotherapy plus ISRT is an excellent treatment for patients with low-risk, non-bulky, early-stage DLBCL with a 2-year progression-free and overall survival over 90 % [21]. Randomized trials testing the value of RT are only available from the pre-rituximab era [19, 22–24]. They have been criticized for many reasons [25], and they are of limited interest after the introduction of rituximab.
The NCCN Guidelines mention as an alternative six cycles of R-CHOP with or without subsequent ISRT [26]. Full chemotherapy is probably advisable in patients with risk factors, such as bulky disease or an elevated LDH, although no randomized trials exist to support this. However, full chemotherapy comes at the price of an increased risk of cardiac failure from anthracyclines [27]. Even with full chemotherapy, omission of RT will lead to a decrease in the chance of durable local control [28].
Treatment Volume and Technique
In the combined modality setting, modern immunochemotherapy is relied upon to eradicate microscopic disease. RT is delivered as consolidation therapy after the systemic chemotherapy. The detailed guidelines on modern RT for nodal non-Hodgkin lymphoma have been recently published by the International Lymphoma Radiation Oncology Group (ILROG) [20].
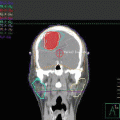
The clinical target volume (CTV) that should be irradiated to the prescribed dose should encompass the initial macroscopically involved lymphoma volume (GTV) before any intervention. The pre-chemotherapy-staging PET/CT scan of the patient is seen in Fig. 2.1. The GTV was contoured on this scan, the PET positive volume was contoured in light blue, and the whole GTV as seen on CT was contoured in red (see Fig. 2.2).
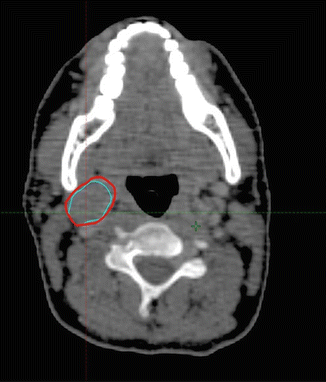

Fig. 2.2
Pre-chemotherapy PET/CT scan. PET+ volume: light blue. GTV: red
The patient’s planning CT scan after the end of the immunochemotherapy was performed with the patient in roughly the same position as the pre-chemotherapy-staging PET/CT scan. This allowed fusion of the pre- and post-chemotherapy images, and the GTV was imported to the planning CT image (see Fig. 2.3).
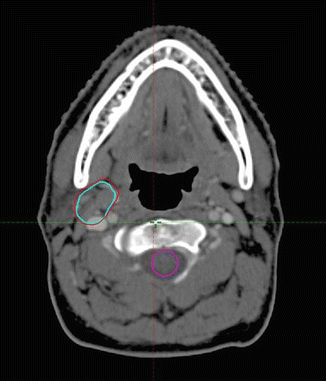

Fig. 2.3
Post-chemotherapy-planning CT scan: Pre-chemo GTV: red. Pre-chemo PET+ GTV: light blue. (Pre- and post-chemotherapy images were fused with some adjustment for slightly different positions in the two images)
The CTV was then contoured on the post-chemotherapy, planning CT scan using information from the pre-chemotherapy PET/CT scan, but taking into account tumor shrinkage and other anatomic changes. The CTV encompasses the GTV and the tissue volume which contained the lymph node, which was removed for diagnosis. The CTV was modified to account for anatomical changes during chemotherapy, excluding normal structures such as muscles that were clearly never involved. The CTV was also modified and sometimes enlarged to account for uncertainties in the image fusion and the position of the patient. The CTV in this patient is shown in pink in Fig. 2.4 .
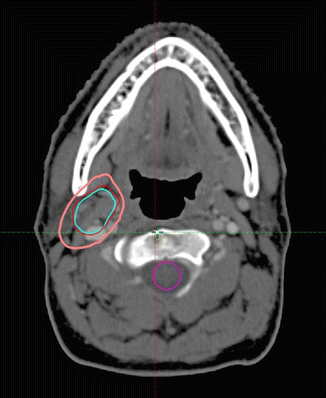

Fig. 2.4
Post-chemotherapy-planning CT scan. CTV: pink. Pre-chemotherapy GTV: red. Pre-chemotherapy PET + volume: light blue
As there is very little movement in the neck, no internal target volume (ITV) was contoured. A planning target volume was created adding a margin of 5 mm to the CTV to account for setup uncertainties in patient positioning and alignment of the beams during treatment planning and through all treatment sessions, see Fig. 2.5 .


Fig. 2.5
Post-chemotherapy-planning CT scan. CTV: pink. PTV: Light blue
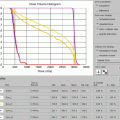
Treatment planning was then performed, using volumetric arc treatment, and the prescribed dose volume conforms excellently to the PTV (see Fig. 2.6a–c ).
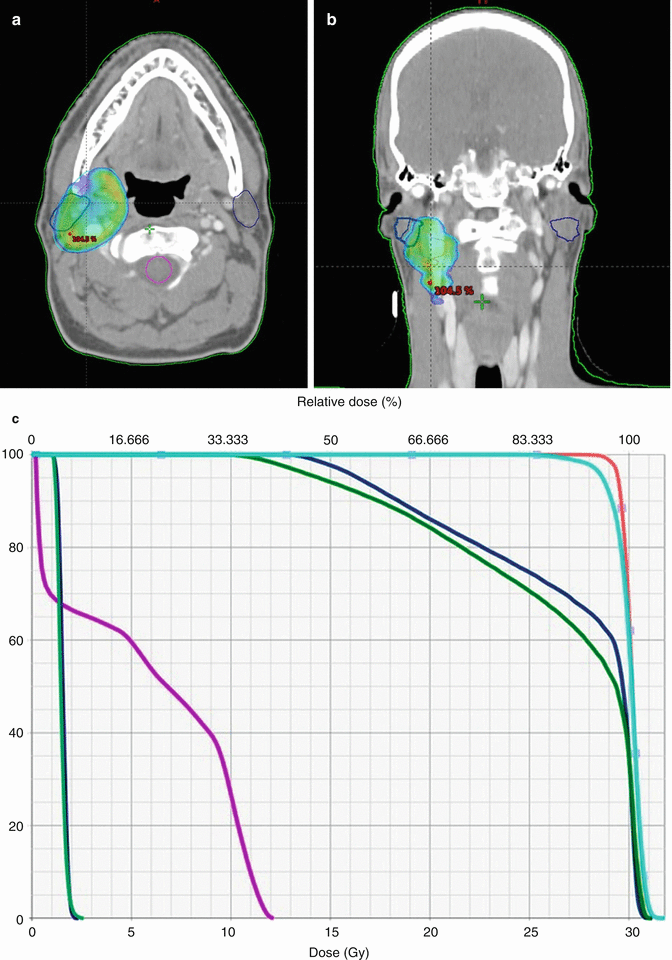

Fig. 2.6
(a) Treatment plan, showing doses above 95 % of prescribed dose. (b) Treatment plan in coronal view. (c) Dose/volume histogram. CTV: pink. PTV: light blue. Spinal cord: purple. Right and left parotids: dark blue. Right and left submandibular gland: green
The prescribed radiation dose was 30 Gy in 15 fractions with five fractions per week. The choice of dose was based on the large prospective, randomized trial from the United Kingdom, showing no difference between doses of 30 Gy and 40 Gy in high-grade lymphomas [29].
Posttreatment Considerations
As can be seen from the treatment plan, some of the right side of the pharynx will receive close to the prescribed dose. This means that, when the patient is about 1 week into the treatment, some pain and discomfort may be elicited from this area, in particular when the patient eats. This condition should be treated with analgesics. It is an acute side effect, and it is expected to resolve about 2 weeks after the end of therapy.
The lymphoma was originally located very close to the right parotid and submandibular glands; hence, particular attention was given to spare these glands as much as possible.
About 50 % of the right submandibular gland receives the prescribed dose of 30 Gy. Fortunately, the submandibular gland tolerates doses up to 36 Gy, and the patient is not expected to experience any reduction in the saliva from the right submandibular gland. The left submandibular gland receives very little radiation.
About 80 % of the right parotid receives 26 Gy or more, which is the constraint normally used in head and neck cancer, and the saliva production from these glands will be somewhat decreased after RT. However, as the left parotid gland receives very little radiation, it is expected that there will be very little xerostomia after treatment. Still, the patient should be instructed in good oral hygiene and regular dental examinations. The radiation dose is so low that osteoradionecrosis is not an issue.
Most patients like this one, who are treated to small volumes with conformal techniques and to modest radiation doses, experience very little long term side effects from the treatment. As mentioned above, a patient like this one, with stage IA DLBCL, GCB type, no risk factors and no bulky disease, has a very good chance (over 90 %) of being permanently cured with brief immunochemotherapy followed by ISRT to 30 Gy [21, 28, 30].
Clinical Presentation: Diffuse Large B-Cell Lymphoma, Bulky Abdominal Mass, and Primary Refractory Disease
A 58-year-old female with no previous medical history presented with abdominal pain and nausea. On examination, there was an ill-defined mass in the right upper quadrant. An infection work-up including virology was negative. A CT scan of the abdomen revealed a large abdominal mass 14.3 × 10.3 cm in the axial dimension and 13 cm in the craniocaudal dimension with medial displacement of the right kidney. There was external compression of the inferior vena cava without complete obstruction. A CT-guided percutaneous biopsy showed diffuse large B-cell lymphoma (DLBCL).
Pathology
Microscopic examination of the mediastinal mass biopsy showed sheets of malignant large lymphocytes which were CD79a+, CD20+ confirming B-cell origin. Further immunohistochemistry confirmed activated B-cell subtype of DLBCL, which is MUM1+, BCL2+, BCL6+, p53+, CD30+/−, CD10+/− with proliferation fraction (Ki67) > 90 %. In situ hybridization showed no MYC, IgH, BCL2, or BCL6 rearrangements. The subclassification of DLBCL is discussed under the previous case.
Staging and Prognostic Factors
The patient was referred to the lymphoma team and was staged with clinical examination, blood tests (including CBC, serum biochemistry, LDH), whole-body FDG-PET/CT, and bone marrow biopsy (BMB). PET/CT revealed stage II disease with large abdominal mass spanning over the upper and lower mesenteric area with increased FDG uptake (SUVmax 18.5). There was no evidence of involvement above the diaphragm (see Fig. 2.7). Prognostic indices are discussed under the previous case.
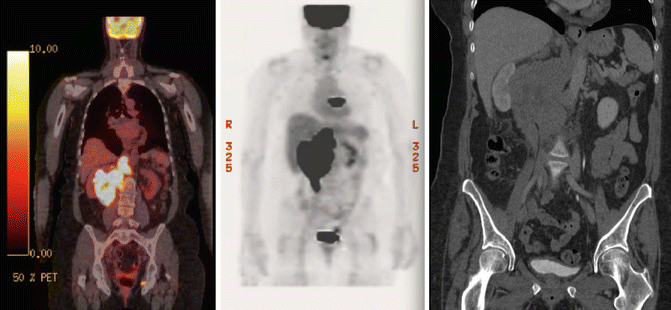

Fig. 2.7
Initial PET/CT and CT abdomen showing right bulky abdominal mass
Treatment Management
The case was discussed in the lymphoma multidisciplinary meeting (MDM), and the histological diagnosis and the stage of disease (IIA bulky) were confirmed. The International Prognostic Index (IPI) was 1 (elevated LDH), i.e., low-risk group, and the age-adjusted IPI was also 1. A treatment plan was recommended to administer R-CHOP-21 immunochemotherapy given every 3 weeks for six cycles followed by consolidation radiotherapy (RT) to the bulky disease site.
The treatment of choice for advanced DLBCL (stage III + IV or early stage with bulky disease) is immunochemotherapy with R-CHOP-21 (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone given on day 1 every 21 days) for six cycles. R-CHOP has been shown to be superior to CHOP alone in phase 3 randomized trials [31–33]. Although CHOP given every 14 days (CHOP-14) is superior to CHOP-21, the same does not hold true when rituximab is added. A large phase 3 randomized study showed that there was no difference between R-CHOP-21 and R-CHOP-14 [34]. Eight cycles of chemotherapy have not been found to be better than six cycles [35].
This patient had bulky disease which is a recognized risk factor, although it is not included in the IPI which classifies her as “low risk” (IPI 0–1) [18]. A significant proportion of recurrences occur in previous sites of involvement, particularly the bulky sites. RT to sites of bulky disease has been traditionally used as a consolidation after chemotherapy. Single institution series report significantly better PFS and OS in patients who receive RT to initial bulky sites with local control usually in the region of 90–100 % [28, 36, 37]. However, with improvements in chemotherapy outcome with the addition of rituximab, the value of consolidation RT has been questioned. The MInT study, which tested CHOP-like chemotherapy with or without rituximab in young patients (<60) with good prognosis and showed a definite benefit from adding rituximab in these low-risk patients, was analyzed with respect to the presence of bulky disease. The analysis included 823 patients and revealed that rituximab reduced but did not eliminate the poor prognostic effect of bulky disease [38]. An updated analysis with 6 years follow-up showed that RT seemed to eliminate the adverse effect of bulky disease [39]. More recent evidence from the German RICOVER-60 study suggests that consolidation RT to sites of bulky disease (≥7.5 cm) or extranodal sites improves PFS (3-y PFS 88 v 62 %, p < 0.001) and OS (3-y OS 90 v 65 %, p = 001) in R-CHOP-treated patients over 60 years of age [40]. Another German study (UNFOLDER study, DSHNHL 2004–3; ClinicalTrials.gov identifier NCT00278408) evaluated the role of consolidation RT in patients aged 18–60 with an age-adjusted IPI score of 1 or score 0 with bulky tumor (≥7.5 cm). Patients were randomized into 14-day versus 21-day cycles of R-CHOP and consolidative RT versus observation after chemotherapy. The no-RT arms were closed when interim analysis revealed significantly worse EFS with the omission of RT (3-year EFS 65 % vs. 81 % with combined modality therapy) [12th international conference on malignant lymphoma, Lugano, Switzerland, 2013]. The final results of this study are eagerly awaited, but the current evidence supports a role for consolidation RT to sites of bulky (≥7.5 cm) disease. Therefore a recommendation to give consolidation RT for the site of bulky disease was made by the multidisciplinary team.
An alternative approach would be to decide on consolidation RT on the basis of response to full course of chemotherapy as assessed by PET/CT with RT only added if there is evidence of residual FDG activity. This approach has not been tested in clinical trials and there is evidence suggesting that the presence of a residual mass (>2 cm) in the context of complete metabolic response confers an increased risk of relapse compared to patients with no residual mass [41].
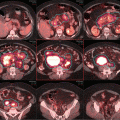
The patient received three cycles of R-CHOP at full dose, and a repeat PET/CT scan showed reduction in the size of the mass (10 × 7.5 cm). There was also partial metabolic response with reduction in the FDG uptake (SUVmax 11.7) and the residual uptake was scored as Deauville score 5 (see Fig. 2.8). She received further three cycles and a repeat PET/CT scan after a total of six cycles showed further reduction of the uptake (SUVmax 8, Deauville score 4) and the size of the mass (see Fig. 2.9).
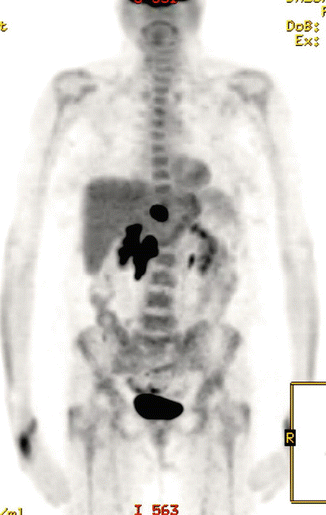
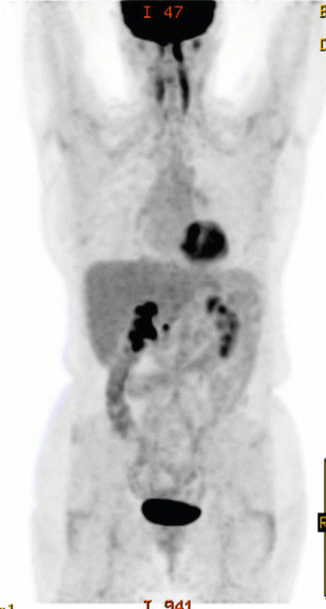

Fig. 2.8
Large residual mass with Deauville 5 after three cycles of R-CHOP

Fig. 2.9
PET/CT after completing 6 R-CHOP showing further reduction in the size and FDG activity in the disease
Interim imaging is usually done during a course of chemotherapy to monitor response to treatment. PET/CT is better than CT in showing early response during a course of chemotherapy [42]. Patients with early complete metabolic response tend to have a better outcome than patients with residual activity. However, the difference between the two groups is variable in various studies in DLBCL [43–48]. Treatment modification (response-adapted therapy) based on interim PET (iPET) scanning is currently being tested in several randomized studies in Hodgkin lymphoma and aggressive non-Hodgkin lymphoma. To date there has been no evidence that an early change of treatment improves outcome in DLBCL, and there is no established alternative therapy, so the information of iPET is only of prognostic value and should not be used to change treatment unless it shows no response or progressive disease. Therefore treatment with the most effective regime (R-CHOP) was continued despite the presence of residual activity on iPET.
The case was discussed again in the lymphoma MDM, and a plan for salvage treatment was made. Primary refractory DLBCL is usually defined as disease progression or failure to achieve remission during or shortly after (<6 months) chemotherapy and carries a poor prognosis even with salvage therapy. The standard salvage therapy after failure of R-CHOP involves inducing a remission with a salvage chemotherapy regime followed by high-dose chemotherapy and autologous hematopoietic stem cell transplant (SCT) for fit patients. However, the CORAL study showed that the effectiveness of salvage chemotherapy after R-CHOP is worse than after CHOP as it appears that R-CHOP-failures define a worse prognostic group (response rate was 51 % v 83 % in rituximab-treated and rituximab-naive patients) which translated into a worse outcome (3y EFS of 21 vs. 47 %, respectively) [49]. In addition, patients who relapsed within <1 year and received rituximab in first-line therapy had a particularly poor outcome. None of the commonly used salvage regimes (e.g., DHAP, ESHAP, ICE, GPD, IGEV) has proven to be better than the rest, and there is a need to improve the effectiveness of salvage chemotherapy in rituximab-treated patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree