Erythropoietic protoporphyria (EPP), the third porphyric disorder with only cutaneous manifestations, is not uncommon, with an estimated prevalence of 1 per 75,000 to 200,000. Since the first clear description of the disease by Magnus et al. in 1961,
173 hundreds of cases have been reported throughout the world.
20,
174,
175,
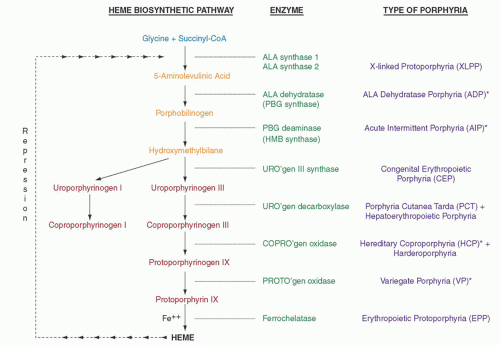
176 Defects in the gene encoding ferrochelatase (FECH), the last enzyme in the heme biosynthetic pathway (
Fig. 26.1), underlie most cases and result in accumulation of free protoporphyrin, mainly in erythroid tissue. The cutaneous manifestations are distinctive, but considerable individual variation is noted in clinical severity as well as in biochemical abnormalities. Three patterns of inheritance are recognized for the disorder (
Table 26.2), and two variants occur.
Molecular Basis and Pathogenesis
Subnormal FECH activity is found in all tissues examined from patients with EPP, namely bone marrow,
177 reticulocytes,
178 liver,
179 cultured skin fibroblasts,
179 and lymphocytes.
180 However, the protoporphyrin accumulates principally, if not entirely, in erythroid cells,
181 whose contribution to heme production far exceeds that of all other tissues. Although the normal relative activity of FECH in erythroid tissue, in contrast to liver, is the second lowest among the enzymes of the heme biosynthetic pathway and is only three times higher than 5-aminolevulinate synthase,
19 defective FECH in EPP does not appear to be rate limiting until late in erythroid development.
177 Patients often exhibit a mild hypochromic-microcytic anemia as a consequence of the FECH deficiency that in turn appears to limit iron assimilation.
177,
182,
183,
184 Protoporphyrin begins to accumulate in bone marrow erythroblasts just before the nucleus is lost,
185 and reticulocytes and young erythrocytes probably are the major source of protoporphyrin in plasma on its rapid release from these cells.
186,
187 Because of its low water solubility, the protoporphyrin is not excreted in urine but is taken up by the liver and excreted exclusively through the biliary tract.
Based on clinical features and FECH activity in many pedigrees, inheritance of this porphyria was first believed to most often follow an autosomal dominant pattern with incomplete penetrance.
183,
188 In a few families, autosomal recessive inheritance was evident.
174,
189 However, FECH activity in tissue lysates from patients with clinical EPP is always only 20% to 30% of normal,
20,
181 not the 50% value expected for an autosomal-dominant enzyme deficiency. Neither levels of FECH activity nor levels of erythrocyte and fecal protoporphyrin consistently correlate with severity of symptoms. Moreover, most obligate carriers of the disorder have no symptoms. Their erythrocyte and fecal protoporphyrin levels are usually normal, and tissue FECH activity is approximately 50% of normal.
20,
179,
181 To explain these findings, it was proposed that more than one allele is involved in the full expression of the disease.
174,
190Upon the cloning and characterization of the human
FECH gene,
191 which is located on chromosome 18 (18q21.3),
192 at least 187 different molecular defects have been identified and are highly heterogeneous, commonly as missense/nonsense mutations, nucleotide deletions and insertions, and intronic point mutations near intron/exon splice sites in one
FECH allele.
10,
192a Most often, the defects lead to frameshifts or deletions, resulting in a truncated protein (“null allele”). The entire
FECH gene was absent in one patient as a result of a chromosome 18q deletion.
193 Two types of regulatory defects in the gene have also been reported, impairing transcription due to hypermethylation of the promoter region
194 or a point mutation affecting transcription factor binding in the promoter.
195 In about 4% of cases a mutation is present on each allele, defining recessive inheritance,
10,
196 where the majority are missense mutations. Although these few double heterozygotes clarify the phenotypic differences between symptomatic and asymptomatic family members, all other manifesting cases usually coinherit a “low-expression” normal
FECH allele
trans to a deleterious mutant allele.
197,
198 The low-expression allele is highly associated with a specific ancestral haplotype involving a single nucleotide polymorphism site in the
FECH gene (IVS3-48T/C) that influences the use of a constitutive aberrant acceptor splice site in the gene.
199 The aberrantly spliced RNA fraction is degraded, decreasing the steady-state level of FECH mRNA expressed by the allele by ≥20%. Hence the term
“pseudodominant EPP” came into use for patients with a
FECH mutation
trans to a hypomorphic IVS3-48C allele, to distinguish them from those with
autosomal recessive EPP who are hetero-or homoallelic for
FECH mutations. The frequency of the hypomorphic IVS3-48C allele differs widely between ethnic groups, ranging from 67.8% in Japanese to <1% in West Africans, and in general correlates with prevalence of clinical EPP among individuals with a mutant
FECH allele in the different populations.
176 When a low-expression allele is not found, a dominant-negative effect of
FECH mutants may be operative.
200 Because the enzyme activity is restricted to its homodimers,
201 a mutant monomer may generate nonfunctional homodimers and impaired or unstable heterodimers, resulting in residual enzyme activity of <50% of normal.
202,
203 Thus, the ultimate effect of a specific mutation in the
FECH gene depends on how it affects the integrity of the protein and whether the nonmutated allele is expressed at a lower level to reduce FECH activity to below a threshold of around 30% of normal as found in clinically overt EPP.
More recently a third pattern of inheritance of EPP that is X-linked was described and is referred to as
X-linked protoporphyria (XLPP).
204 In the United Kingdom and in the US, the disorder accounts for 2% and 10% of unrelated EPP patients, respectively.
192a,
205 FECH enzyme activity is normal but erythrocyte protoporphyrin concentrations are higher than in EPP due to FECH deficiency. Around 40% is zinc protoporphyrin, indicating that the supply of the iron substrate as well as ferrochelatase activity becomes rate limiting. To date, five distinct mutations have been identified in exon 11 of the erythroid-specific 5-aminolevulinate synthase gene (
ALAS2) (
Fig. 26.1), which resides on the X chromosome, and lead to predicted amino acid sequence disruption or deletion of up to 40 C-terminal amino acids of the enzyme.
192a,
204,
205a The recombinant mutants have two- to three-fold increased activity,
205b explaining the protoporphyrin overproduction. This gain-of-function in the enzyme contrasts with all other previously described
ALAS2 mutations that decrease enzyme activity and cause X-linked sideroblastic anemia (
Chapter 24). It also reflects a critical role of the C-terminal structure of ALAS2 for its activity and thus erythroid heme synthesis.
About 5% of EPP families in a large cohort were mutation-negative for
FECH and
ALAS2.
205 However, in these the disease was strongly associated with inheritance of the IVS3-48C allele and decreased FECH activity, suggesting that most may have mutations in regions of the
FECH gene that are not included in current strategies for mutation detection. In one case mild photosensitivity was ascribed to the IVS3-48CC genotype alone.
205The pathophysiology of EPP is mediated by the accumulated protoporphyrin. As it leaks out of erythroid cells into plasma, it gains entry into tissues. It is extracted solely by the liver and secreted unchanged into the bile. The liver is capable of clearing large amounts of protoporphyrin, but its secretion across the canalicular membrane and into the bile appears to be rate limiting.
206 Yet despite microscopic evidence of hepatobiliary changes in many EPP patients, cholestasis leads to cirrhosis and hepatic failure in only a few (<5%).
207 This complication tends to be an abrupt event, is unrelenting, and is not predictable from prior biochemical features or the clinical course of the patient. However, it occurs more commonly in compound heterozygotes for two mutations
196 and in XLPP
204 than in heterozygotes. Profoundly reduced ferrochelatase activity predisposes to liver failure.
208 In an analysis of 112 EPP patients, all 18 who developed liver disease carried a null allele mutation, whereas none of 20 patients having missense mutations had liver disease as yet.
209 Undetected molecular derangements
194 or undefined hepatic factors,
210 including a greater hepatic source of the excess protoporphyrin related to the defect, can be postulated. Excess alcohol intake
211 and viral hepatitis,
212 as well as hyperthyroidism,
212a also accentuate the genetic disorder.
In the skin, the hydrophobic protoporphyrin transfers to endothelial cells of capillaries to produce the light-induced skin damage in patients with EPP.
213,
214 Porphyrin-sensitized, oxygen-dependent histochemical reactions and the activation of complement eliciting an inflammatory response are involved in the pathogenesis.
22,
23 The extensive double-bond structure of the protoporphyrin renders it particularly photoactive, resulting in the unique acute epidermal phototoxicity,
173 in contrast to the porphyrins that accumulate in the other porphyrias with cutaneous photosensitivity. The activated porphyrin also stimulates fibroblast proliferation, accounting for a characteristic waxy thickening of the sun-exposed skin
23
Variant Erythropoietic Protoporphyria
The presence of an abnormal transcript of mitoferrin 1 (MFRN1) associated with reduced FECH activity was identified in a series of 7 patients with the EPP phenotype but without genetic defects in FECH in 6 of them, although no cause for the aberrant splicing of MFRN1 mRNA was found in the MFRN1 sequence.
215 Four patients also had a previously reported C-terminal deletional ALAS2 mutation, and 4 cases had advanced liver disease. MFRN1, which transports iron into mitochondria, is highly expressed and regulated in erythroid cells.
216 A molecular complex of FECH protein, MFRN1 and ABCB10 (a MFRN1 stabilizer) integrates mitochondrial iron import with heme synthesis.
217 Thus, aberrant MFRN1 can contribute to the EPP phenotype in some patients, apparently by reducing FECH activity.
Late-onset Erythropoietic Protoporphyria Variant
EPP that is clinically indistinguishable from the inherited forms has developed in later years in the setting of a myelodysplastic syndrome
218 including the sideroblastic anemia subtype (
Chapter 24),
19 or a myeloproliferative disorder.
219 Two cases were associated with severe cholestatic liver disease.
219,
260 In several instances acquired deletion of one
FECH gene was demonstrated.
218,
219,
220 An associated low-expression allele or a mutation in the other
FECH allele, or altered erythroid heme biosynthesis in the dysplastic clone, likely contributes to the clinical expression in such cases. One case with late-onset XLPP has also been reported.
220a