Fig. 8.1
Brain imaging in a 71-year-old woman with PCNSL. (a) Axial CT without contrast reveals a dominant mass in the left frontal lobe. (b) Axial T1 post-contrast image demonstrates the heterogeneously enhancing large left frontal mass as well as an enhancing lesion in the right ventricle. (c) Corresponding T2-FLAIR axial image illustrating vasogenic edema with mass effect on the left lateral ventricle
Clinical Presentation
Patients with PCNSL often have presenting symptoms that reflect the periventricular location of the lymphoma. In a retrospective review of 248 cases of intracerebral PCNSL, 70 % of patients presented with a focal neurologic deficit, 43 % had neuropsychiatric symptoms, and 33 % had signs of increased intracranial pressure [4]. Given that most tumors are located deep in the white matter, seizures are not as common as in other cases of primary brain neoplasms, with only 14 % of patients presenting with seizures in this series.
The eye, specifically the vitreous and/or the retina, is a common site of initial disease and a potential location for relapse in PCNSL. Roughly 20–25 % of patients with PCNSL will have ocular involvement at diagnosis [5]. Primary intraocular lymphoma (PIOL) affects the vitreous or retina in the absence of the brain, leptomeninges, spinal cord, or systemic disease. For patients with PIOL, brain relapse is the rule, as 50–80 % of patients will have cerebral lymphomatous involvement, often within 2 years of PIOL diagnosis [6]. Patients often present with floaters, blurry vision, or painless decrease in visual acuity [7, 8]. Bilateral ocular involvement is common [5].
Histopathology
Greater than 95 % of PCNSL tumors are CD20+ DLBCL; however other histologic lymphoma variants can affect the CNS, including T-cell lymphoma, Burkitt’s lymphoma, and indolent B-cell lymphomas (most notably marginal zone lymphoma of mucosa-associated lymphoid tissue, MALT) [9–11]. Indeed, according to the WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue, the term PCNSL refers only to DLBCL affecting the CNS in immunocompetent patients with exclusion of other histologies [12].
The DLBCL cells in PCNSL express typical B-cell markers, including CD19, CD20, and CD79a. MUM1 expression is present in greater than 90 % of cases, and BCL6 expression is also common [13]. In nodal DLBCL, gene expression profiling has identified two distinct cell of origin (COO) phenotypes, the “activated B-cell” (ABC) and “germinal center B-cell” (GCB) types that are predictive of disease aggression and outcome [14, 15]. The immunohistochemical Hans classifier, based on CD10, BCL6, and MUM1 expression, is routinely used as gene expression profiling surrogates to distinguish the two subgroups in the setting of systemic DLBCL [16]. While recent data does suggest a predominance of the non-GCB phenotype among PCNSL cells, this classification is of limited clinical utility, as no association of COO subtype with clinical outcome has been demonstrated among patients with PCNSL [17, 18].
Myc expression has also been evaluated in PCNSL tumor cells given the poor prognosis of PCNSL compared to systemic DLBCL. Myc rearrangements, as detected by fluorescent in situ hybridization (FISH), occur in roughly 10–15 % of DLBCLs and confer poor clinical outcome, especially when paired with rearrangements of the BCL-2 gene (i.e., “double-hit lymphomas”) [19, 20]. In PCNSL, despite the low frequency of Myc rearrangements (3–9 %), Myc protein overexpression is common (73–92 %) suggesting other mechanisms for Myc overactivity [17, 18, 21]. However, in the limited data available, Myc overexpression did not correlate with adverse clinical outcome. Taken together, this suggests that prognostic markers used in nodal DLBCL are of limited utility in PCNSL. This is likely due to differences in disease biology.
Diagnosis and Work-Up
When there is suspicion for PCNSL, the initial radiographic study of choice is a contrast-enhanced cranial MRI. Despite the absence of a pathognomonic MRI characteristic, there are several radiographic findings suggestive of PCNSL [22]. Lesions are typically solitary (in 2/3 of cases) involving the supratentorial brain with ventricular or meningeal surface contact. Edema is prominent; however it is less than observed in malignant gliomas or metastatic lesions. Given the hypercellularity and high nuclear to cytoplasmic ratio of PCNSL tumor cells, the predominant mass is usually isointense on T1 and T2 sequences and exhibits restricted diffusion on diffusion-weighted imaging. Diffuse and homogeneous contrast enhancement is the rule. In cases of immunosuppressed patients with PCNSL, ring enhancement is frequent. Necrosis, hemorrhage, and calcifications are rare.
Stereotactic-guided biopsy is the method of choice for obtaining tissue for definitive diagnosis. If possible, it is highly recommended that glucocorticoid administration be avoided prior to biopsy, as there is a potential for dramatic steroid-induced tumor regression that will result in a nondiagnostic biopsy [23]. In the absence of a contraindication to lumbar puncture, a cerebrospinal fluid (CSF) sample should be obtained. CSF studies include cytology, flow cytometric analysis, cell count, and protein and glucose levels. Concurrent systemic involvement should be excluded with bone marrow biopsy and positron emission tomography-computed tomography (PET-CT) imaging with or without contrasted CT imaging of the chest, abdomen, and pelvis. A detailed ophthalmologic evaluation with a slit lamp examination should be performed to screen for intraocular involvement that often manifests as a cellular infiltration of the vitreous and subretinal tumor cell deposits. Serum studies including HIV, blood urea nitrogen (BUN), creatinine, lactate dehydrogenase (LDH), and hepatitis B and C serologies are also part of baseline work-up evaluation. Ultrasound of the testis should be considered in elderly men who present with DLBCL involving the CNS.
Case 1
The patient initiated a systemic therapy with rituximab, methotrexate, procarbazine, and vincristine (R–MPV). Restaging MRI after 2 cycles of therapy demonstrated improvement (Fig. 8.2a). She completed a total of five cycles of therapy. Repeat MRI revealed a complete regression of the known lymphoma involvement within the left frontal lobe and right ventricle (Fig. 8.2b). She was then referred for consolidative low-dose whole brain radiation therapy (WBRT).
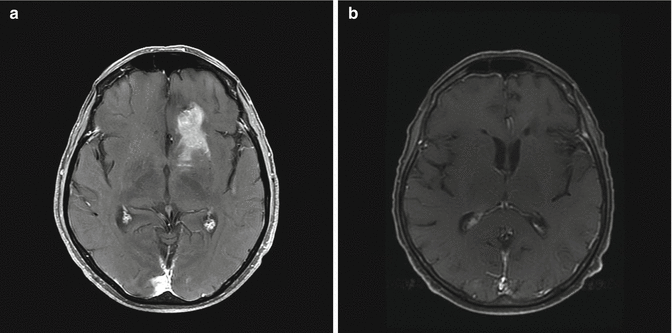

Fig. 8.2
MRI after methotrexate-based chemotherapy. T1 post-contrast axial image demonstrates a partial response after 2 cycles of R-MPV (rituximab, methotrexate, procarbazine, and vincristine) (a) and a complete response after 5 cycles (b)
Treatment
Surgery
It has been widely accepted that aggressive surgical resection has little role in the management of PCNSL given a potential increased risk of permanent neurologic deficit coupled with no evidence of improvement in overall survival [4, 24]. However, this standard has been recently challenged by a retrospective analysis of a large phase III randomized trial, the German PCNSL Study Group-1 (GPSG-1) trial. In this analysis of several hundred patients, compete resection was associated with an increase in progression-free survival (PFS) [25]. In light of this observation, many centers will consider a complete surgical resection of well-circumscribed single lesions in non-eloquent areas. Otherwise, stereotactic-guided biopsy should be considered standard of care, given the lower risk of postoperative morbidity.
Radiation Therapy Alone
Historically RT has been the cornerstone of therapy for PCNSL, a highly radiosensitive tumor. Responses to whole brain RT (WBRT) are rapid and robust with an overall response rate (ORR) of greater than 90 %. Unfortunately however these responses are short lived. The Radiation Therapy Oncology Group (RTOG) conducted a phase II prospective trial between 1983 and 1987 of WBRT alone for patients with PCNSL (RTOG 83–15) [26]. Forty-one patients were enrolled and received RT to the whole brain to a dose of 40 Gy followed by a 20 Gy boost to gross disease. The results were disappointing with a median overall survival (OS) of only 11.6 months. The dominant pattern of failure was local, with 61 % failure rate within the radiated brain field in contrast to 7 % distant failure rate. One- and two-year OS rates were 48 % and 28 %, respectively. Of the 25 documented local recurrences, 22 (88 %) were within the 60 Gy region. Given the inability to further dose escalate without excessive severe toxicity such as brain necrosis, these data highlighted the need for therapy beyond RT alone to improve outcomes among patients with newly diagnosed PCNSL.
Combined Modality Therapy
Given the disappointing results of the RTOG 83–15 trial, subsequent studies focused on the potential of combined modality therapy (CMT) to improve outcomes. In RTOG 88–06, cyclophosphamide, doxorubicin, vincristine, and dexamethasone (CHOD) were administered prior to 41.4 Gy in 1.8 Gy fractions to the whole brain followed by a boost of 18 Gy, for a cumulative tumor bed boost of 59.4 Gy [27]. Unfortunately results from this prospective study were on par with outcomes obtained with RT alone. The median OS was 16.1 months with a 2-year OS rate of 42 %. It is hypothesized that the ineffectiveness of CHOP like regimens relates to the poor penetration of these agents across the blood-brain barrier (BBB).
The dilemma of inadequate BBB penetration of systemically administered chemotherapeutic agents has been addressed with the implementation of high-dose Mtx-based regimens. Rapid infusion of greater than 3 g/m2 Mtx intravenously (IV) results in consistent CSF concentrations that have antitumor activity in the CNS [28, 29]. Leucovorin must be administered to prevent bone marrow and end organ damage (especially kidneys), while lymphoma cells in the CNS are offered little rescue given the poor BBB penetration of leucovorin. In one of the first studies to evaluate high-dose Mtx-based regimens in patients with PCNSL, DeAngelis and colleagues at the Memorial Sloan Kettering Cancer Center (MSKCC) evaluated pre-RT IV and intra-Ommaya Mtx followed by cranial RT (40 Gy to the whole brain followed by 14.4 Gy boost) and consolidative high-dose Ara-C among 31 patients with PCNSL [30]. In a nonrandomized fashion, outcomes were compared with 16 patients treated with RT alone. Median OS was improved among patients treated with combined modality therapy (42.5 months) versus RT alone (21.7 months). The median time to recurrence was also improved (41 months versus 10 months). These improvements in OS were reproducible in other studies, with a median OS of 32–36 months reported when patients were treated with combined modality therapy [31–33].
The improvement in clinical outcome with Mtx-based CMT did not come without a cost. With longer follow-up, profound neurotoxicity was appreciated, with patients older than 60 being at the highest risk. In long-term follow-up of the 31 patients treated with CMT at MSKCC, delayed neurotoxicity was reported in 10 patients (32 %) [34]. At 48-month follow-up, 100 % of patients 60 years or older developed neurotoxicity compared with 35 % of patients less than 60. For patients greater than 60 years of age, symptoms began at a median of 13.2 months (range 6–52 months) and consisted of dementia, urinary incontinence, and gait ataxia often requiring custodial care. The neurotoxicity was fatal in three patients. MRI findings consisted of atrophy and diffuse white matter changes in the absence of disease recurrence. Given the high incidence of long-term neurotoxicity after Mtx and high-dose whole brain RT (40 Gy with 14.4 Gy boost), the focus of subsequent studies shifted to the omission or delay of WBRT.
Omission of WBRT
In the largest randomized trial conducted in PCNSL, the G-PCNSL-SG-1 trial sought to evaluate whether high-dose Mtx-based chemotherapy alone was not inferior to the same chemotherapy regimen followed by whole brain RT to 45 Gy in 30 fractions of 1.5 Gy daily without a boost. 551 patients were enrolled at 74 German centers, but only 318 patients were treated according to protocol. Among these 318 patients, the median OS was 32.4 months among patients that received WBRT, and 37.1 months in patients treated with chemotherapy alone (p = 0.71). Median progression-free survival (PFS) was improved among patients treated with WBRT but was not statistically significant (18.3 months versus 11.9 months, p = 0.14). Treatment-related neurotoxicity was lower among patients who had RT omitted (49 % versus 26 %). The authors concluded that the PFS benefit of WBRT must be weighed against the increased risk of neurotoxicity, given the lack of demonstrable OS benefit. Many in the medical oncology community regarded the results of the trial as a justification for permanent omission of WBRT as part of upfront therapy for patients with PCNSL. However this trial is fraught with many limitations that restrict the ability to make definitive conclusions regarding the role of consolidative WBRT. The trial was drastically underpowered (~60 %) to address the primary hypothesis, and therefore unplanned subset analyses were even further underpowered. Poor protocol adherence was common with major violations noted in 30 % of patients. For instance, 29 % of patients who did not achieve complete response (CR) in the no RT arm actually received WBRT, while 24 % of patients assigned to receive cranial RT did not receive it. Additional limitations of the trial included suboptimal chemotherapy use, the absence of quality assurance, and insufficient neurotoxicity evaluation. Finally only 58 % of the original cohort was included in the analysis which undoubtedly undermines the results of the study. It has also been suggested that the unbalanced effect from salvage therapy between the arms that favored the experimental no radiation cohort also undermines conclusions drawn from this trial [35].
Reduced Dose WBRT
In an effort to maintain the potential benefit of consolidative WBRT in improving PFS while limiting risk of long-term neurotoxicity, the group at MSCKK pioneered a reduced dose WBRT (rdWBRT) approach after high-dose Mtx-based polychemotherapy. In the multicenter phase II trial of 52 patients with median age of 60 years (range 30–79) patients were treated with 5–7 cycles of rituximab, Mtx (3.5 g/m2), procarbazine, and vincristine (R-MPV) followed by rdWBRT to 23.4 Gy in 1.8 Gy fractions and 2 cycles of consolidative high-dose Ara-C if CR was achieved. 31 patients achieved CR and received rdWBRT after R-MPV. For this group of patients, the median PFS was 7.7 years, and the median OS was not reached at a median follow-up of 5.9 years. 2-year PFS was 77 % and 3-year OS was 87 %. Rigorous neurocognitive testing was a component of the trial, and no severe long-term neurotoxicity was demonstrated. Disease progression occurred in 35 % of patients treated with rdWBRT (n = 11); two patients developed ocular relapse and nine patients had parenchymal brain relapse. Given these impressive results with minimal neurotoxicity, the role of rdWBRT is being evaluated in a randomized fashion by the RTOG (RTOG 1114).
Autologous Stem Cell Transplant
Efforts to identify strategies to augment therapy in the absence of WBRT continue at many centers. Recently the interest in consolidative high-dose chemotherapy and autologous stem cell transplant (ASCT) has increased. Theoretically the high concentration of chemotherapy drugs given IV will allow for concentrations in the CSF adequate to provide cytotoxic effects. Initial studies using a common regimen in systemic DLBCL, BEAM (BCNU, etoposide, Ara-C, melphalan), have been disappointing with 3-year event-free survival (EFS) of 25 % and 3-year OS of 60 % [36]. When conditioning regimens containing agents known to penetrate the CNS are utilized (such as thiotepa), the outcomes improve. In a single publication reporting on the outcome of two prospective single arm studies that treated patients with Mtx-based chemotherapy followed by high-dose carmustine and thiotepa and ASCT with or without WBRT, the median OS was 104 months with 5-year OS of 70 %[37]. The group at MSKCC recently published their results with R-MPV followed by high-dose chemotherapy and ASCT with TBC (thiotepa, busulfan, and cyclophosphamide) [38]. Among 32 patients, 81 % proceeded to frontline ASCT. With a median follow-up of 45 months, median PFS and OS were not reached, and 2-year PFS and OS were 79 % and 81 %, respectively. To date there is no evidence of neurotoxicity although follow-up time is limited. WBRT was omitted among patients who received ASCT. The results of R-MPV followed by rdWBRT compare favorably with this trial; however it should be noted that in the ASCT study, there were three transplant-related deaths, while the rdWBRT phase II study was without mortality related to consolidative WBRT. Two ongoing randomized trials being conducted by the International Extranodal Lymphoma Study Group and ANOCEF/GOELAMS will provide key data regarding the clinical benefit and toxicity associated with consolidative WBRT versus ASCT after Mtx-based polychemotherapy. (NCT01011920 and NCT00863460).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree