Fig. 12.1
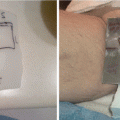
Localized mycosis fungoides (MF) of the chin
A complete head–to–toe skin examination was performed of the patient’s face, hair, scalp, neck, chest, back, abdomen, buttocks, bilateral upper extremities, bilateral lower extremities, hands, feet, palms, soles, digits, and nails. Only the 5 × 5 cm purplish plaque on the chin area was noted, for a body surface area (BSA) assessment of 1 % plaque and modified Severity-Weighted Assessment Tool (mSWAT) score of 2, Stage IA.
Pathology, Staging, and Prognostic Factors
Diagnosis of MF is challenging; with the lack of a diagnostic gold standard and the many benign conditions, MF can mimic in its early stages. Diagnosis requires correlation between the clinical presentation along with histopathologic and immunophenotypic findings of the skin biopsy, including seeking to identify a clonal T-cell population clustered at the basement membrane of the epidermis. The malignant clone should bear the immunophenotype of activated, skin-homing CD4+ helper T cells that interact with antigen-presenting dendritic (Langerhans) cells in the epidermis [4]. A typical immunophenotype is CD2+, CD3+, CD4+, CD45RO+, CLA+, and CD8− (cytotoxic T cell) [5].
Early MF typically presents in patch phase, eventually progressing to plaques, tumors, and erythroderma (>80 % body surface involvement with confluent patches/plaques) (Fig. 12.2). With progression, there is loss of epidermotropism, lymphoid infiltration increasing in density and invasion into the deeper reticular dermis, and ultimately the development of tumor. The Pautrier’s microabscess is considered pathognomonic but occurs in less than 20 % of early lesions. The World Health Organization- European Organization for Research and Treatment of Cancer (WHO-EORTC) established the pathologic classification scheme for CTCL including MF [6–8]. Variants include folliculotropic and granulomatous slack skin MF.
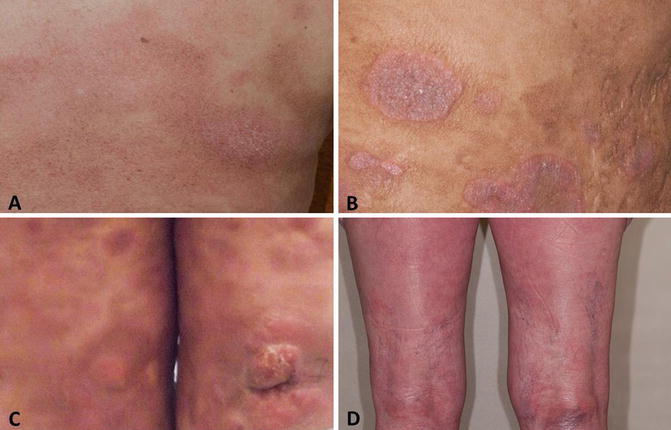

Fig. 12.2
Patch (a), plaque (b), tumor (c), and erythroderma (d) lesions
Staging and Prognostic Factors: Early Stage Case
A thorough history should demonstrate attention to duration, change, and distribution of lesion(s) and an understanding of symptoms including pruritis, pain, exfoliation, fissures, bullae, and perineal/perianal discomfort or evidence of involvement. The skin examination is critical not only for RT planning but also for staging and characterization of the percent cutaneous involvement by BSA and mSWAT scores. Skin photographs are important for documentation and anticipation of the chronic waxing and waning of disease over time and treatment. Adenopathy and organomegaly should also be documented. After biopsy, complete staging evaluation for this patient included complete blood count (CBC) with differential, comprehensive metabolic panel (CMP), serum chemistries (including renal and hepatic function), and lactate dehydrogenase (LDH), all of which were normal for this patient. Concerning lymph nodes, particularly those ≥1.5 cm, may require excisional biopsy. PET/CT scan and bone marrow biopsy are not required in patients presenting with early stage, limited disease, but should be considered in patients with a higher burden of skin involvement or other concerning findings such as clinical abnormalities or large-cell transformation on pathology. Peripheral blood flow cytometry is typically performed to assess expression of CD2, CD3, CD4, CD5, CD7, CD8, CD20, and CD45RO.
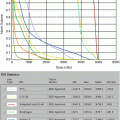
Current staging is based on the TNMB system, as proposed by the European Organization for Research and Treatment of Cancer (EORTC)/International Society for Cutaneous Lymphomas (ISCL). This staging includes MF and Sézary syndrome (SS) and considers skin, nodal involvement, and clonal and tumor burden in the blood [9]. T stage consistently demonstrates strong prognostic significance. Patients with limited patch and plaque involvement (stage IA, T1 N0 M0) demonstrate 10-year survival comparable to matched controls, while a dose response in the survival curve is demonstrated in patients with extensive patches and plaques (T2), tumors (T3), and erythroderma (T4), with median 11-, 3.2-, and 4.6-year survival, respectively [10]. In contrast, the independent prognostic significance of nodal involvement on overall survival is less clear, though overall stage is still correlated with outcome [11]. Other prognostic factors previously identified in studies include older age, large-cell transformation, elevated LDH [12], increased soluble interleukin-2 receptor levels [13], lower percentage of CD8+ tumor-infiltrating lymphocytes [14], or T-cell clonality within the cutaneous infiltrate, blood, and/or nodes [15–18].
Treatment and Management: Early Stage Case 1
For the patient’s stage IA MF, a definitive radiation treatment approach was offered. The radiation treatment plan targeted the visible sole plaque of the skin of the chin plus a margin for penumbra and setup error (1.5 cm beyond all visible and palpable abnormality), using local appositional electrons to a total dose of 20 Gy in 10 fractions. With the consideration of decreasing toxicity to normal tissue and with the understanding that repeated courses of radiotherapy might be likely in the future, the lowest possible electron energy directed at only the involved site was chosen. Appositional 9 MeV electron treatment was adequate to cover the thickness of the lesion. Cotton rolls were placed between the lip and the gingiva to decrease the electron backscatter, avoiding excessive toxicity to the mucosa (see Fig. 12.3 ). Patients will often have undergone a CT scan during their workup, and this imaging can be helpful to determine the thickness of the lesion to inform electron energy. Otherwise, for nodules or irregular skin surfaces, CT simulation may be helpful for choosing the electron energy that ensures adequate depth.
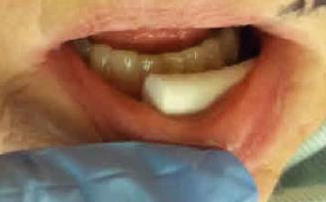

Fig. 12.3
Cotton roll between the lip and gingiva displaces the lip and decreases the electron backscatter, in order to decrease risk of treatment-associated radiation mucositis
Presentation of MF with a single cutaneous lesion is rare, with most early stage patients, though presenting with limited skin surface area involvement, still having multiple lesions. The treatment strategy for MF depends on whether it is early vs. advanced or refractory in presentation [19]. For early disease, first-line treatment strategy is based on sequential skin-directed therapies, including RT, with the goal of providing the most durable response, with the least toxicity. As the disease becomes increasingly refractory, systemic, targeted, or combined therapies are second-line considerations, though RT remains an option.
For patients with disease limited to the skin, the skin-directed therapies including corticosteroids, mechlorethamine (nitrogen mustard), carmustine (BCNU), bexarotene gel (rexinoids), psoralen plus ultraviolet A (PUVA), ultraviolet B (UVB), and either localized or total skin electron radiotherapy all render high rates of control, particularly in patients presenting with localized uni-lesional MF or <10 % BSA involvement [20–22]. Long-term disease-free survival still ranges from 30 to 50 % in prior studies of these patients. Patients with more extensive cutaneous involvement still typically achieve initial control with these therapies, but long-term cure is unlikely [23, 24].
Radiation Field, Dose, and Technique: Early Stage Case 1
Radiation remains a highly effective treatment for MF, though determining the optimal dose is challenging [25]. The dose response with RT was well documented both for local RT and total skin electron beam therapy (TSEBT), and as a result, historically, RT doses >30 Gy were considered standard. More recently, the high rates of effective cutaneous control for lower doses has been increasingly appreciated, as high as 88 % overall response rate in a series of patients treated with 12 Gy TSEBT with mild and reversible toxicities [26, 27]. Accordingly, there is increasing consensus that the incremental local control benefit of higher RT doses may not outweigh the concomitant incremental toxicity—with a consequent shift toward low-dose RT as a standard approach. The International Lymphoma Radiation Oncology Group (ILROG) consensus field and dose guidelines consider local RT doses ranging from 6 Gy to >30 Gy as acceptable, with an emphasis on recommending lower doses for palliation and doses from 20 to 24 Gy for uni-lesional MF. The recommended target for treatment is the lesion plus 1.0–2.0 cm margin for local palliation and the entire skin for TSEBT, using electron technique (customarily 6–9 MeV with bolus for patch, and higher energies for thicker plaques or tumors and exophytic lesions) [28].
Posttreatment Considerations: Early Stage Case 1
The early stage MF patient developed mild erythema and dry skin with RT, completely resolved at 4–week follow–up. At the last follow–up, 20 months after treatment, the lesion remained in complete remission (Fig. 12.4 ). Unfortunately, the patient developed another similar small lesion on the left arm, which was biopsy-proven MF. This lesion is currently well controlled with topical corticosteroids only. Typical follow–up visits every 3–6 months include a history and physical examination including comprehensive skin examination, blood work (including a CBC and LDH), swab and culture of any lesions concerning for infection/colonization, and repeat imaging only as needed.
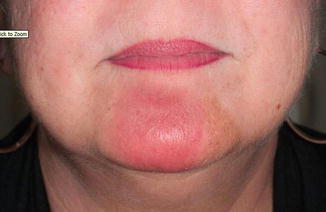

Fig. 12.4
MF lesion at 8 weeks follow-up, demonstrating an excellent clinical response
Clinical Case 2: Patient Presenting with Stage I, Tumorous Lesion
A healthy 52-year–old female hairdresser noted an itchy lesion over the left ear. She managed it for months using topical steroids. However, the lesion kept growing, eventually forming a tumor (see Fig. 12.5 ). After 18 months, biopsy confirmed MF. Careful skin examination showed another lesion in close proximity on the left ear, as well as one on the back of the neck measuring 1.5 × 2 cm. Staging workup showed no evidence of disease in the marrow, nodes, or internally. The patient was referred to receive definitive radiation therapy. The patient was simulated with an open neck position, with the area to be targeted clinically traced with radioopaque wires. The treatment was planned using a single appositional electron field with 6 MeV electrons, and in an effort to avoid the parotid gland, skin collimation was applied (see Fig. 12.6 ). To overcome the irregularity of the skin, a tissue-equivalent material was used to compensate for the gaps and form a uniform surface. A 1 cm tissue-equivalent bolus was placed over the lesion to ensure that 100 % of the dose would reach the surface (see Fig. 12.7 ). A total dose of 12 Gy in six treatments was delivered. The patient was discharged with moisturizers to apply to the treated area. A picture of the lesion was taken almost on a weekly basis until complete disappearance of the lesion on week 12 (see Fig. 12.8 ).
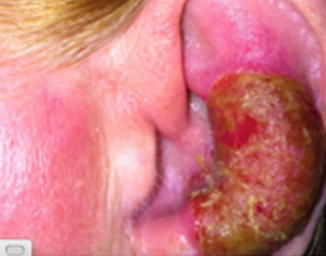
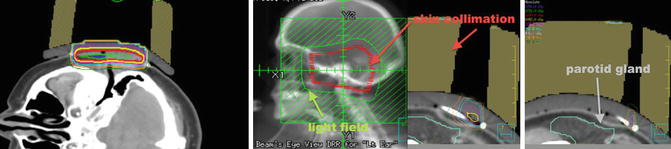
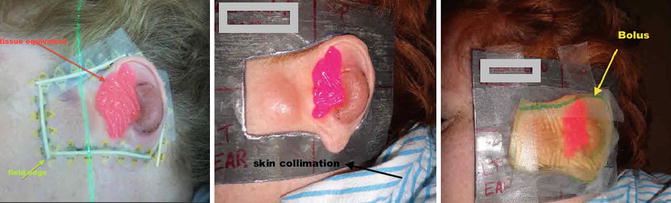
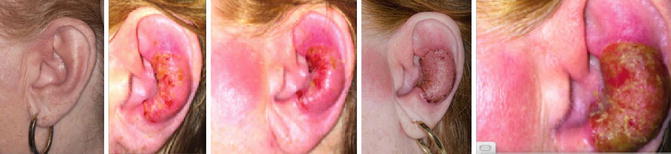

Fig. 12.5
Left ear lesion forming an ulcerative tumor. Another lesion is seen in front of the tragus

Fig. 12.6
Isodose lines encompassing the target (left); skin collimation placement and light field (green) surrounding the skin collimation (red) (middle photo); skin collimation blocking dose to the parotid gland (right)

Fig. 12.7
Daily setup with bolus (yellow), tissue equivalent (pink), and the skin collimation to protect the surrounding organs including the parotid

Fig. 12.8
Lesion prior to radiation (far right) and the progression of the response until the last one on week 12 (left) with complete disappearance
The patient did well for a year before she presented with another lesion over the face that was again treated with radiation. Today almost 18 months after treating her ear, the later site is still well controlled. This case is a good example of how lowering the dose can still offer a durable local control.
A key caution is to allow lesions to heal, even if healing appears slow, sometimes taking several months. As long as a continuous improvement is seen, we recommend continued observation. Patients not infrequently demonstrate a natural history consisting of local lesions only that respond very well to either topical treatment or low-dose radiation. Especially for such patients, the treatment strategy should avoid unnecessary radiation to surrounding critical organs, for instance in this case the parotid gland.
Advanced Stage MF
Clinical Presentation: Advanced Stage Case 3
A 60–year–old Caucasian woman presented with a 5–year history of progressive pruritic rash, originally thought to be psoriasis, but was eventually biopsy-proven MF. On initial presentation BSA patch was 22 %, plaque 0 %, tumor 4.5 %, and mSWAT 40. Tumors were present on the arms, legs, and buttocks, with weeping, ulcerated lesions over the arms and feet; see example photos in Fig. 12.9. The patient had a palpable right inguinal lymph node. A staging PET–CT (Fig. 12.10) demonstrated extensive cutaneous hypermetabolism consistent with the clinically noted skin lesions. There were several hypermetabolic right inguinal lymph nodes, the largest measuring 2.8 cm with SUV 3.9. Peripheral flow studies were negative, ruling out SS. Baseline LDH was within normal limits.
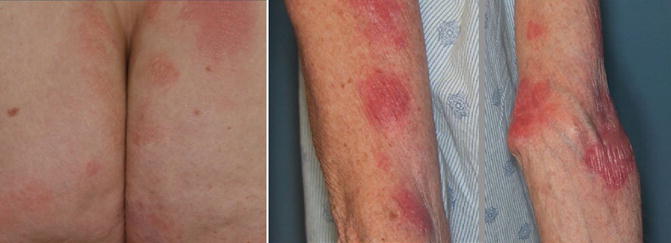
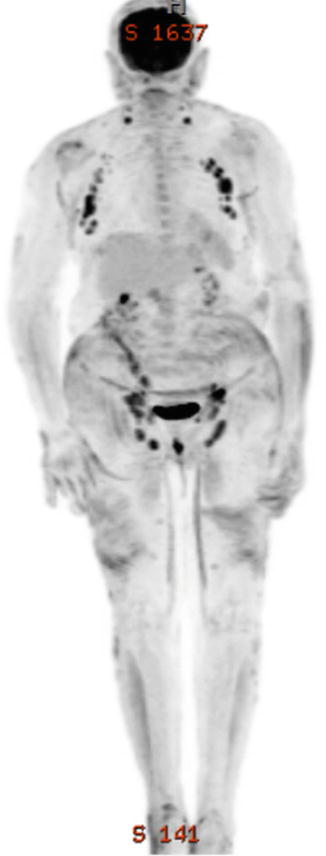

Fig. 12.9
Lesions over the arms and buttocks

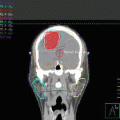
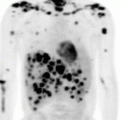
Fig. 12.10
PET/CT images of Case 1 showing lymph nodes in the neck, axillae, and groins
Pathology: Advanced Stage Case
The skin biopsy demonstrated a dense dermal atypical lymphocytic infiltrate. Many of the cells were large with vesicular nuclei, irregular nuclear contours and prominent nucleoli, consistent with large-cell transformation. Immunohistochemical studies demonstrated CD3+ and CD4+ T cells with CD4/CD8 ratio >10:1. Approximately 10 % of lymphocytes expressed CD30+ in a 2+ membranous pattern. The patient also received a biopsy of the concerning inguinal lymph node, which demonstrated involvement with MF. Therefore, the patient was Stage IVA.
Large-cell transformation can occur, in up to 39 % of patients. Transformation is associated with higher stage, potentially more aggressive behavior, and CD30+ in 30 % and CD20+ in 45 % of cases [29, 30]. In advanced disease, MF can involve the lymph nodes, blood, bone marrow, and visceral organs. Sézary syndrome (SS) is characterized by erythroderma plus malignant circulating T cells (as determined by absolute Sézary cell count, flow, or polymerase chain reaction) [31].
Staging and Prognostic Factors: Advanced Stage Case
Particularly for advanced stage patients, 15 % have lymph node involvement at diagnosis, and size ≥1.5 cm is considered clinically abnormal [32, 33]. Visceral involvement, including involvement of the lungs, central nervous system, oral cavity, and oropharynx, is rarer and co-segregates with advanced skin involvement, nodal disease, and blood involvement [32, 34]. The frequency of bone marrow involvement ranges between 6 and 28 % of patients in prior studies [7, 8].
MF can progress to an erythrodermic stage and SS, or SS can arise independently. Patients can clinically present with diffuse edema and tumorous involvement of the face (leonine facies), and severe fissures of the palms and soles, accompanied by symptoms of intense pruritus and pain. In a retrospective study of 1502 patients, one of the largest cohorts assembled for MF, advanced T, N, M, and B stages were all independent predictors of worse survival, as were older age and male sex. Large-cell transformation and tumor distribution were independent predictors of disease progression [35]. Another cohort study of 1275 patients stage IIB to IV risk stratified patients into low, intermediate, and high risk for mortality based on a prognostic index combining Stage IV, age >60 years, large-cell transformation, and increased LDH. Five-year survival for high-risk patients was 28 % in this analysis [36]. A similar prognostic index, the cutaneous lymphoma international prognostic index (CLIPi) for MF and SS demonstrated validating findings [37].
Treatment and Management: Advanced Stage Case
The patient in case 3 demonstrated a progressively refractory course. After failing topical therapies, he was started on gemcitabine, which he received for four cycles. He achieved a partial response but was unable to tolerate further therapy due to the associated nausea and fatigue. For treatment of this advanced stage case patient, oral bexarotene and topical nitrogen mustard treatment were then attempted, unfortunately with frank progression of plaques and tumors with total BSA 39.5 % and mSWAT 67.7. The patient was referred for TSEBT and concomitantly referred for workup anticipating allogeneic stem cell transplant.
Systemic chemotherapy is used in refractory disease, though with variable response rates and unfortunately less durable response for advanced and relapsed/refractory cases [38]. Gemcitabine treatment was associated with 10 % complete response and 70 % overall response rates in a prior study [39]. Other considerations include vorinostat and romidepsin, which are histone deacetylase (HDAC) inhibitors. A durable response can be achieved in a subset of patients, even lasting years in prior studies [40, 41]. Development of targeted therapies continues to be on the horizon for long-term management of MF, especially for the challenging case of advanced and refractory disease.
Radiation Field, Dose, and Technique: Advanced Stage Case 3
The ILROG guidelines accept a wide range of doses for TSEBT to be acceptable, from 8 to 36 Gy [28]. After TSEBT, patients with early stage disease can achieve as high as 90 % 5-year relapse-free survival, while only 30–50 % of patients with advanced disease remain without disease at 5 years. TSEBT targets the epidermis (varying from 0.05 to 0.5 mm thick, thickest in distal extremities) and dermis (varying from 2 to 4 mm, thickest in hands and feet) [26]. According to EORTC TSEBT consensus guidelines, the 80 % isodose line should be ≥4 mm deep. This technique places the dose maximum at 1 mm, the 80 % isodose line at 6 mm, and the 20 % isodose line at 12 mm [42], thus satisfying the EORTC criteria for TSEBT [43]. Photon contamination due to bremsstrahlung scattering in the machine head, intervening air, scatterers or degraders, and patient, is acceptable at 1.2 % [42]. Using six treatment positions shifts, the isodose curves toward the skin surface due to the obliquity of the incident electrons.
Overall approach
At our institution, we use a modern linear accelerator using the dual-field, six treatment position technique: The machine delivers a 9 MeV beam to the patient standing 3 m from the electron source, behind a Lucite plate for x-ray attenuation. Effective energy at the skin is approximately 5 MeV. The gantry angle is set at 113 and 67° to produce dual fields (upper and lower fields). There are a total of six treatment positions, each treated with the upper and lower fields: anteroposterior (AP), posteroanterior (PA), right and left anterior oblique (RAO, LAO), and right and left posterior oblique (RPO, LPO) (Fig. 12.11). The purpose of multiple positions is to “unfold” the skin creases, to expose the “shadowed” areas as much as possible and improve lateral dose homogeneity. The upper and lower fields improve dose homogeneity in the vertical dimension. At our institution, a patient typically cycles twice for each field in a single week (2 Gy × 2 fractions for a total of 4 Gy delivered to the total skin each week). Therefore, a 12 Gy treatment course is typically completed in 3 weeks, while a 32 Gy treatment course would last 8 weeks. We standardly do not exceed 2 Gy per fraction due to concern for excess toxicity [44].


Fig. 12.11
Total skin electron beam treatment positions
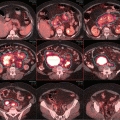
To date, the conventional wisdom has preferred high-dose regimens (>30 Gy) for patients with extensive disease, higher stage, or erythroderma. Figure 12.12 shows an example of advanced cases where a dose of >30 Gy is typically used.
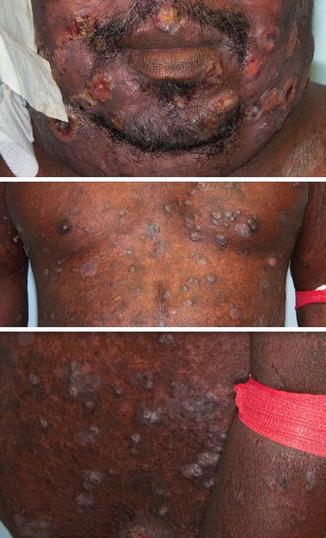

Fig. 12.12




Advanced disease where >12 Gy TSEBT is considered, either in the setting of anticipated transplant or non-transplant candidate refractory to multiple lines of therapy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree