T
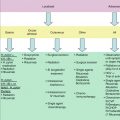
T1: Solitary skin involvement
T1a: A solitary lesion <5 cm diameter
T1b: A solitary >5 cm diameter
T2: Regional skin involvement: multiple lesions limited to one body region or two contiguous body regions*
T2a: All-disease encompassing in a <15-cm-diameter circular area
T2b: All-disease encompassing in a >15- and <30-cm-diameter circular area
T2c: All-disease encompassing in a >30-cm-diameter circular area
T3: Generalized skin involvement
T3a: Multiple lesions involving two noncontiguous body regions
T3b: Multiple lesions involving >3 body regions
N
N0: No clinical or pathologic lymph node involvement
N1: Involvement of one peripheral lymph node region† that drains an area of current or prior skin involvement
N2: Involvement of two or more peripheral lymph node regions† or involvement of any lymph node region that does not drain an area of current or prior skin involvement
N3: Involvement of central lymph nodes
M
M0: No evidence of extracutaneous non-lymph node disease
M1: Extracutaneous non-lymph node disease present
19.3 Definitions and Clinical Features
19.3.1 Primary Cutaneous Marginal Zone Lymphomas (PCMZL)
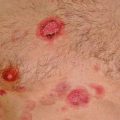
Primary cutaneous marginal zone lymphomas of MALT type (PCMZL), previously known as primary cutaneous immunocytomas, comprises 24 % of all primary cutaneous B-cell lymphomas (Senff et al. 2007). They present as single or multiple lesions, with multifocal lesions being more common (72 %) (Hoefnagel et al. 2005a). MZL affects most commonly adults between third and fifth decade of life with a male to female ratio of 2.1. MZL presents as red to violaceous infiltrated cutaneous and subcutaneous plaques or multifocal nodules with a diameter of less than 2 cm (Golling et al. 2008). Over half of patients (55 %) have lesions on the trunk with upper and lower extremities being involved in 37 and 27 % of patients, respectively. The head and neck are involved in 14 % of patients. The tumors display slow growth and usually do not ulcerate. Biopsies should be deep in order to reflect the extent of the infiltrate. In Europe there have been cases of CMZL associated with Borrelia burgdorferi infection (Hoefnagel et al. 2005a; Aberer et al. 2011). An association of primary cutaneous MALT lymphomas with infectious etiologies (similar to H. pylori and gastric MALT lymphomas) has been postulated for many years, and Borrelia burgdorferi has been suggested and found in a fraction of cases in some series; however, recent reports are contradictory (Cerroni et al. 1997; Goodlad et al. 2000a; Ponzoni et al. 2011; Wood et al. 2001). Although still debatable, it has been suggested that MZL is associated with chronic inflammatory process or infections (Zendri et al. 2005; May et al. 2005). H pylori infection is often found in MALT lymphomas of the gastric mucosa and intestine, and remission has been induced by treatment of the underlying infection. This may also hold true for a subset of patients with cutaneous B-cell lymphomas (Bogle et al. 2005).
19.3.2 Primary Cutaneous Follicle Center Lymphoma (PCFCL)
PCFCL is the most common type of primary cutaneous B-cell lymphomas, making up 57 % of cases in a recent large review. The median age at diagnosis is 58 years with a male/female ratio of 1.8 (Senff et al. 2007). It presents as an erythematous papule, plaque, or nodule most commonly located on the trunk or head/neck. Lesions may be single or multiple but are localized when multiple. It is only rarely seen on the upper (2.3 % of patients) or lower (6.4 % of patients) extremities. The latter is at times difficult to differentiate from DLBCL, LT.
19.3.3 Primary Cutaneous Diffuse Large B-Cell Lymphoma, Leg Type (PCDLBL-LT)
PCDLBCL-LT comprises approximately 20 % of primary cutaneous B-cell lymphomas and has several distinctive clinical features. Compared to the above two types, this lymphoma occurs in an older population (median age 78 years) and has a striking female predominance (M:F ratio of 0.5). As the name implies, it presents most commonly (88 % of patients) on the lower extremity. However, it can occur at other sites including the head/neck, trunk, and upper extremities in 5–12 % of patients (Willemze et al. 2005; Senff et al. 2007). Clinically it presents as a nodule, either singly or as multiple regional lesions. Multifocal disease is seen in 20 % of cases. Uncharacteristic to other cutaneous lymphomas, PCDLBCL-LT will often disseminate to nodal and visceral site, which likely portends a transition in its already aggressive behavior (Grange et al. 2007; Vermeer et al. 1996).
19.4 Pathology
PCMZL manifests as a mid-dermal lymphoid infiltrate that can extend into the superficial dermis and deep dermis/subcutis. As in other marginal zone lymphomas, reactive germinal centers are usually present but may become colonized and eventually obliterated. The cells are small with condensed chromatin, slight nuclear irregularities, and moderate-to-abundant amounts of pale cytoplasm. These marginal zone cells are often centered on the residual lymphoid follicles and expand into the surrounding dermis (Fig. 19.1). Admixed plasma cells may be present. These can represent plasmacytic differentiation of the lymphoma or an inflammatory component. Dutcher bodies, if present, would favor the former and investigation of the plasma cell component by immunohistochemistry or in situ hybridization for kappa and lambda immunoglobulin light chain expression is advisable. Although lymphoepithelial lesions (destructive infiltration of epithelium by clusters of lymphoma cells) are common in MALT-type lymphomas at sites such as stomach or salivary glands, they are not frequently seen in PCMZLs. When present they are usually seen in hair follicle epithelium. Some cases may have an extensive reactive, nonneoplastic lymphoid infiltrate and may be the type composed of class-switched PCMZL (Edinger et al. 2010). An eosinophilic infiltrate has been described in cases originating in Asia (Takino et al. 2008).
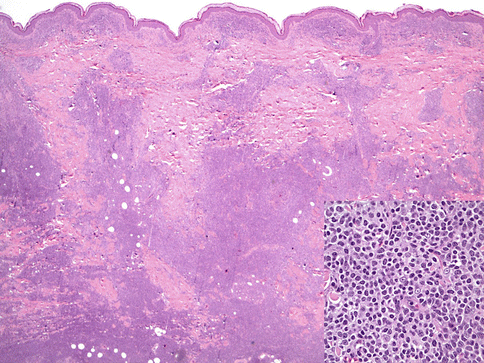

Fig. 19.1
Primary cutaneous marginal zone lymphoma. A dense lymphoid infiltrate is seen (hematoxylin and eosin, H&E, 20×). The inset shows the cytologic features of marginal zone cells (H&E 400×)
PCFCL has a heterogeneous appearance. The common features are a dense dermal lymphoid infiltrate of follicle center cells (varying proportions centrocytes and centroblasts). The architecture may be follicular, follicular and diffuse, or completely diffuse. Follicles may have attenuated or absent mantle zones, are not polarized, and lack tingible body macrophages. In diffuse examples that are composed of predominantly centroblastic cells, the histopathology may be that of a diffuse large B-cell lymphoma in other sites; however, the appropriate diagnosis given the anatomic site is still PCFCL (Fig. 19.2) (Cerroni and Kerl 2001a, b; Goodlad et al. 2002; Mirza et al. 2002). Thus, this lymphoma is defined more by the type of cells (follicle center cells) rather than architecture.


Fig. 19.2
Primary cutaneous follicle center lymphoma. A dense dermal lymphoid infiltrate is present (Fig. 19.2, H&E 400×) and is composed of follicle center-type cells (Inset, H&E 400×)
PCDLBL-LT shows a diffuse architecture and is entirely composed of large immunoblastic or centroblastic cells with round nuclear contours and variably prominent nucleoli (Fig. 19.3) (Vermeer et al. 1996). The dominant round cell morphology contrasts with PCFCL. Mitotic figures are easily found and characteristically there are few infiltrating small reactive lymphocytes.
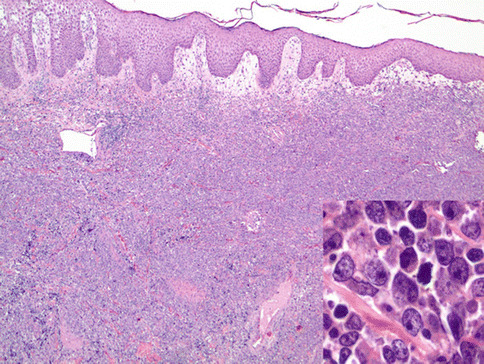

Fig. 19.3
Diffuse large B-cell lymphoma, leg type. A dense dermal lymphoid infiltrate is present and is composed of monotonous round large cells
19.5 Immunophenotype
The immunophenotype of PCMZL is CD19+, CD20+, CD5−, and CD10− with monotypic immunoglobulin light chains often demonstrable in paraffin sections (Fig. 19.4). The cells are often class switched (IgG, IgA, IgE), unlike other types of extranodal marginal zone B-cell lymphomas. BCL2 is expressed in most cases (Edinger et al. 2010; Servitje et al. 2002; Cho-Vega et al. 2006). The characteristic presence of reactive follicles can be highlighted by a CD21 stain that marks follicular dendritic cells.


Fig. 19.4
Primary cutaneous marginal zone lymphoma. The upper left panel shows a remnant germinal center (upper left) with clusters of centroblastic cells. The marginal zone component extends into the surrounding dermis. A CD21 stain (upper right) showed one of many remnant follicular dendritic cell network. CD20 is expressed (lower left) and a BCL6 stain (lower left) shows that residual germinal center B cells are present but undergoing colonization by the neoplastic cells
PCFCL expresses pan-B-cell markers CD19 and CD20 but also coexpresses BCL6. Other germinal center B-cell markers are also often expressed including CD10 and HGAL (Xie et al. 2008). Unlike nodal follicular lymphoma, in which BCL2 expression is a hallmark that reflects a t(14;18)(q32;q21), PCFCL is characteristically negative for BCL2 (Fig. 19.5). However, in examples that are follicular, especially when predominantly small cleaved cells, BCL2 is expressed in approximately 40 % of cases (Mirza et al. 2002; Xie et al. 2008).


Fig. 19.5
Primary cutaneous follicle center lymphoma immunohistochemistry. The cells express CD20 (upper left), CD10 (upper right), and BCL6 (lower left) but are negative for BCL2 (lower right)
PCDLBL-LT also expresses CD19 and CD20. Unlike PCFCL, expression of BCL2 is the rule and the post-germinal center B-cell maker MUM1 is usually expressed. BCL6 is expressed by most cases, but CD10 is not (Fig. 19.6) (Xie et al. 2008; Geelen et al. 1998; Grange et al. 2004; Hoefnagel et al. 2003; Sundram et al. 2005).
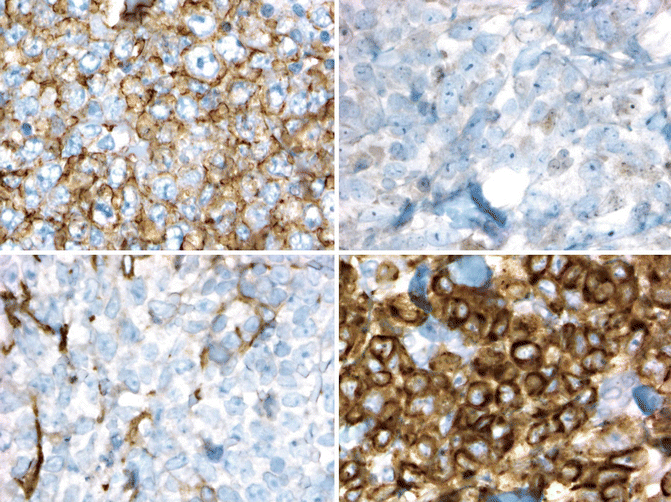

Fig. 19.6
Diffuse large B-cell lymphoma, leg type. Immunohistochemistry shows that the cells express CD20 (upper left) and BCL2 (lower right) but negative for BCL6 (lower right) and CD10
19.6 Molecular Genetics
Application of modern PCR-based methods for determining monoclonality in formalin-fixed tissue has greatly increased our ability to diagnose cutaneous lymphomas. At least 85 % of cases demonstrate monoclonality(Morales et al. 2008). However, since monoclonality can be seen rarely in reactive processes, interpretation in the context of histopathologic and immunophenotypic findings is essential (Morales et al. 2008; Fujiwara et al. 2013; Nihal et al. 2000). t(11;18)(q21;q21) involving API2–MALT1 and t(3;14)(p14;q32) involving FOXP1 and IGH@ are seen in less than 10 % of cases. The t(14;18)(q32;q21) also involving IGH@ and MALT1 is present in less than 15 % of cases (Cho-Vega et al. 2006; Streubel et al. 2004). Molecular studies looking for a causative microorganism similar to H. pylori in gastric marginal zone lymphomas have raised the possibility of Borrelia burgdorferi; however, detection of this organism has not been consistent and a potential role has not been established (Ponzoni et al. 2011; Cho-Vega et al. 2006; Goodlad et al. 2000b; Roggero et al. 2000). The IGH@-BCL2 translocation typically seen in nodal follicular lymphoma can be seen in 0–40 % of cases of PCFCL with a follicular growth pattern (Mirza et al. 2002; Streubel et al. 2006; Cerroni et al. 2000). Variation may be related to technique (Streubel et al. 2006). Gene expression profiling studies have shown that the profile resembles germinal center-like diffuse large B-cell lymphomas (Hoefnagel et al. 2005b).
PCDLBL-LT lacks translocations seen in MALT-type lymphomas or follicular lymphoma. However, translocations of BCL6, MYC, and IGH@ and amplification of BCL2 are commonly seen. Deletion in the region of cell cycle inhibitors CDKN2A and CDK2NB (chromosome 9p21.3) or promoter methylation is frequent and associated with poor outcome (Dijkman et al. 2006; Hallermann et al. 2004). Gene expression profiling shows a distinct profile from PCFCL and similarity to activated B-cell type of diffuse large B-cell lymphoma (Hoefnagel et al. 2005b).
19.7 Differential Diagnosis
The differential diagnosis of PCMZL is often a form of cutaneous lymphoid hyperplasia (CLH) that is B-cell rich due to the presence of reactive follicles. The follicles in CLH should contain preserved mantle zones and the overall immune architecture is preserved, with distinct B- and T-cell areas. Expansion of B cells with a marginal zone appearance away from follicles and demonstration of monotypic plasma cells or monoclonality by molecular methods strongly support lymphoma. As noted above, monoclonality by PCR-based methods can be seen in reactive conditions. In difficult cases, demonstration of the same clone in another lesion or subsequent lesion (identical clone separated in time or space) can help confirm a diagnosis of lymphoma. MZL is at times difficult to differentiate from pseudolymphomas, although increased general awareness between dermatologist and establishment of new markers have facilitated a more accurate diagnosis (Jenni et al. 2011). Skin manifestation of B-CLL can precede its systemic presentation by weeks to months (Cerroni et al. 1996); therefore, the appropriate diagnosis is important since the treatments are completely different. The detection of an immunoglobulin light chain restriction and immunohistochemical staining for CD5, CD23, and CD43 can be helpful in the differentiation of these two entities (Levin et al. 2012). The plasma cell-rich variants of MZL can resemble skin infiltrates by a plasma cell myeloma, but the latter entities can be recognized by adequate staging (Kempf et al. 2012).
PCFCL with a follicular pattern can be differentiated from cutaneous follicular hyperplasia by presence of monomorphous follicles, lack of polarization, and absence of tingible body macrophages in PCFCL. Diffuse forms of PCFCL are usually composed predominantly of large cells, and the diffuse infiltrative pattern of B cells makes CLH unlikely. Differentiation of a diffuse type of PCFCL composed of large cells from PCDLBCL-LT is done on clinical grounds (propensity for the leg of older women), morphology (sheets of centroblasts and immunoblasts), and immunophenotype (B cells that usually express MUM1 and BCL2).
Of course, clinical correlation and staging is required to confirm that the lymphomas represent primary cutaneous disease. It should be noted that bone marrow involvement can be found in up to 11 % of patients with follicle center lymphoma presenting in skin, arguing for routine bone marrow staging studies in these patients (Senff et al. 2008a).
19.8 Prognosis
The 5-year survival rate of 90–95 % for indolent cutaneous BCL is indicative of excellent prognosis. Although cutaneous relapses occur, dissemination to other organs is rare (Cerroni et al. 2000; Garcia et al. 1986).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree