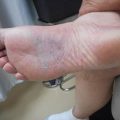
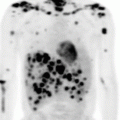
Fig. 10.1
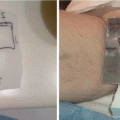
MCL involving the inferior bulbar conjunctiva of the left eye
A careful history and physical examination was performed. The patient had an excellent performance status. He had no symptoms attributable to the eye. He denied constitutional symptoms of fever, night sweats, and weight loss. The conjunctival lesion was the only abnormality appreciated on physical examination. Special attention was paid to the nodal basins, liver, and spleen, all of which were unremarkable.
Pathology, Early-Stage Case
An incisional biopsy of the conjunctival mass was performed. It should be noted that a fine-needle aspiration is typically insufficient to make the diagnosis of MCL. The biopsy revealed a glandular epithelium, largely replaced by lymphocytes with features typical of MCL, which arises from immature B-cells. The neoplastic cells tend to be small to medium sized, with irregular nuclear contours, inconspicuous nucleoli, and scant cytoplasm.
This patient’s neoplasm was noted to have a diffuse pattern of growth. Three histological patterns (mantle zone, nodular, and diffuse) and three cytological variants (classical, blastoid, and pleomorphic) have been described. Data suggest that these pathological differences correlate with clinical behavior, for example, pleomorphic and blastoid variants demonstrate a particularly aggressive course [1, 2].
The immunohistochemistry results for this case were typical for MCL. The cells were positive for B-cell markers (including CD19, CD20, and PAX5), CD5, CD43, and cyclin D1; they were negative for CD10 and CD23. The translocation t(11;14) was detected by fluorescent in situ hybridization (FISH). This genetic anomaly is regarded as the primary genetic event in MCL. It places the cyclin D1 gene downstream of the IgH promoter, resulting in the overexpression of cyclin D1 and consequent accelerated passage through the cell cycle [1]. It should be noted, however, that a small subset of MCL cases is negative for the t(11;14) translocation and for cyclin D1 overexpression [3, 4]. A proportion of these cases bear translocations involving cyclin D2 and cyclin D3 [5, 6]. SOX11 expression may be useful for the identification of the cyclin D1-negative subtype [7].
Staging and Prognostic Factors, Early-Stage Case
A complete staging evaluation was performed. Bloodwork, including a complete blood count (CBC) with differential, comprehensive metabolic panel (CMP), β2-microglobulin, and lactate dehydrogenase (LDH), was within normal limits. A contrast-enhanced PET-CT scan revealed no other sites of disease. A bone marrow biopsy, which must be performed in all cases, was negative for lymphoma by histology and flow cytometry. An esophagogastroduodenoscopy and colonoscopy with random biopsies showed no evidence of lymphoma. Evaluation of the gastrointestinal tract is critical to establish the diagnosis of limited stage disease, as occult involvement by MCL is detected in the majority of patients [8]. CNS involvement is exceedingly rare; therefore, CSF analysis and neuroimaging should be reserved for patients with neurological symptoms, blastoid histology, or Ki-67 > 30 % [9, 10]. Thus, in this patient, a complete staging evaluation, including bloodwork, PET-CT scan, bone marrow biopsy, and gastrointestinal endoscopy, confirmed that his disease was limited to the left conjunctiva.
Only 10 % of MCL cases are early stage at diagnosis [11]. Anytime the diagnosis of localized MCL is suspected, a thorough evaluation must be performed to exclude the presence of occult systemic disease. The proportion of patients diagnosed with limited stage disease has been decreasing over time, likely due to more sensitive assays of the bone marrow and more frequent evaluation of the gastrointestinal tract, both of which are commonly involved by disease [11, 12].
As observed in this case, extranodal disease is common in MCL. The most frequently involved sites are the gastrointestinal tract, head and neck, and reticuloendothelial system. Less commonly involved extranodal sites include the lung, skin, musculoskeletal system, and breast. Data suggest that the primary site of disease involvement is a predictor of survival. For example, patients with primary disease of the head and neck have superior survival, compared to those with primary nodal disease (P < 0.001) [13].
Treatment and Management, Early-Stage Case
For his stage IAE MCL of the left conjunctiva, this patient was treated with three cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone). He had a complete clinical and radiographic response to chemotherapy. Then, he received consolidative RT to the initially involved site.
Because early-stage MCL is rare, data regarding its management and outcomes are limited. A large series, reported by the International Lymphoma Radiation Oncology Group (ILROG), consists of 160 stage I–II MCL patients treated at 13 institutions. The majority of patients (59 %) received chemotherapy followed by consolidative RT. The remaining patients were treated with chemotherapy alone (23 %) or definitive RT alone (18 %). The most common chemotherapy regimen was R-CHOP or R-CHOP-like, and RT was to a median dose of 35 Gy (range 12–45 Gy). The authors reported favorable outcomes for the cohort, irrespective of the modality of treatment given, with disease-free and overall survival rates of 65 and 76 % at 5 years and of 44 % and 63 % at 10 years, respectively [14]. These findings demonstrate that patients with limited stage MCL can achieve long-term disease control even with radiation therapy alone and de-escalation of therapy should be considered in early-stage presentation.
A smaller series from the British Columbia Cancer Agency suggests an important role of RT in the management of limited stage MCL. The authors reported on 26 patients with non-bulky-stage IA–IIA MCL. They observed superior progression-free survival in patients whose initial therapy consisted of RT ± chemotherapy, when compared to those who did not receive RT (68 % vs. 11 % at 5 years, P = 0.002). Furthermore, there was a trend toward improved overall survival (71 % vs. 25 % at 6 years, P = 0.13). All patients with PFS beyond 6 years had received RT as a part of their upfront management [15]. ILROG trial.
Researchers from Princess Margaret Hospital reported on 21 patients with stage I–II MCL who were managed with curative intent. Among the 17 patients treated with a combination of chemotherapy (typically CHOP or R-CHOP) and RT (median 35 Gy), the overall response rate was 100 % (15 complete response, 1 unconfirmed complete response, 1 partial response). For five patients, a partial or unconfirmed complete response after chemotherapy was transformed to a complete response after RT. Local control was maintained in all but one patient, whose disease relapsed both locally and distantly. Distant relapse was common. The 5-year progression-free survival rate was 43.8 %, with a median time to event of 3.2 years. The median overall survival time was 6.4 years [16]. It is worth to mention that these patients were subsequently included in the aforementioned ILROG study.
Lastly, an analysis using the National Cancer Database (NCDB) assessed outcomes of early-stage MCL. The authors identified 2539 patients with stage I–II MCL. Of these, 70 % were treated with chemotherapy alone, 11 % with RT alone, and 19 % with a combination of chemotherapy and RT. Patients who received combined modality therapy experienced significantly better overall survival than patients treated with either chemotherapy or RT alone (3-year overall survival 79.8 % for chemotherapy + RT, 67.8 % for chemotherapy alone, 72.4 % for RT alone, P < 0.001). After correction for indication bias through inverse probability treatment weighting, combined modality therapy was found to reduce the risk of overall mortality compared with monotherapy (hazard ratio 0.65, P = 0.029) [17].
Taken together, these studies suggest that the incorporation of RT into management is associated with improved outcomes in limited stage MCL. Patients who are fit should receive chemo-immunotherapy followed by consolidative RT. RT alone is appropriate in patients considered unsuitable for systemic therapy [1].
Radiation Field, Dose, and Technique, Early-Stage Case
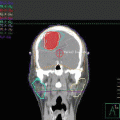
In this case, consolidative RT targeted the entire left conjunctiva. Technique varies between institutions, some uses electron, and others use photon with intensity-modulated radiation therapy (IMRT). In case 1, treatment was delivered using a single appositional 16-MeV electron beam. The total dose of 24 Gy was given at 2 Gy per fraction (Fig. 10.2a). Custom skin collimation was used to sharpen the penumbra and protect adjacent structures; in this case since we used 16 MeV, the eyelid served as a bolus, and patient was treated with closed eyes. It should be looked at on a case-by-case basis to make sure that the target is well covered. It is also felt that when the conjunctiva is involved, the whole orbit has to be treated as opposed to when the lacrimal gland is involved, only targeting the lacrimal gland is acceptable.
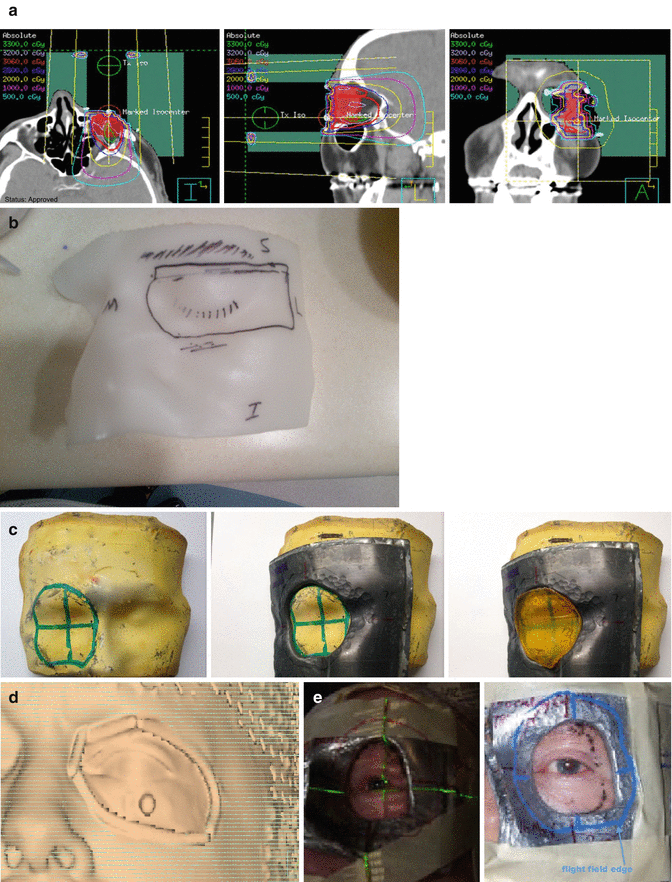

Fig. 10.2
(a) Axial, sagittal, and coronal slices from an RT plan that targeted the left conjunctiva, using a single appositional 16-MeV electron beam. The total dose of 24 Gy was given at 2 Gy per fraction. Custom skin collimation was used to reduce penumbra and protect adjacent structures. (b) Aquaplast mold used to template the face. (c) Clay mold showing how to mark for the skin collimation (1), the skin collimation (2), and (3) the bolus added to bring the dose superficially. (d) Skin rendering used to mark the skin collimation on the clay mold. (e) Patient daily setup
The step of planning starts with the simulation where the physician outlines the tentative area of interest using CT wires; this area can be later easily identified by the planning team as the presumed edge of the skin collimation. CT simulation scan will be obtained, the eye will be contoured as the CTV, and plan will be generated taking into consideration that a skin collimation will be used. At the same time, the skin collimation making will start as follows: an aquaplast material is used to template the face and the eye during the simulation (Fig. 10.2b) and will be used as the negative to produce a clay material (Fig. 10.2c 1, 2, 3), the skin collimation will be drawn on the clay by the physician, and the shape of the skin collimation will be copied from the skin rendering from the treatment planning system (Fig. 10.2d). The skin collimation thickness will be produced according to the electron energy used. It is advisable on day one to verify with the area treated by applying the skin collimation and generating the field using CT wires and rerun the plan on the new scan to make sure we are not missing the target (Fig. 10.2e).
The ILROG RT guidelines for nodal and extranodal non-Hodgkin lymphomas are a valuable reference for the radiation oncologist treating MCL [18, 19]. As described in the guidelines, an involved site RT (ISRT) approach should be used, in which the gross tumor volume (GTV) equates to the clinically and radiographically evident tumor, the clinical target volume (CTV) encompasses the GTV as well as potential sites of microscopic disease, the internal target volume (ITV) considers uncertainties in position of the CTV within the patient, and the planning target volume (PTV) accounts for differences in patient positioning with each treatment. In patients treated with consolidative RT after a complete response to chemotherapy, RT should target the pre-chemotherapy site of disease, taking into consideration changes in anatomy following disease response. In patients who are not candidates for or decline chemotherapy, RT may be used as a single modality; in this setting, a more generous area may be irradiated to account for adjacent microscopic disease.
MCL responds to low RT doses. M’kacher et al. demonstrated the exquisite radiosensitivity of MCL cells in vitro [20]. These findings have been supported by clinical experience. When RT is used with curative intent, a dose of 24–30 Gy is recommended in most cases; however, as described below, lower doses may be preferable in certain settings [18].
Posttreatment Considerations, Early-Stage Case
This patient developed dryness and irritation of the eye near the end of RT. His symptoms responded well to lubricating eye drops and had improved significantly by 1 month after treatment.
After the completion of therapy, he was seen in routine follow-up every 3–6 months for 5 years, and annually thereafter. Each follow-up visit included a history and physical examination, bloodwork (including a CBC, CMP, and LDH), and a contrast-enhanced CT scan of the neck, chest, abdomen, and pelvis. Six years after the completion of treatment, this patient remained clinically and radiographically without evidence of disease.
Advanced-Stage Mantle Cell Lymphoma
Clinical Presentation, Advanced-Stage Case 1
A 69-year-old man presented with a sore throat. He was prescribed several courses of antibiotics, which did not provide relief. Ultimately, he was referred to an otolaryngologist, who appreciated an oropharyngeal mass. A biopsy of the mass confirmed MCL.
The patient had an excellent performance status. His only symptom was throat pain. He denied fevers, night sweats, and weight loss. A physical examination was notable for lymphadenopathy of the neck, axillae, and inguinal regions. The largest node, in the left neck, measured 6 cm. No hepatosplenomegaly was appreciated.
Pathology, Advanced-Stage Case 1
This patient’s oropharyngeal biopsy revealed MCL, blastoid variant. The pharyngeal mucosa was extensively replaced by lymphoma. The neoplastic cells were positive for CD5, CD20, PAX5, and cyclin D1; they were negative for CD10. The anti-Ki-67 antibody showed a proliferation rate of 90 %. Translocation t(11;14) was detected by FISH.
Blastoid or blastic variant MCL describes cases with a homogeneous population of cells displaying lymphoblastic morphology. It represents 10–15 % of MCL cases. Patients with blastoid variant MCL experience particularly short durations of response after chemotherapy and poor overall survival [2].
Staging and Prognostic Factors, Advanced-Stage Case 1
A complete staging evaluation was remarkable for an elevated white blood cell count (WBC, 19,000/μL) and LDH level (1400 IU/L). A PET scan showed a hypermetabolic mass involving the oropharynx and multi-compartmental adenopathy above and below the diaphragm. A bone marrow biopsy revealed involvement by disease, with MCL comprising 10 % of the cellularity.
The MCL International Prognostic Index (MIPI) is a validated prognostic stratification tool for patients with advanced-stage MCL. The formula takes into account age, performance status, LDH level, and WBC to divide patients into low-, intermediate-, and high-risk groups, which have markedly different outcomes. In the initial publication, the median survival of the high-risk group was 29 months, of the intermediate-risk group was 51 months, and of the low-risk group had not been reached at a median follow-up of 32 months (5-year overall survival rate of the low-risk group was 60 %) [21, 22]. Variations of the MIPI have been introduced. The simplified MIPI is calculable at the bedside, with similar concordance but lower discriminatory power. The biologic MIPI incorporates the Ki-67 proliferation index, which improves the discriminatory power for progression-free survival [23]. These scores may be used to develop individualized, risk-adapted treatment plans. These formulas placed this patient in the high-risk category, so his outcome was expected to be poor, and aggressive management was indicated.
Treatment and Management, Advanced-Stage Case 1
For his high-risk disease, this patient received induction treatment with four cycles of rituximab and dose intensified CHOP (maxi-CHOP) alternating with rituximab and high-dose cytarabine. His disease responded completely. Then, he underwent autologous stem cell transplantation (ASCT) with a conditioning regimen of carmustine, etoposide, cytarabine, and melphalan (BEAM). This regimen, published by the Nordic Lymphoma Group [24], is one of a variety of approaches used to intensify therapy and improve outcomes in MCL.
CHOP chemotherapy, used historically to treat MCL, was associated with poor outcomes. Early studies demonstrated that the addition of rituximab to CHOP improved the overall response rate (94 % vs. 75 %), complete response rate (34 % vs. 7 %), and median time to treatment failure (21 months vs. 14 months); however, no improvement was observed in progression-free survival (25 % at 2 years) or overall survival (76 % at 2 years) [25]. Given these poor outcomes, intensification of therapy has been studied. One approach that has demonstrated promising outcomes is first-line consolidation with high-dose chemotherapy (HDC) and ASCT. Multiple groups have reported their favorable experiences [26, 27]. As one example, the Nordic group published the regimen that was used to treat this patient. In their trial, MCL patients who responded to induction therapy with rituximab and maxi-CHOP alternating with high-dose cytarabine were subsequently consolidated with HDC/ASCT. This regimen resulted in excellent 6-year progression-free and overall survival rates of 66 % and 70 %, respectively [24]. Further follow-up showed an impressive median event-free and overall survival of 7.4 years and over 10 years, respectively [28]. An alternative approach that does not incorporate ASCT is rituximab in combination with fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyper-CVAD), alternating with high-dose methotrexate and cytarabine (R-hyper-CVAD/MA). This intensive regimen has yielded favorable outcomes. For example, in the SWOG 0213 study, R-hyper-CVAD/MA was associated with a median progression-free survival of 4.8 years and overall survival of 6.8 years; the 2-year progression-free and overall survival rates were 63 % and 76 %, respectively [29].
This patient tolerated intensified chemotherapy and ASCT well. However, 3 weeks after ASCT, he developed rapidly progressive oropharyngeal swelling. A tonsillar biopsy confirmed refractory MCL. Of note, in the Nordic Study, high MIPI score and Ki-67 expression level were independent predictors of poor outcome [28]. This patient had both of these risk factors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree