POSTINFECTIOUS ENCEPHALOMYELITIS
KAREN L. ROOS AND AUGUSTO MIRAVALLE
Postinfectious encephalomyelitis is an acute monophasic disorder of the central nervous system (CNS) that occurs within days to weeks of a viral illness or a vaccination. The antecedent viral illness is typically either an upper respiratory tract infection or a nonspecific febrile illness. In the past, most cases were associated with the exanthematous diseases (vaccinia, measles, varicella, and rubella) (1). Although postinfectious encephalomyelitis has a clear temporal relationship with infection or immunization, it is not the result of primary neural tissue invasion by an organism. Infectious agents are rarely identified or recovered from neural tissue (2). The disease is instead an immune-mediated disease triggered by an infectious agent or an immunization.
HISTORY
One of the earliest descriptions of postinfectious encephalitis was recorded in 1790 of a 23-year-old woman who developed symptoms of encephalitis following smallpox (3). This was followed by reports of several neurologic disorders following smallpox infection (4). In 1905, a case of encephalitis after the jennerian cowpox inoculation was reported in France, and another case was observed in the London Hospital in 1912 (5). The disease was recognized as a well-defined entity in 1922, when 11 fatalities due to postinfectious encephalitis were reported in Great Britain (4).
Paralysis and encephalitis is a well-known complication of animal brain tissue–derived rabies vaccine. In developed countries, these have been replaced by the use of commercial tissue culture rabies vaccines, but animal brain tissue–derived rabies vaccines continue to be used in many areas of the world.
In 1941, Weston Hurst (6) described a syndrome with similar clinical presentation to postinfectious encephalomyelitis but with a worse prognosis. This entity was characterized by the presence of petechial hemorrhages around blood vessels, intense numbers of polymorphonuclear leukocytes, perivenular demyelination, necrosis, and fibrin deposits on pathologic examination. This disease was called acute hemorrhagic leukoencephalomyelitis (6).
On the basis of the preceding illness, postinfectious encephalomyelitis has also been called parainfectious, postexanthematous, postvaccinal, and postinfluenzal encephalomyelitis. In reference to the pathology, this illness is also known as acute disseminated encephalomyelitis (ADEM), perivascular myelinoclasis, perivenous encephalitis, acute demyelinating encephalomyelitis, immune-mediated or hyperergic encephalomyelitis, and disseminated vasculomyelinopathy (7). These terms are used interchangeably.
ETIOLOGY
The incidence of postinfectious encephalomyelitis from different causes has been reported to be between 0.4 and 0.8 per 100,000 of population, with a median age of onset of 4.5 to 7.5 years in pediatric studies and 33.5 years in a study of adult patients (8). The disease has a seasonal peak in winter and spring, consistent with its putative infectious etiologies (9,10).
Although postinfectious encephalomyelitis can occur spontaneously, most often it follows a precipitating event (Table 22.1). As stated, the major preceding event is either a viral infection or a vaccination. Measles, varicella, rubella, mumps, and influenza A and B viruses have all been associated with the development of postinfectious encephalomyelitis. The other major inciting event is vaccination, particularly in cases involving enveloped viruses such as smallpox (vaccinia virus) and rabies. In addition to viral infection and vaccination, other less common etiologies associated with postinfectious encephalomyelitis are bacterial infections and autoimmune and hematologic disorders (5,11–33).

Viral Etiologies
Postmeasles encephalomyelitis is the most common CNS complication of measles virus infection, with an estimated incidence of 1 to 2 in 1,000 cases of measles. The onset of symptoms of postmeasles encephalomyelitis is variable. Typically, after the rash is fading, fever suddenly returns, associated with headaches, vomiting, and signs of meningeal irritation. Headache is invariably an early feature and often relieved by lumbar puncture. If the spinal cord is involved, there is backache, progressive lower extremity weakness, and urinary retention (13).
Postinfectious encephalomyelitis as a complication of varicella virus infection is rare and occurs in about 1 in 10,000 cases of chickenpox. The onset of symptoms is usually between 4 and 14 days after the appearance of the rash, with sudden fever, ataxia, seizures, drowsiness, stupor, and obtundation (13).
Postinfectious encephalomyelitis is thought to occur in approximately 1 out of 5,000 children with rubella infection. Signs and symptoms of neurologic involvement occur within the first week after the onset of the rash. The presentation is usually very severe with convulsions and sudden loss of consciousness. Headache and meningeal signs are also common (13).
Postinfectious encephalomyelitis has been reported in association with hepatitis C virus infection and as a primary manifestation of human immunodeficiency virus (HIV) infection (14–16).
Vaccination
At present, less than 5% of all postinfectious encephalomyelitis cases follow immunization. Postvaccinal encephalomyelitis has been associated with immunization for rabies, hepatitis B, influenza, Japanese B encephalitis, diphtheria/pertussis/tetanus, measles, mumps, rubella, pneumococcus, polio, smallpox, and varicella (Table 22.1) (34). Postvaccinal encephalomyelitis usually occurs 7 to 14 days after vaccination, but cases have been reported as early as 1 day and as late as 23 days following vaccination (35). The risk is usually increased directly with increasing age of primary vaccination after the first year of life (36). In general, postvaccinal encephalomyelitis occurs more frequently in primary vaccinees than in revaccinees. Complications in revaccinees occur in individuals who have not been vaccinated for many years, and therefore react like primary vaccinees, or in individuals who have acquired immunodeficiency disorders (37). During the 1947 smallpox outbreak in New York City, the reported incidence of postvaccinal encephalomyelitis was 1 in 100,000 (35). In 1968, 5,594,000 primary smallpox vaccinations and 857,400 revaccinations were given in the United States. The overall incidence of postvaccinal encephalomyelitis was 2.9 per 1 million primary vaccinations. None of the revaccinees developed postvaccinal encephalomyelitis. The case-fatality rate of postvaccinal encephalomyelitis between 1959 and 1966 was approximately 25% in the United States (38) and 30% to 50% in Europe (4,39).
The incidence of encephalitis associated with the live attenuated measles virus vaccine is thought to be 1.16 per 1,000,000 doses, with most cases occurring in the second week after immunization (19). Postvaccinal encephalomyelitis has been associated with the poliovirus vaccine (19), the Japanese encephalitis vaccine (20), the tetanus toxoid vaccine (21), and the recombinant hepatitis B vaccine (24).
Other Infectious Agents
Streptococcus pyogenes has been reported as a causative agent of postinfectious encephalomyelitis associated with acute glomerulonephritis (23). Postinfectious encephalomyelitis has been reported as a complication of Legionella pneumophila infection (24), following leptospirosis (25), typhoid fever (26), and in the recovery phase from Rocky Mountain spotted fever (28).
A postmalaria neurologic syndrome has been described, characterized as acute onset of convulsions, acute confusional state, dysphasia, acute psychosis, tremor, myoclonus, and ataxia in patients recovering from Plasmodium falciparum malaria. Giemsa-stained smears of peripheral blood must be negative at the time of symptom onset, distinguishing this syndrome from cerebral malaria, which occurs during parasitemia. The development of the syndrome can be up to 9 weeks (median, 4 days) from eradication of the systemic parasitemia (29). Postinfectious encephalomyelitis has been reported as a complication of Mycoplasma pneumoniae infection (30), after autologous stem cell transplantation (31), and in association with lupus (32) and autoimmune hemolytic anemia (33). Whether this is simply a chance association or these diseases have a specific role in the pathogenesis is unclear.
CLINICAL PRESENTATION
The presentation of postinfectious encephalomyelitis is usually characterized by abrupt onset of neurologic symptoms days to weeks after a viral illness or vaccination. Nevertheless, a clear preceding infection or vaccination cannot be found in up to one third of children and half of adults presenting with disease (8,40). In those cases, systemic symptoms, including fever (43% to 52%), headache (45% to 58%), malaise, and myalgias may occur shortly before the appearance of neurologic signs and symptoms (41).
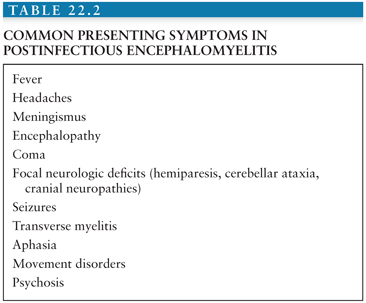
Because of the widespread involvement of the optic nerves, brain, and spinal cord, postinfectious encephalomyelitis usually presents as a polysymptomatic, monophasic, multifocal neurologic demyelinating disease. Obtundation and depressed consciousness, in addition to unilateral or bilateral long tract signs (85%), acute hemiparesis (76%), and ataxia (59%), are the most common presentations. Cranial nerve deficits may be present because of involvement of the corticobulbar fibers to the motor nuclei of the cranial nerves. These signs may be associated with an altered level of consciousness ranging from lethargy to coma (42). Focal or generalized tonic-clonic seizures and psychosis may also be part of the initial presentation (43). Postinfectious encephalomyelitis can be distinguished clinically from acute viral encephalitis by the predominance of subcortical white matter involvement. In contrast, viral encephalitis usually presents with predominantly cortical features, including confusion, aphasia, and convulsions. Other common presenting signs and symptoms are listed in Table 22.2. Interestingly, presenting symptoms may vary in pediatric versus adult-onset postinfectious encephalomyelitis. Motor deficits can occur in both adult and pediatric cases. However, sensory deficits and polyradiculoneuropathies are more frequently found in adults, whereas seizures predominate in pediatric cases.
Even though postinfectious encephalomyelitis usually displays a monophasic disease course, rare cases of relapsing postinfectious encephalomyelitis have been described. In order to fulfill definition of “recurrent postinfectious encephalomyelitis,” the second clinical event should occur at least 3 months from the initial event, without involvement of new clinical areas or magnetic resonance imaging (MRI) evidence of dissemination in time. It has also been suggested that in order to distinguish recurrent postinfectious encephalomyelitis from multiple sclerosis (MS), the second event should not occur while the patients is receiving steroid treatment (44). Multiphasic postinfectious encephalomyelitis is a term that has been assigned to recurrent postinfectious encephalomyelitis cases where the second event represents a polysymptomatic presentation with involvement of a different anatomic area. In those cases, MRI must show new areas of involvement with complete or partial resolution of previous lesions (10,44). Long-term clinical and imaging follow-up has shown the resolution of lesions with no long-lasting neurologic impairments in most of these multiphasic cases (41).
There is a long-standing controversy about whether a second episode of postinfectious encephalomyelitis should be called MS and treated accordingly. As a general rule, patients who develop clinical evidence of dissemination in space and time along with evidence of chronic demyelination in the CNS will likely develop MS. The classic scenario is the patient, typically a child, who is diagnosed with postinfectious encephalomyelitis following a viral infection, recovers, and then after some time develops recurrent symptoms with or without an antecedent viral infection. Two criteria are helpful in making the correct diagnosis: (a) The development of new symptoms representing distinct areas of demyelination not involved in the original episode favors the diagnosis of relapsing-remitting MS, and (b) the appearance of new lesions on neuroimaging supports the diagnosis of MS (45). It is worth remembering that brain lesions of MS patients usually increase in size and number during the course of the illness (41). In addition, chronic MS lesions appear as black holes on T1-weighted images, whereas black holes are not seen on T1-weighted images in patients with postinfectious encephalomyelitis. As patients recover from postinfectious encephalomyelitis, there is evidence of complete or partial resolution of lesions on neuroimaging. Oligoclonal bands should not persist in the cerebrospinal fluid (CSF) of patients with postinfectious encephalomyelitis, but will either persist or appear over time in the CSF of patients with MS (41).
Acute hemorrhagic leukoencephalitis is considered a hyperacute form of postinfectious encephalomyelitis and has been reported to occur in 2% of pediatric cases (46). On physical examination, there may be meningismus, obtundation, and lethargy, in addition to upper motor neuron signs, brainstem findings, transverse myelitis, and cranial neuropathies. In general, maximum deficits are reached in the first week from onset. Recovery usually starts to become clinically evident after the first week, with complete resolution of deficits and MRI lesions within 3 months (10). Although the prognosis is in general favorable, as high as 30% of patients require intensive care, with an estimated mortality rate of 20% (10). Despite the neuropathologic differences between postinfectious encephalomyelitis and acute hemorrhagic leukoencephalitis, it is possible that they represent a gradient of severity of the same pathologic process.
PATHOGENESIS
The pathology of postinfectious encephalomyelitis can be reproduced in the animal model of experimental allergic encephalomyelitis. This is a demyelinating disorder of the CNS induced in animals by immunization with myelin extracts, proteins, or peptides found in myelin. In an effort to reproduce in animal models the lesions described in postvaccinal encephalomyelitis, Rivers and colleagues (47) in 1933 injected homogenates of normal rabbit brains into monkeys. After 6 months, several monkeys developed a lymphocytic infiltration and demyelination of the CNS tissue (47).
Experimental allergic encephalomyelitis can be passively transferred to healthy animals by immune lymphocytes (48). These activated T cells assume a novel functional phenotype after transfer into a recipient animal that allows them to migrate to the CNS and pass through the blood–brain barrier. This migration briefly precedes the onset of clinical experimental allergic encephalomyelitis. There is a minimal interval of 3 days between the intravenous administration of pathogenic T cells and the onset of clinical experimental allergic encephalomyelitis (49).
T cells can potentially react with a wide variety of molecular structures, but normally they do not react against self-antigens. However, some encephalitogenic CD4 and CD8 T lymphocytes can be found in the blood, thymus, and secondary lymphoid tissues of apparently healthy individuals, but through the action of suppressive cytokines, they usually do not attack the CNS. T cells become pathogenic only if activated. One possible scenario to explain the development of postinfectious encephalomyelitis is that an infecting microbe expresses a peptide that is structurally similar to myelin basic protein (MBP). This epitope can trigger the activation of self-reactive T cells by a mechanism known as molecular mimicry. Once activated, these cells can multiply and mature into effector T cells, producing mediators and cytokines that can react to normal self-antigens. In addition, some T cells express more than one specific antigen receptor. One receptor type could be specific for the myelin antigen and the other for the microbial antigen. Exposure to the microbial antigen could activate the T cell, which by virtue of its myelin-specific alternative receptor could attack the CNS (49).
Another mechanism proposed to explain the pathogenesis of postinfectious encephalomyelitis is the activation of self-reactive immune cells by the release of cytokines by virus-mediated death of host cells. Penetration of these self-reactive immune cells into the brain or spinal cord leads to the characteristic pathology of postinfectious encephalomyelitis.
The role of circulating humoral factors in the pathogenesis of postinfectious encephalomyelitis is still unclear, but several lines of evidence suggest that antibody production by the host may aid in limiting or preventing the disease presumably by binding to MBP and inhibiting the access of autoreactive T lymphocytes. Prostaglandins of the E series secreted by blood monocytes and cerebral glial cells inhibit the immune response in experimental allergic encephalomyelitis by downregulation of monocytes and T cells and reduce the clinical and histologic abnormalities of experimental allergic encephalomyelitis in rats (12).
DIAGNOSIS
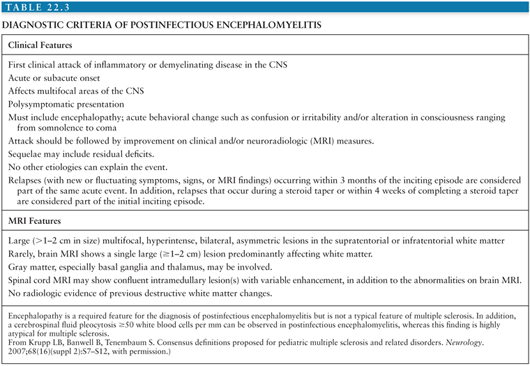
The diagnosis of postinfectious encephalomyelitis is based on clinical history, the findings on neurologic examination, neuroimaging abnormalities of demyelination, and CSF analysis. Several attempts have been made to establish a series of clinical features that will increase the likelihood of the diagnosis of postinfectious encephalomyelitis. The International Pediatric Multiple Sclerosis Study Group has developed a series of criteria for the diagnosis of postinfectious encephalomyelitis (Table 22.3). These criteria have been developed based on selected review of the literature and expert panel discussion. The specificity, sensitivity, and biologic validity to the diagnosis of postinfectious encephalomyelitis when using these guidelines have not been evaluated to date.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree