Pneumonia Syndromes
General Concepts and Methods
Pneumonia should be suspected when there is a history of cough, fever, and difficult or rapid breathing. Physical findings such as inspiratory crackles, decreased breath sounds, dullness to percussion, or grunting or painful breathing usually are reliable enough to be taken as presumptive evidence of pneumonia—physical examination of the chest is discussed further in Chapter 7. In general, the signs and symptoms that have a high degree of sensitivity (e.g., fever and tachypnea) lack specificity, while those that have a high degree of specificity (e.g., rales and pleuritic pain) lack sensitivity.1
Thus, if pneumonia is suspected, a chest x-ray should usually be obtained; the radiologic findings are usually regarded as definitive for confirmation of the presence and location of pneumonia, and they provide some assistance in distinguishing the probable cause.
The term pneumonitis literally means inflammation of the lungs; sometimes it is used interchangeably with pneumonia, and sometimes it is used to mean minimal pneumonia. The phrase pulmonary infiltrate is more precise and problem-oriented than pneumonia and may be used when the clinician suspects a noninfectious process. A recent review defines pneumonia in children as the presence of fever, acute respiratory symptoms, or both, plus evidence of parenchymal infiltrates on chest radiography.1
The most frequent etiologies for acute pneumonias discussed in this chapter are listed in Table 8-1.
Classification
The historic classification of pneumonias in the 1920s used the terms “typical” for classic pneumococcal pneumonia and “atypical” for almost all other pneumonias. When chest radiology became easily available in the 1930s, “silent pneumonia” and “walking pneumonia” were recognized, particularly during screening of military recruits. “Walking pneumonia” is now largely a lay term.
Pneumonias are now best classified as specific syndromes, using several variables:
Onset and course: Pneumonia can be acute or chronic, progressive or improving, recurrent or a first episode.
Severity: Pneumonia can be classified on the basis of severity, as estimated by clinical observations or by quantitation of respiratory acidosis and hypoxemia.
Anatomic pattern: Pneumonia can be classified as lobar, multilobar, segmental, subsegmental, lobular, interstitial, perihilar, nodular, or miliary. Combinations of these forms are also possible.
Additional anatomic features: Pleural thickening or effusion, cavitation, pneumatoceles, or pneumothorax may be present.
Extrapulmonary features: Eosinophilia in the peripheral blood smear and underlying chronic disease may be present. Pneumonia complicating cystic fibrosis or malignancy is discussed in Chapter 22.
Etiology: Most pneumonias in children are caused by infectious agents: viruses, bacteria (including mycobacteria), mycoplasmas, fungi, and protozoans. Etiologic diagnoses are not as easy to determine or as accurate as is sometimes implied, and proof of the etiology is not obtained in most cases.
To make a therapeutic decision in children, radiologic findings of the chest alone are not sufficient or accurate enough to distinguish a viral from a bacterial etiology.2 However, the age of the child is a very important factor in selecting antibiotic therapy. The additional pulmonary features are also helpful, as confirmed viral pneumonias are rarely lobar, although they may be sublobular, and almost never produce hilar adenopathy, effusion, pneumothorax, pneumatoceles, or cavitation.3
It is helpful to give an accurate description for
the primary diagnosis or statement of the problem. The etiologic diagnosis should usually be expressed as a probability, along with a statement of other reasonable possibilities. Examples of such preliminary clinical diagnoses include:
the primary diagnosis or statement of the problem. The etiologic diagnosis should usually be expressed as a probability, along with a statement of other reasonable possibilities. Examples of such preliminary clinical diagnoses include:
Acute lobar pneumonia; probably pneumococcal, possibly Haemophilus influenzae.
Severe bilateral interstitial pneumonia; probably caused by influenza virus.
Recurrent right middle-lobe pneumonia; probably pneumococcal; consider partial bronchial obstruction.
Persistent right peripheral pneumonia with hilar adenopathy; probably tuberculous.
Bilateral interstitial pneumonia complicating acute leukemia; consider cytomegalovirus.
The remainder of this section discusses the general principles involved in obtaining microbiologic specimens in pneumonias and the frequency of various etiologic agents. Subsequent sections discuss the various syndromes, such as lobar pneumonia, and the etiologic agents most commonly associated with each. The physiologic principles involved in pneumonia, including respiratory insufficiency, are discussed in Chapter 7.
TABLE 8-1. MOST FREQUENT ETIOLOGIES OF ACUTE PNEUMONIA SYNDROMES | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Etiologic Sources
Attempts to obtain a definitive etiologic diagnosis in pneumonia involve the use of culture, histology (smear or biopsy), skin tests, or serology. The significance of culture results in pneumonia depends on the source of the culture and the probability of finding the infectious agent from that source in normal individuals. One way to classify organisms is whether they may sometimes colonize the respiratory tract in the absence of disease. For organisms that do not colonize the respiratory tract, their isolation can be considered diagnostic of infection, regardless of the clinical setting (Box 8-1).
While considering such procedures as lung puncture or flexible bronchoscopy, the physician must weigh the balance between the likely value of the procedure and the risk to the patient.4 In most clinical situations, a conclusive etiologic diagnosis
by a definitive procedure is not essential for optimal treatment and thus may entail an unnecessary risk to the patient. However, in immunocompromised children, invasive techniques such as lung biopsy may be indicated. Prospective randomized studies of risks and benefits have not been done even in adults.4
by a definitive procedure is not essential for optimal treatment and thus may entail an unnecessary risk to the patient. However, in immunocompromised children, invasive techniques such as lung biopsy may be indicated. Prospective randomized studies of risks and benefits have not been done even in adults.4
BOX 8-1 Classification of Pathogens Based on Whether Isolation of the Organism from the Respiratory Tract is generally Diagnostic of Infection
| Diagnostic | Nondiagnostic |
| Bacteria | |
| Mycobacterium tuberculosis Legionella pneumophila Francisella tularensis Bordetella pertussis Nocardia spp. Mycoplasma spp. | Nontuberculous mycobacteria All other bacteria |
| Viruses | |
| Influenza Parainfluenza Respiratory syncytial virus Hantavirus Measles Enterovirus Adenovirus | All other viruses |
| Fungi | |
| Histoplasma capsulatum Blastomyces dermatiditis Coccidioides immitis Cryptococcus neoformans Pneumocystis jiroreci (carinii) | Candida species Aspergillus species |
| Parasites | |
| Toxoplasma gondii Strongyloides stercoralis | All others |
Conclusive Culture Sources
Cultures of blood, pleural fluid, material obtained by lung puncture, or lung biopsy specimens are usually considered conclusive.
Occasionally, one encounters statements about pneumonia that are not substantiated by any accurate data and in fact are examples of reverse logic. For example, the statement that 20% of patients with pneumococcal pneumonia have pneumococcal bacteremia requires some conclusive evidence that the other 80% without bacteremia had pneumococcal pneumonia. Such statistics are usually not supported by any conclusive tests such as lung puncture. However, it can be accurate to say that 20% of a group of patients with lobar pneumonia had pneumococcal bacteremia.
Occasionally Significant Culture Sources
Material obtained from flexible fiberoptic bronchoscopy,5,6 tracheostomy secretions,7 rigid bronchoscopy aspiration, and transtracheal aspirations may occasionally be useful in identifying the etiologic agent. Transtracheal aspiration, although used in adults, has rarely been used in children because of their small, growing tracheas. In one study of children, the correlation between the types of bacteria recovered from transtracheal aspiration and those recovered from lung puncture was only fair.8 Reported complications include transient hemoptysis, mediastinal or subcutaneous emphysema, and cardiac arrest, perhaps secondary to anoxia, vagal reflex, or vomiting with aspiration.9
Cultures of Dubious Significance
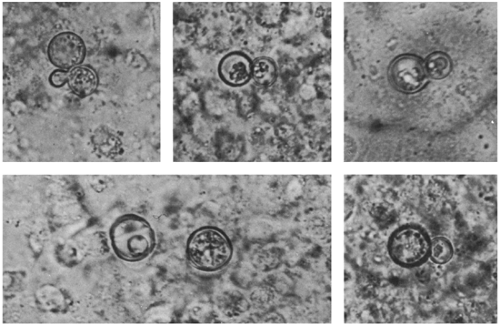
Direct orotracheal aspirates sometimes are useful in newborns with pneumonia.10 Sputum culture is usually not useful, as children seldom produce sputum before about 6 years of age. After this age, specimen collection can sometimes be facilitated by techniques to induce sputum production, such as nebulized hypertonic saline. Occasionally, younger children do cough up and spit out tracheal secretions, especially if they have chronic pulmonary disease. However, sputum is an unreliable source for culture in children with acute pneumonia, because more than one potential pathogen can often be found even in normal individuals. In chronic pneumonias, even a 2-year-old may cough up purulent sputum, which may reveal budding yeasts (Fig. 8-1) or bacterial pathogens (as in cystic fibrosis).
Cultures of endotracheal tube secretions in patients on mechanical ventilators are similarly fraught with interpretational difficulties. One study suggests that endotracheal tube aspirates with negative Gram stains or with greater than 10 squamous epithelial cells per low-power field should be rejected.11 Obtaining surveillance cultures from endotracheal tubes of all patients on mechanical ventilation does not predict the etiology of subsequent invasive disease.12 Other than for epidemiologic purposes, this practice should be abandoned.
Nose or nasopharyngeal cultures usually yield a
high frequency of potential pathogens and are not useful in diagnosing bacterial pneumonia. They may occasionally be useful if a viral pathogen, such as respiratory syncytial virus (RSV) or influenza, is suspected. Throat cultures are not useful in the etiologic diagnosis of pneumonia.
high frequency of potential pathogens and are not useful in diagnosing bacterial pneumonia. They may occasionally be useful if a viral pathogen, such as respiratory syncytial virus (RSV) or influenza, is suspected. Throat cultures are not useful in the etiologic diagnosis of pneumonia.
Gram Stain
A Gram stain of clinical material is often helpful. When the stain is done on material from a moderately significant culture source, it may help guide empiric therapy. The results of Gram staining may also aid interpretation of cultures obtained from sites that are often contaminated, such as the endotracheal tube, which may be cultured when ventilator-associated pneumonia is suspected. Endotracheal tubes are nearly always colonized with flora common to the intensive care unit, and therefore cultures are usually positive. If the Gram stain shows many neutrophils and a single bacterial morphology, the cultured organism is more likely to be a pathogen than if the Gram stain shows few white cells and mixed flora.
Detection of Antigens
Assaying sputum, pleural fluid, or serum for antigens is neither useful nor approved for the diagnosis of pneumonia. In 1999, the Food and Drug Administration (FDA) approved a rapid assay for the detection of pneumococcal antigen in the urine. However, this assay lacks specificity in children and is not recommended for use in that population.13
Bronchoalveolar Lavage
Bronchoalveolar lavage (BAL) often is a useful procedure in immunocompromised hosts with pulmonary infiltrates. In non-neutropenic patients with diffuse pulmonary infiltrates, the sensitivity of BAL is 80–90% and is highest in detecting Pneumocystis jiroveci (formerly carinii) pneumonia (PCP) in patients with human immunodeficiency virus (HIV) infection.14 It is somewhat less sensitive in neutropenic cancer patients, and thus a negative result cannot rule out the possibility of an infectious cause of the infiltrates. For any patient population, the diagnostic yield in the case of focal infiltrates is relatively low.
Biopsy of the Lung or Pleura
Lung biopsy may be done thoracoscopically, by thoracotomy (open biopsy), or by needle. Open
lung biopsy is most useful for the diagnosis of tuberculosis (TB) or of opportunistic microorganisms such as Pneumocystis in an immunocompromised host16 or in chronic pneumonias (described later). It can be done using a limited thoracotomy (i.e., no rib resection).17 A small pilot study in 13 immunocompromised patients with pneumonia showed that open lung biopsy yielded superior diagnostic information compared with BAL 5 of the patients had diagnoses established by open lung biopsy only.18 However, we have occasionally seen patients in which the reverse was true (i.e., diagnosis was made by BAL with a negative lung biopsy).
lung biopsy is most useful for the diagnosis of tuberculosis (TB) or of opportunistic microorganisms such as Pneumocystis in an immunocompromised host16 or in chronic pneumonias (described later). It can be done using a limited thoracotomy (i.e., no rib resection).17 A small pilot study in 13 immunocompromised patients with pneumonia showed that open lung biopsy yielded superior diagnostic information compared with BAL 5 of the patients had diagnoses established by open lung biopsy only.18 However, we have occasionally seen patients in which the reverse was true (i.e., diagnosis was made by BAL with a negative lung biopsy).
BOX 8-2 Normal Range of Bronchoalveolar Lavage Differential Cell Counts in Children*
| WBC | 7 × 104/mL to 50 × 104/mL |
| Macrophages | 84–93% |
| Lymphocytes | 7–13% |
| Neutrophils | 1–4% |
| Eosinophils | 0–0.2% |
| *Values are the approximate range of median values from five studies. | |
Transbronchial lung biopsy using a flexible fiberoptic bronchoscope has been useful in distinguishing tumor from opportunistic infection in adults.19 The use of this technique in children is limited because of an increased risk of bleeding and pneumothorax compared with adults.14 In addition, transbronchial biopsy is contraindicated in patients receiving mechanical ventilation because of the risk of tension pneumothorax.20
Percutaneous needle biopsy under fluoroscopic guidance for histologic diagnosis is sometimes done in adults,21 especially when malignancy is a consideration, but it is rarely done in children. Pleural biopsy is discussed in the section on pneumonia with effusion.
Thoracoscopy
Thorascopy has been used in children to rule out PCP, usually using sedation and local anesthesia.22 It is also useful in evaluating intrathoracic tumors but is sometimes complicated by pneumothorax or bleeding. Its primary role is in decortication of loculated empyema and is discussed in the section on pneumonia with effusion.
Computed Tomography and Ultrasound
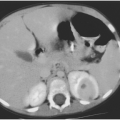
The use of computed tomography (CT) and ultrasound procedures is discussed in the sections on the syndromes where they are helpful. For example, ultrasound is most useful in detecting pleural effusion and in guiding thoracentesis. CT scanning is certainly more sensitive than plain chest films in picking up pleural effusions;23 typically, however, any effusion small enough to be detected only by CT scanning is probably too small to be clinically significant. CT scanning also improves visualization of abscesses, and affords the clinician a better view of the mediastinum and its structures.24
Serum Antibodies
Serologic diagnosis is usually retrospective, except for cold agglutinins, which usually appear after one week of illness, often at about the time the patient seeks medical attention. Testing of paired sera is most useful for the diagnosis of Chlamydia pneumoniae, Mycoplasma pneumoniae, Q fever, and psittacosis. Serologic methods can be used for the detection of respiratory syncytial virus (RSV), influenza virus, parainfluenza virus, and adenovirus infection, but rapid antigen testing or viral culture methods are preferable.
Skin Tests
The intermediate-strength tuberculin test is very useful for the diagnosis of tuberculous pneumonia. However, serologic tests are better than skin tests for the diagnosis of current pneumonia caused by Histoplasma or Coccidioides species. Furthermore, skin tests for fungi may boost the antibody titer, resulting in a misleading rise.
Acute Focal Pneumonia
Acute lobar or segmental pneumonia is usually pneumococcal in all groups beyond the newborn period. It was once called typical pneumonia, in contrast to atypical pneumonia.
The following characteristics can be considered typical: significant fever above 102°F (38.8°C) is usually present; chills often are noted in older children and adults; and often, there is a toxic appearance, defined as appearing acutely ill, anxious, and distressed. Also, definite chest signs are usually present and lateralized including consolidation, which is defined by dullness to percussion, decreased breath
sounds, increased fremitus (vibration felt on palpation of the chest wall, produced by the spoken voice), and, sometimes, bronchophony (increased intensity and clarity of voice sounds heard over a bronchus surrounded by consolidated lung tissue). Fine end-inspiratory rales are often present. Rhonchi are often described, which may be loud enough to make the end-inspiratory rales difficult to discern. Pleuritic pain or splinting may be noted.
sounds, increased fremitus (vibration felt on palpation of the chest wall, produced by the spoken voice), and, sometimes, bronchophony (increased intensity and clarity of voice sounds heard over a bronchus surrounded by consolidated lung tissue). Fine end-inspiratory rales are often present. Rhonchi are often described, which may be loud enough to make the end-inspiratory rales difficult to discern. Pleuritic pain or splinting may be noted.
The pain in lower-lobe pneumonia is sometimes mistaken for abdominal pain or is referred to the abdomen. The patient may be unwilling to breathe deeply to aid auscultation. Shallow breathing (splinting) and a rapid breathing rate are sometimes the only definite signs suggesting that the illness involves the chest. Grunting may be noted in infants or young children. Older children may fail to breathe deeply when asked to do so or complain of pain and cough when they do. On occasion, children with a round (spherical) pneumonia due to S. pneumoniae will present with fever only.
Pleurisy is often used as a lay term or older medical term for pleuritis or pleural pain. Pleurodynia refers to chest pain that is usually pleuritic but without pneumonia or pleural effusion and is characteristically caused by coxsackie B virus infection.25
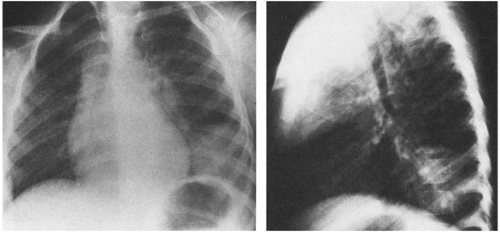
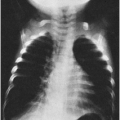
In typical pneumonia, the chest roentgenogram shows a dense infiltrate. It may appear segmental or lobar or even spherical26 (Fig. 8-2) but typically does not have multiple fluffy areas or bilateral thin linear infiltrates. Response to antibiotic therapy is often (but not always) dramatic, with the temperature falling from 104°F (40°C) to 98°F (36.7°C) after the first dose and remaining normal.
Possible Causes
The best evidence for the frequency of various causes of lobar pneumonia is that based on lung puncture or blood culture studies.
Streptococcus pneumoniae
The pneumococcus is almost always the cause of classic typical pneumonia as described earlier. However, the pneumococcus can also produce other forms of pneumonia, such as pneumonia that fails to respond promptly to antibiotics, pneumonia with effusion, and even interstitial pneumonia.27 A retrospective review of 85 children between the ages of 5 months and 16 years with bacteremic pneumococcal pneumonia found that 70% of children had the “typical” illness described before, with high fever, leukocytosis (white blood cell [WBC] greater than 15,000/mcL), and lobar or segmental consolidation on chest x-ray.28 Interestingly, however, one-fourth of the patients presented without any respiratory symptoms. Thirty-eight percent of patients had gastrointestinal tract symptomatology, but only 5 (6%) of the 85 children had gastrointestinal symptoms in the absence of respiratory symptoms.
Routine use of the conjugated heptavalent pneumococcal
vaccine will decrease the incidence of pneumococcal pneumonia, but it will not eliminate it. In a large, randomized controlled trial, the incidence of radiographically-confirmed pneumonia among vaccinees was decreased by 35%.29
vaccine will decrease the incidence of pneumococcal pneumonia, but it will not eliminate it. In a large, randomized controlled trial, the incidence of radiographically-confirmed pneumonia among vaccinees was decreased by 35%.29
H. influenzae Type B
The prevalence of H. influenzae type b (Hib) as a cause of pneumonia in children has greatly decreased because of widespread use of conjugated Hib vaccine. It is still a possible cause of lobar or segmental pneumonia in the unvaccinated child and is frequently accompanied by pleural effusion.30,31 The onset is usually gradual but can be acute. Otitis media is frequent.30 Purulent conjunctivitis is occasionally present. Hib can produce lobar or segmental pneumonia in older children and adults. The response to amoxicillin in recognized cases is typically poor. H. influenzae non-type b is also an occasional cause of pneumonia, especially in children younger than 10 years.
Uncommon Causes
Staphylococcus aureus is an uncommon cause of lobar pneumonia (see Box 8-3).32 It should be considered in young or debilitated infants or when there is effusion or pneumatoceles, as discussed in a later section.
Group A streptococcal pneumonia often is associated with pleuritic pain and marked leukocytosis.33 Often the organism cannot be recovered from the throat culture. A scarlatiniform rash may be present. Empyema and pneumatoceles occasionally occur, and S. aureus infection may be suspected. Group A streptococcal pneumonia tends to occur following a viral infection (especially varicella) and to have a fairly severe and protracted course.34 In stark contrast to pneumonia caused by the pneumococcus, fever in Group A streptococcal pneumonia persists for days to weeks even in the face of appropriate antimicrobial therapy. Pneumonia caused by toxin-producing strains of S. aureus or Group A streptococcus may result in toxic shock syndrome (see Chapter 11).
Box 8-3 Causes of Acute Focal Pneumonia
| Usual Streptococcus pneumoniae |
| Uncommon H. influenzae type b (< 5 years old) Nontypable H. influenzae S. aureus Group A streptococcus Mycoplasma pneumoniae Chlamydia pneumoniae |
| Rare Francisella tularensis Mycobacterium tuberculosis Respiratory viruses (usually lobular): RSV, parainfluenza, adenovirus Meningococcus Enteric bacteria |
Mycoplasma pneumoniae has to be considered as a possible cause of lobar pneumonia.35 It is discussed further in the section on atypical pneumonia syndromes.
Primary pulmonary TB occasionally causes an acute lobar pneumonia, and in such a case, the tuberculin test is almost always positive at the time the pneumonia occurs.36 All children with acute lobar pneumonia that is poorly responsive to empiric antibacterial therapy should have a tuberculin skin test placed. Tuberculosis also can be the basis for a segmental bacterial pneumonia, particularly in the right middle lobe, or for obstruction of a major bronchus by a lymph node. Histoplasma or other systemic fungi also can do this and should be considered in endemic areas.
The right middle-lobe syndrome and other recurrent or chronic lobar pneumonias are discussed later in this chapter.
Klebsiella pneumoniae is rarely documented as a cause of pneumonia in children by blood culture or lung puncture except in association with neonatal sepsis, nosocomial infection, or in immunocompromised hosts.
Meningococcal pneumonia without meningitis has been documented by blood cultures but is uncommon in children.37 It may be more frequent in military populations 16–30 years of age and may follow influenza or adenovirus pneumonia. Serogroup Y is more likely to be associated with pneumonia than are the other serotypes. Septic shock may occur, but the typical purpuric rash may be absent.
Francisella tularensis is also a possible cause of acute focal pneumonia.38 Most cases of tularemia follow the bite of a tick or a deerfly, and cause a systemic disease plus local inflammation at the site of the bite. The pneumonic form of tularemia usually occurs after inhalation of airborne organisms.
Typically in these cases, there is an interesting exposure history; for example, beating a dead infected rabbit with sticks can be a cause of aerosolization. Pleuropulmonary involvement also occurs in typhoidal tularemia, which in children is sometimes acquired by ingestion of the agent.39 Enteric bacteria such as E. coli, Enterobacter, and Pseudomonas aeruginosa are extremely rare causes of pneumonia unless there is an underlying disease or nosocomial acquisition in an intensive care unit.
Typically in these cases, there is an interesting exposure history; for example, beating a dead infected rabbit with sticks can be a cause of aerosolization. Pleuropulmonary involvement also occurs in typhoidal tularemia, which in children is sometimes acquired by ingestion of the agent.39 Enteric bacteria such as E. coli, Enterobacter, and Pseudomonas aeruginosa are extremely rare causes of pneumonia unless there is an underlying disease or nosocomial acquisition in an intensive care unit.
Psittacosis with a lobar segmental pneumonia may present with an acute onset of chills and high fever. Patients with psittacosis usually do not have a leukocytosis.
Treatment
Antibiotic Therapy of Presumed Pneumococcal Pneumonia
Due to the ever-increasing numbers of penicillin-resistant pneumococci, therapy with ceftriaxone or cefotaxime should be instituted for an acute lobar pneumonia presumed to be due to the pneumococcus and requiring intravenous therapy (Table 8-2). If one is fortunate enough to isolate the organism from the blood, a pleural effusion, or another reliable sample (discussed earlier), sensitivity testing should be done, and therapy switched to penicillin for sensitive strains. For patients requiring intravenous therapy, administering penicillin by continuous infusion is an effective and cost-effective approach. The dose is generally 150,000–250,000 units/kg/day (with a maximum of 24 million units per day).40 This provides constant serum levels that easily exceed the minimum inhibitory concentration (MIC) for all but highly penicillin resistant strains.41 The outcome in patients with pneumococcal pneumonia is similar whether the organism is sensitive or resistant to penicillin.42
TABLE 8-2. INITIAL THERAPY OF PNEUMONIA | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
It is important to remember that resistance is relative and can often be overcome by higher doses of antibiotics. Success also depends on the ability of the antibiotic to achieve therapeutic levels at the site of infection (e.g., lung tissue or meninges). Confusion is compounded when some reports refer to both highly-resistant strains and intermediately-resistant strains as nonsusceptible, when in reality infections with intermediately-resistant strains are often easily treated by higher doses of penicillin or other beta-lactam antibiotics. The recent interpretive standards published by the National Committee for Clinical Laboratory Standards (NCCLS) address this issue by citing different MIC cutoffs depending on whether the isolate is from cerebro-spinal
fluid (CSF) or some other source (see Table 8-3).43 In general, it is rarely necessary to add vancomycin for therapy of pneumococcal pneumonia. Poor response to therapy is more likely due to inadequate drainage of an empyema. In contrast, as discussed in Chapter 9, the addition of vancomycin is advocated for meningitis caused by strains that are intermediate or resistant to cephalosporins.
fluid (CSF) or some other source (see Table 8-3).43 In general, it is rarely necessary to add vancomycin for therapy of pneumococcal pneumonia. Poor response to therapy is more likely due to inadequate drainage of an empyema. In contrast, as discussed in Chapter 9, the addition of vancomycin is advocated for meningitis caused by strains that are intermediate or resistant to cephalosporins.
TABLE 8-3. INTERPRETIVE STANDARDS FOR S. PNEUMONIAE | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
One study of 26 children with culture proven pneumococcal pneumonia and complicated parapneumonic effusions found that children with penicillin-resistant isolates were younger than those with susceptible organisms. Bacteremia was also more common.44
Patients with presumed pneumococcal pneumonia who are well enough to be managed as outpatients can be given high-dose amoxicillin (80 mg/kg/day) or cefuroxime axetil, with close follow-up. Tetracycline should not be used for treatment of presumed pneumococcal pneumonia. Macrolides have relatively poor anti-pneumococcal activity as well. Fluoroquinolones are commonly used for community-acquired pneumonia in adults and adolescents. A report suggests that recent exposure to a fluoroquinolone antibiotic increases the risk of fluoroquinolone resistance and treatment failure of pneumococcal pneumonia.45
Patients who have a late diagnosis, who are seriously ill, or who have underlying disease should not be treated as outpatients.
Treatment of Young Children
Moderately ill children of preschool age should be admitted and treated with a parenteral antibiotic effective against both S. pneumoniae and beta-lactamase producing strains of H. influenzae. The third-generation cephalosporins are stable against these beta-lactamases and are therefore a good choice.
Complications
Persistent Pneumonia
Persistent pneumonia is defined by persistence of consolidation on chest roentgenogram for more than 1 month. Pleural thickening, paralysis of the diaphragm, or atelectasis may explain some cases. Other causes of persistence of lobar or segmental densities are described in the section on chronic pneumonia.
Hemolytic-uremic Syndrome
Hemolytic-uremic syndrome (HUS) is an important, albeit uncommon, complication of pneumococcal pneumonia. Although occurring via a different mechanism, it presents similarly to other forms of HUS, with anemia, thrombocytopenia, and acute renal failure. Neuraminidase produced by S. pneumoniae removes N-acetylneuraminic acid from cell surface glycoproteins, exposing the T-antigen on red blood cells, platelets, and glomeruli. Immunoglobulin M (IgM) antibodies that are present in most human plasma react with the exposed T-antigen, resulting in hemolysis and damage to glomerular endothelial cells.48
Typically, cases occur in young children (1–2 years old) with severe or even fulminant pneumococcal pneumonia. Most patients have a positive direct Coomb’s test.49
Because plasma products contain IgM antibodies that can activate the T-antigen, their use is contraindicated. In addition, if transfusions are necessary,
the red blood cells should be washed to reduce the amount of IgM present.50
the red blood cells should be washed to reduce the amount of IgM present.50
Poor Response to Antibiotic Therapy
This occasionally occurs in uncomplicated pneumococcal pneumonia. Patients with low white blood cell counts and multilobar involvement have the highest mortality rate. Poor clinical response may also occur in patients infected with highly penicillin-resistant strains of the pneumococcus. Occasionally, patients with pneumonia severe enough to require mechanical ventilation will have an initial response to therapy but then develop recrudescent fever. Such patients are often found to have secondary nosocomial pneumonia with a different organism (usually a gram negative).
Rare but serious complications include pericarditis, meningitis, endocarditis, arthritis, and peritonitis. Pleural effusion and empyema are described in the following section and should always be considered when there is failure of a lobar pneumonia to respond to adequate therapy (Fig. 8-3).
Effect on Lung Function in Adulthood
This question of the effect on lung function in adulthood was addressed by a prospective cohort study of 1392 British children followed from their births in 1958. Of these, 193 children had a history of pneumonia by the age of 7 years. At 35 years of age, pulmonary function testing was performed in all subjects. A history of pneumonia was associated with a small but significant decrease in forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC), even after controlling for a history of wheezing and multiple other confounding factors. The effect was no greater for the subjects who had pneumonia at younger than 2 years than for those who had pneumonia between ages 2 and 7. It remains unclear whether pneumonia causes the deficit in lung function or whether pneumonia is more common among children who have poorer lung function before the disease.51
Follow-Up Chest Radiograph
In one study of 70 children, about 20% had residual pulmonary infiltrates 3–4 weeks after an acute pneumonia, and all who returned for follow-up had cleared the infiltrate within 3 months.52 It was concluded that routine follow-up chest films are not necessary for children unless there is persistent respiratory difficulty or failure to thrive.
Acute Pneumonia with Effusion or Pneumatocele
Definitions
Pleural effusions in children typically accompany pneumonia, although they may be a result of noninfectious diseases. A pleural effusion in the setting of a known pneumonia is best referred to as a parapneumonic effusion.
Distinctions are traditionally made between effusions as exudates or transudates, which can be defined by the concentration of protein and lactic dehydrogenase (LDH). Exudates typically have a pleural fluid protein concentration greater than one-half that of the serum and usually also have an elevated LDH (greater than 200 units or greater than two-thirds of the upper limit of normal for serum LDH).53 Other factors have also been used to classify an effusion as an exudate, such as glucose less than 40 mg/dl and pH less than 7.2.54
As a rule, children who have fluid in the chest
secondary to congestive heart failure, chronic renal failure, or low serum protein have these known predisposing causes, so that the finding of a transudate by analysis of the pleural fluid is not a surprise.
secondary to congestive heart failure, chronic renal failure, or low serum protein have these known predisposing causes, so that the finding of a transudate by analysis of the pleural fluid is not a surprise.
Empyema is traditional term for a thick purulent pleural effusion. An empyema may be simple, i.e., with free-flowing pus, or loculated. In addition, many exudates that are a result of an infectious disease in the chest at first appear straw-colored and serous and become thicker and cloudier over time. Whenever an entire hemi-thorax appears opaque, a large pleural effusion or hemothorax should be suspected.55
Radiographic Studies
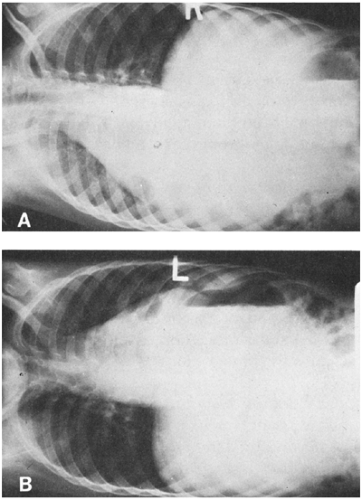
Pleural effusion can be defined on a practical clinical basis as any fluid in the pleural space, as determined by thoracentesis. This definition should be distinguished from the radiologic criterion for a pleural effusion, because much more fluid (about 50 mL in children) is necessary to be visible on a chest film. Furthermore, demonstration of a pleural effusion by chest roentgenogram depends on proper positioning of the patient. A lateral decubitus or upright view is better than the supine view for demonstration of an effusion (Fig. 8-3).
A number of other imaging methods are useful to detect pleural fluid. Perhaps portable ultrasonic evaluation is the most convenient for detecting small amounts, which then can be tapped conveniently.56 Ultrasound can also help differentiate effusion from consolidation.57 Computed tomography is exceedingly useful in studying unusual pleural effusions detected by other methods, particularly in determining possible underlying disease.58
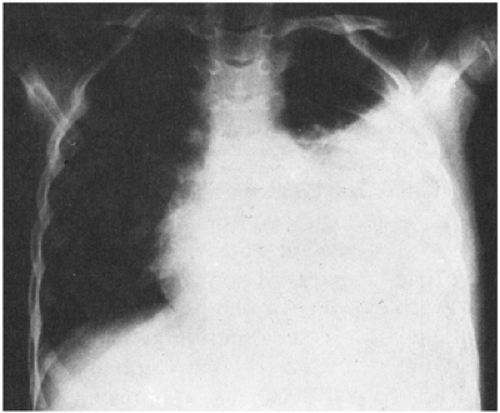
In adults, it usually takes approximately 200–500 mL of fluid to produce blunting of the costophrenic angle in an erect postero-anterior radiograph. This amount of fluid typically produces a noticeable increase in the density of the lower lung zone on the supine radiograph.58 As the amount of effusion increases according to the upright film, the density in the supine radiograph also increases in stepwise fashion. The classic findings of increased density over the entire hemithorax with apical capping occurs only with a large pleural effusion (Fig. 8-4).59 Another useful sign on the frontal film is the thorn sign, a thorn-like protrusion of fluid at the lateral end of the minor fissure.60
Fluid recovered by thoracentesis can be regarded as pleural fluid if it does not have the same hematocrit as the patient’s venous blood. The gross appearance of pleural fluid may be bloody (suggesting traumatic hemothorax or entry of the needle into a blood vessel), blood-tinged (often a result of slight bleeding from the procedure), cloudy and purulent (suggesting an exudate from an infectious process), or serous (suggesting a transudate). Patients who have had recent thoracic surgery may have a chylous effusion (milky-appearing) from disruption of the thoracic duct.
Even if the fluid obtained is not grossly purulent, it should be Gram-stained and cultured. A white cell count and differential study is useful. Measurement of protein content and LDH can be cancelled if the Gram stain shows bacteria.
Diagnostic Procedures
Several procedures may be of value in patients with suspected pleural effusions. Thoracentesis is the most common procedure if fluid is obviously present. Lung puncture is a closely related procedure, since attempts at lung puncture often yield unsuspected pleural fluid. Needle biopsy of the pleura is rarely done in children.
When pleural fluid is suspected, or even if the problem is distinguishing lung abscess or pneumonia from pleural effusion, thoracentesis should be done promptly (see Box 8-4).
Thoracentesis
Whenever there is radiologic evidence of pleural fluid in a patient with pneumonia, some of the fluid
should be removed for Gram stain and culture.61 Thoracentesis is rarely associated with complications.62 However, overly-rapid removal of large volumes may cause a sudden shift of the mediastinum with reflex changes in heart rate and, occasionally, cardiac arrest. Pneumothorax is rare.
should be removed for Gram stain and culture.61 Thoracentesis is rarely associated with complications.62 However, overly-rapid removal of large volumes may cause a sudden shift of the mediastinum with reflex changes in heart rate and, occasionally, cardiac arrest. Pneumothorax is rare.
BOX 8-4 Management of Parapneumonic Effusions in Children
|
The purpose of the procedure is to obtain enough fluid for diagnostic purposes, but occasionally respiratory excursions are improved by the removal of moderate volumes. Thoracentesis and exact bacteriologic diagnosis are especially needed in a severely ill patient. Even a small amount is useful for diagnostic study.
Fluid Studies
Thoracentesis is especially useful for culture of pus to determine the identity of the infecting organism so antibiotic therapy can be tailored. If the Gram stain is quickly available and reveals definite bacteria, the cell count, differential, protein measurement, glucose, and other studies add little. Both aerobic and anaerobic cultures should be ordered.
Rapid Antigen Testing
Intrapleural Fibrinolytic Therapy
Empyemas treated with closed chest tube drainage frequently loculate and then require a surgical procedure to remove the congealed pus. Several small trials have studied the idea of instilling fibrinolytic agents (such as urokinase) through the chest tube to prevent or even treat loculation.64,65,66
In children, only case series are reported.67 In adults, this practice seems to be safe and to have a modest effect in shortening hospital stay and decreasing the need for surgical intervention. A systematic review concluded that because of the small size of these randomized trials there was insufficient evidence to support the routine use of intrapleural fibrinolytic therapy in the management of empyema.68 A large, multicenter trial is currently underway.
Needle Biopsy of the Pleura
Percutaneous needle biopsy of the pleura is especially useful in the diagnosis of tuberculous pleurisy in adults. The procedure has also been used in children.69
Infectious Etiologies of Pleural Effusion
Sterile infectious effusions are relatively frequent. In a recent review of 76 cases of complicated parapneumonic effusions in children, 32 (42%) were sterile.70 The most commonly identified agents were S. pneumoniae (31 patients), S. aureus (7 patients), and Group A streptococcus (5 patients). The proportion of cases caused by S. aureus increased from 6% in 1996–2000 to 30% in 2001. The more recent cases of S. aureus pneumonia were also more likely to be caused by methicillin-resistant strains.70
Thin Effusions
If the pleural fluid exudate is relatively clear, several etiologies are more likely (Box 8-5). Tuberculosis is a possibility if the fluid appears serous but the protein concentration is high. In tuberculous effusion, which reflects hypersensitivity, the tuberculin test is almost always positive.71 Partially treated bacterial pneumonia with effusion is also a frequent cause of exudate that is not grossly purulent.
Box 8-5 Causes of Pneumonia with Effusion
| Common S. aureus S. pneumoniae Mycoplasma pneumoniae (small effusion) Group B streptococci (newborn) Sterile effusions (usually because of prior antibiotics) |
| Uncommon Group A streptococci Other streptococci H. influenzae type b Nontypable H. influenzae Systemic fungi Anaerobic enteric bacteria Tuberculosis (adolescent) |
| Rare F. tularensis Pasteurella multocida Atypical measles Adenovirus (newborn) Paragonimiasis (immigrant from Far East) Aerobic enteric gram-negative rods Hepatitis B Hepatitis A Yersinia, Listeria, Chlamydia Cat scratch disease Amebiasis Dengue fever Leptospirosis |
| Noninfectious Congestive heart failure Nephrotic syndrome Cirrhosis (late complication of cystic fibrosis) Vitamin A intoxication Malignancy Low serum protein diseases Snake envenomation Lymphatic abnormalities |
Mycoplasma pneumoniae can cause small effusions in children.72 In a study of young adults with mycoplasmal and viral pneumonia, small pleural effusions were found in about 20% of the 59 patients with serologic evidence of nonbacterial pneumonia.73 Pleural effusions were seen in 6 (21%) of 29 patients with Mycoplasma, 1 (14%) of 7 with adenovirus, 1 (25%) of 4 with influenza virus pneumonia, and in 4 (21%) of 19 patients with elevated titers to cold agglutinins 73 Another study of 56
patients with moderately severe disease from M. pneumoniae revealed that pleural effusions were detectable in 8 (14)%.74 However, other studies have indicated that the frequency of pleural effusions in Mycoplasma pneumoniae in young adults is closer to 5%.75
patients with moderately severe disease from M. pneumoniae revealed that pleural effusions were detectable in 8 (14)%.74 However, other studies have indicated that the frequency of pleural effusions in Mycoplasma pneumoniae in young adults is closer to 5%.75
Adenovirus, particularly type 7, can cause massive pleural effusions and disseminated disease, which may be fatal.76 Epstein-Barr virus (EBV) is sometimes associated with a pleural effusion.77 In infants and young children, the illness may not resemble infectious mononucleosis except for the presence of atypical lymphocytosis.78 When a pleural effusion accompanies typical infectious mononucleosis in teenagers, a concurrent mycoplasmal pneumonia may be present.
A small pleural effusion can occur with viral hepatitis and may precede jaundice.79 In an effusion secondary to hepatitis B, which is an immune-complex reaction, both blood and pleural fluid are positive for hepatitis B surface antigen, and serum transaminases are elevated even if jaundice has not yet appeared. Pleural effusion during the course of hepatitis A virus infection has been reported.80 Cat scratch disease can produce a pleural effusion and anicteric hepatitis.81 Leptospirosis can be associated with a pleural effusion; other features of the disease may lead to the correct diagnosis.82 Yellow nail syndrome is an exceedingly rare lymphatic abnormality that may cause pleural, as well as pericardial, effusions.83
Purulent Effusions
Frankly purulent pleural fluid (empyema) is almost always caused by bacterial pneumonia. Before antibiotics were available, the pneumococcus or beta-hemolytic streptococci were the most common causes of empyema.84,85,86 For a time thereafter, Staphylococcus aureus became the most common cause of empyema, but in recent years S. pneumoniae has again become prominent. The percentage of patients with pneumococcal pneumonia who have parapneumonic effusions is not as high as that seen with S. aureus (approximately 80% of children with S. aureus pneumonia have effusions);32 but cases of pneumococcal pneumonia outnumber those of staphylococcal pneumonia by a large margin. In one series, 88% of culture-positive cases of parapneumonic effusion were due to S. pneumoniae.87 A more recent study suggest that S. aureus may once again be surpassing the pneumococcus as a cause of empyema.88 As mentioned earlier, Group A streptococcus is an occasional cause. Rare causes include F. tularensis,89 H. influenzae type b, and gram-negative enteric bacteria such as Pseudomonas or Salmonella.90,91
The viridans group of streptococci and diphtheroids (which are normal mouth flora) are rare causes of empyema associated with aspiration pneumonia, particularly in adults. Pasteurella multocida has caused empyema in a child with underlying pulmonary disease and exposure to animals.92 Nocardia is a rare cause of pleural effusion, typically in compromised hosts. Other uncommon infectious causes of pleural effusions include Yersinia, Chlamydia trachomatis, and Listeria.93,94,95
Bacteroides or Clostridium species, anaerobic actinomyces, and anaerobic streptococci are occasional causes of empyema (particularly in adults), so that any fluid removed should be cultured anaerobically.96,97 Blastomycosis, histoplasmosis, and coccidioidomycosis can be associated with thin to moderately purulent pleural effusions.98,99,100 These fungi and cryptococci are special risks for immunosuppressed patients.101,102,103,104 However, massive lung disease can sometimes occur in immunologically normal hosts who are exposed to a large inoculum of the fungus. Parasitic causes include paragonimiasis (in Far Eastern immigrants) and amebiasis.105,106
Noninfectious Causes
Noninfectious diseases also must be considered as possible causes of pleural exudates. Malignancy involving the pleura, especially lymphoma and neuroblastoma, sometimes produces effusions resembling those of tuberculosis. Rheumatoid disease, pancreatitis, and pulmonary infarction, which may occur in adults, are rare causes of thin exudates in children. Nitrofurantoin has been reported as a cause of allergic pneumonitis with effusion in adults. Trauma has been reported as a cause of effusion that may contain eosinophils.107 Snake envenomation has been cited as a cause of pleural effusion.108 Vitamin A intoxication is a rare cause in children.109
Intra-abdominal Abscess
Empyema is rarely the result of a perforated appendix.110 Sometimes it is difficult to determine whether fluid is above or below the diaphragm if CT scanning is not readily available. Usually, it is
better to do a thoracentesis and look for pleural fluid first, as removal of pleural fluid often results in the disappearance of radiologically apparent subdiaphragmatic fluid.
better to do a thoracentesis and look for pleural fluid first, as removal of pleural fluid often results in the disappearance of radiologically apparent subdiaphragmatic fluid.
Pulmonary Embolism
Pulmonary embolism (PE) is uncommon in children, and when it occurs the diagnosis is usually delayed. The symptoms of tachypnea, chest pain, dyspnea, and fever are easily confused with pneumonia, which is usually the initial diagnosis. Most children with PE have one of several well-described risk factors, such as recent surgery, immobilization, malignancy, pregnancy, obesity, heart disease, the presence of a central venous catheter, oral contraceptive use, nephrotic syndrome, and sickle cell anemia.111,112,113,114
In children who develop PE without apparent risk factors, nearly all are found to have an antiphospholipid antibody or coagulation regulation protein abnormality (such as protein C or S deficiency).115
Tuberculous Pleural Effusions
Tuberculosis is much less common now in the United States than it used to be, and the relevant studies are now old. However, it remains common in the developing world, and physicians should consider the diagnosis in patients from endemic areas or with a suggestive exposure history. In one study of 202 children with tuberculous pleural effusion, published in 1958, the average duration of cough was 21 days. Difficulty breathing was noticed in only 25 (12%) of the children. The age range for pleural effusions indicated a rather even distribution between 1 and 13 years of age.71 A newer report from Spain looked at 175 children with pulmonary tuberculosis; 22% of them had an effusion. On average, patients who had an effusion accompanying their pulmonary tuberculosis were older than those without effusion (13.5 vs. 7 years). In 41%, the effusion was the sole radiographic manifestation of pulmonary TB. Almost all of them had a tuberculin skin test (TST) greater than 5 mm.116 Pleural effusions in TB are often accompanied by erythema nodosum. Sometimes, pleural effusions develop during chemotherapy for pulmonary tuberculosis.117
The most accurate way to confirm the diagnosis of a tuberculous effusion when it cannot be detected by examining the fluid for acid-fast bacilli is by pleural biopsy.69 In the Spanish study, pleural fluid cultures were positive in 44%, biopsy cultures were positive in 67%, and the pleural biopsy yielded granulomatous changes in 78% of patients.116 Pleural biopsy may not be necessary in a child with a suspected tuberculous effusion if the typical findings of pulmonary tuberculosis are present, especially if the organism can be cultured from another source, such as gastric aspirates.
Mycobacteria other than Mycobacterium tuberculosis can cause effusion, although this is most commonly associated with acquired immune deficiency syndrome (AIDS) in adults. Most often, the organism is of the Mycobacterium avium complex.118
Infectious Causes in the Newborn
In the newborn period, there are several causes of pneumonia associated with a pleural effusion. These include:
Group B streptococci119
Adenovirus with congenital pleural effusion120
E. coli121
Long-term ventilator therapy, especially with multiple courses of antibiotics; this can result in empyema caused by almost any of the nosocomial pathogens found in neonatal intensive care units.
Neonatal pneumonia and other pulmonary diseases in the newborn are discussed further in Chapter 19.
Pneumatoceles
Pneumatoceles occurring with empyema usually indicate staphylococcal pneumonia (Box 8-6).
However, necrotizing pneumonia caused by other bacteria, including H. influenzae, Pseudomonas, and Klebsiella, can result in pneumatoceles.122,123 Group A streptococci can produce pneumatoceles, and the throat culture may be negative for beta-hemolytic streptococci.85 E. coli is an occasional cause of empyema, especially in the newborn period, and can produce pneumatoceles identical to those caused by staphylococci.124 Rarely, septic emboli secondary to Fusobacterium infection produce pneumatoceles and may originate as exudative pharyngitis. Pneumatoceles can also occur with pneumococcal pneumonia, after hydrocarbon aspiration, and in Pneumocystis pneumonia.84,125,126,127
However, necrotizing pneumonia caused by other bacteria, including H. influenzae, Pseudomonas, and Klebsiella, can result in pneumatoceles.122,123 Group A streptococci can produce pneumatoceles, and the throat culture may be negative for beta-hemolytic streptococci.85 E. coli is an occasional cause of empyema, especially in the newborn period, and can produce pneumatoceles identical to those caused by staphylococci.124 Rarely, septic emboli secondary to Fusobacterium infection produce pneumatoceles and may originate as exudative pharyngitis. Pneumatoceles can also occur with pneumococcal pneumonia, after hydrocarbon aspiration, and in Pneumocystis pneumonia.84,125,126,127
Chest Tubes
After thoracentesis has confirmed an empyema, a chest tube should be placed early so that the fluid can be removed thoroughly before it becomes too thick. The chest tube can be put into loculated fluid with guidance from ultrasound.
If it is a free-flowing effusion, as can be determined by lateral decubitus films (Fig. 8-3), the catheter can be put in a comfortable dependent location so that the patient can lie in comfort. Thoracoscopy can also be used for insertion of the chest tube.128
A large chest tube with continuous suction or straight drainage (no suction) should be inserted in all patients with anything more than small amounts of fluid. Small pigtail catheters drain transudates adequately, but should never be used in an attempt to drain parapneumonic effusions.129 Decortication is rarely necessary if adequate chest tube drainage is used early.130 The chest tube need not be inserted immediately if the fluid is serous, until the thoracentesis fluid has been analyzed. However, even if the fluid is thin, if it has the characteristics of an exudate, a chest tube should be put in, as the fluid may become more purulent except when tuberculosis is the cause.
The approach to loculated or thick and poorly draining pleural fluid is the subject of some debate. Studies show that early decortication reduces the duration of hospitalization and the length of illness.88,131,132 However, others have reported that instillation of urokinase through an existing chest tube resulted in increased drainage and avoidance of surgery in 6 of 7133 and in 8 of 9 67 children with loculated pleural effusions. These authors recommend a trial of intrapleural urokinase instillation prior to surgical decortication. Clearly, much work remains to be done in defining the optimal approach to complicated pleural effusions in childhood.
Antibiotic Treatment
Antibiotic treatment should be directed at penicillin-resistant staphylococci and S. pneumoniae unless smear or culture indicates another organism. As a single agent, cefuroxime has reasonable activity against both S. aureus and the pneumococcus, although it is not the agent of choice for either organism. Thus, the combination of oxacillin (for superior S. aureus coverage) and cefotaxime (for superior S. pneumoniae coverage) is often used. In areas with a high incidence of community-acquired methicillin-resistant S. aureus, the use of vancomycin or clindamycin should be considered.134 If the fluid has a fetid odor, the possibility of an anaerobic infection should be considered and clindamycin or metronidazole should be added.54
Complications
Pneumothorax may occur because of a bronchopleural fistula resulting from a break in a bronchial wall. Tension pneumothorax is suggested by a sudden increase in dyspnea and cyanosis. It may occur when the bronchial tear produces a valve-like effect, with entry of air into the pleural space on inspiration, and trapping of air by closure of the passage on expiration. This may produce collapse of the lung and a shift of the heart and mediastinum, especially in infants, who have a more movable mediastinum than do older children. Emergency release of the pressure should be done by insertion of a large needle and withdrawal of the free air until a chest tube can be inserted.
As with other severe infections, anemia is common in severe empyema of any cause; transfusion is only necessary if the child is symptomatic, which is uncommon.135
Atypical Pneumonia Syndromes
Definitions
Criteria for the preliminary diagnosis of atypical pneumonia are the opposite of those for typical lobar pneumonia. The following features are usually regarded as atypical:
Subacute onset. The onset is gradual, with cough for several days before the patient seeks medical
attention. Toxicity is absent, and fever, if present, is low-grade.
Prominent extrapulmonary features. Headache, sore throat, and pharyngeal exudates may be present and are often more prominent than non-productive cough or dyspnea.
Minimal or disparate chest signs. Rales may be bilateral or localized, but there is often a disparity between the auscultatory and radiologic findings. That is, the chest film often shows more extensive involvement than the clinician hears. On the other hand, sometimes the radiologic findings are minimal when the patient is cyanotic with a severe diffusion block.
Chest infiltrate not focal. The infiltrate is patchy or mottled, with various degrees of density, usually without a single dense area of consolidation.136 There may be a wedge-shaped or linear infiltrate or a bilateral interstitial infiltrate.
No clinical response to penicillins or cephalosporins.
No significant leukocytosis.
Slow course. There is gradual improvement, sometimes with a long convalescence.
There are a number of other clinical patterns of pneumonias that are not typical of lobar pneumonia that can be excluded from the group of atypical pneumonia because of distinctive features. These pneumonias are discussed in other sections of this chapter: chronic or recurrent pneumonia, progressive or fulminating pneumonia, pneumonia with eosinophilia (Loeffler’s syndrome), and miliary or multiple nodular pneumonia. Bronchiolitis with pneumonia and pertussis are discussed in Chapter 7.
Classification
It is useful to preserve the term atypical pneumonia as a broad preliminary clinical and radiologic diagnosis that can be subdivided into more specific problem-oriented diagnoses on the basis of certain features. Most patients with atypical pneumonia can be classified into one of the following subgroups or a combination of two of them. As noted earlier, most atypical pneumonias have a subacute onset.
Subacute Minimal Patchy Pneumonia
This subgroup is distinguished by a gradual onset over several days, usually with prominent extrapulmonary symptoms. The chest roentgenogram shows one or more patches of minimal foci of pneumonia. The most common causes are Mycoplasma pneumoniae, Chlamydia pneumoniae, and adenoviruses. Other causes are discussed in the following section on possible etiologies.
Subacute Dense Focal Pneumonia
This subgroup is distinguished by a subacute onset and an unexpectedly dense focal infiltrate that is segmental or smaller. Most of the other features of acute focal pneumonias (typical pneumonia), such as fever, toxicity, marked leukocytosis, and focal chest signs, are absent. Tuberculosis is an important consideration to exclude.
Acute Interstitial Pneumonia
This subgroup is distinguished by the absence of a solid focal or even minimal focal infiltrate. The infiltrate is sometimes described as reticular or patchy. There are many possible causes, most of which are viral and self-limited, but some of which are not viral or are progressive (Box 8-7).
Preliminary Diagnoses to Avoid
Mycoplasmal pneumonia should not be used as a preliminary diagnosis without proof of etiology, because many atypical pneumonias are not caused by M. pneumoniae. Furthermore, it is not possible to distinguish mycoplasmal pneumonia from other atypical types on clinical grounds.138
Nonbacterial pneumonia is a conclusion usually based on such observations as failure to respond to antimicrobial therapy, absence of leukocytosis, and sparse infiltrate. However, the term nonbacterial pneumonia should not be used, because it implies that bacterial causes have been excluded, when in fact this can rarely be done with certainty.
Possible Etiologies
The following etiologies are listed in approximate decreasing frequency of occurrence of the infectious agents within each microbiologic category.
Mycoplasmas
M. pneumoniae is probably the most frequent cause of atypical pneumonia, especially in school-age children and young adults.139,140 Usually, the radiologic pattern is subacute with minimal densities, but occasionally it is an acute bilateral interstitial pneumonia.141
Rarely, it is lobar, as cited in the preceding section on focal pneumonias. The physician is often aware of an outbreak of atypical pneumonia syndrome (“walking pneumonia”) in the community. Typically, there is a long incubation period (2–3 weeks) between illnesses in the same family, a feature that helps with the diagnosis.139 However, cases seen in association with outbreaks may have incubation periods as short as one week. Occasionally, a point-source outbreak occurs,142,143 but usually the illnesses involve separate members of a family over a long period of time.139
Rarely, it is lobar, as cited in the preceding section on focal pneumonias. The physician is often aware of an outbreak of atypical pneumonia syndrome (“walking pneumonia”) in the community. Typically, there is a long incubation period (2–3 weeks) between illnesses in the same family, a feature that helps with the diagnosis.139 However, cases seen in association with outbreaks may have incubation periods as short as one week. Occasionally, a point-source outbreak occurs,142,143 but usually the illnesses involve separate members of a family over a long period of time.139
BOX 8-7 Possible Causes of Atypical Pneumonia Patterns
| Common C. trachomatis (< 4 months) RSV (< 5 years) Mycoplasma pneumoniae (> 5 years) Chlamydia pneumoniae (> 5 years) Adenoviruses Parainfluenza viruses Influenza virus (in epidemics) CMV; varicella-zoster virus (in immunosuppressed) B. pertussis (usually not associated with pneumonia) |
| Uncommon Hypersensitivity penumonitis Drug hypersensitivity Herpes simplex virus Pneumocystis jiroreci (carinii) Chlamydia psittaci H. influenzae S. pneumoniae Systemic fungi Tuberculosis |
| Rare or Unproved Ureaplasma urealyticum (< 3 months) Mycoplasma hominis (< 3 months) Q fever Rhinoviruses; enteroviruses Late-onset rubella syndrome Human metapneumovirus137 SARS-coronavirus |
Otitis media with severe, painful, extremely red tympanic membranes is rarely caused by M. pneumoniae (as discussed previously). Urticarial or nonspecific maculopapular rashes are also associated in some cases.139 Eosinophilia exceeding 5% is not unusual.140 More severe complications are discussed at the end of this section.
It is often taught that mycoplasmal pneumonia has a classic radiographic appearance, which consists of bilateral reticular or interstitial infiltrates, with or without patchy infiltrates. However, almost any x-ray pattern is possible. Brolin and Wernstedt reported the radiographic findings of 56 patients with M. pneumoniae lower respiratory tract infection: 8 (14%) of patients had lobar findings and 21 (38%) had lobar or predominantly alveolar findings. Some had a combination of lobar/alveolar and interstitial infiltrates; if those are added, 36 (64%) of 56 patients had some component of lobar or alveolar infiltration. Twenty (36%) of the patients showed a purely interstitial pattern, and 33 (59%) had some component of interstitial infiltration. Interestingly, 22% had hilar adenopathy and 14% had a pleural effusion.74
M. pneumoniae pneumonia occurs most frequently in individuals 5–12 years old.144 Subclinical infection is probably common before 5 years of age, but the organism is a relatively unusual cause of pneumonia in this group.145 Most young adults have serum antibodies, which suggests a past infection and indicates that past infection is apparently not protective against future reinfection.
Chlamydiae
There are three medically important species in this genus. C. trachomatis is a frequent cause of acute bilateral afebrile pneumonia in infants less than 4 months of age and a less-frequent cause of pneumonia in older children and adults. Because it is sometimes associated with peripheral eosinophilia, it is discussed in the section on pulmonary infiltrates with eosinophilia (PIE syndrome).
C. psittaci infection (psittacosis) is uncommon in the United States, with only about 50 cases reported annually. Nevertheless, it is important to recognize because it responds to tetracycline. There is typically an exposure to parakeets or other birds.146 Large outbreaks have been reported from turkey processing plants.147 Psittacine birds (those with a bent beak) are more likely to be infected, and most birds with psittacosis are ill. C. psittaci infection in humans usually produces fever, chills, and severe headache; arthralgia is sometimes present. Typically, there has been the gradual onset of cough, and there is a patchy infiltrate on chest x-ray. Usually there is no leukocytosis, but eosinophilia is sometimes present. Elevated serum transaminase and alkaline phosphatase can occur.
C. pneumoniae, originally classified as a strain of C. psittaci called the TWAR agent (named after the first two respiratory isolates, TW-183 and AR-39) is now recognized as a fairly common cause of respiratory disease in school-aged children, adolescents, and adults. Studies documenting its prevalence have been hampered by the lack of a diagnostic gold standard. The organism will not grow on cell-free media but is cultivatable in tissue culture. A nasopharyngeal swab is an appropriate specimen. The sample must be transported in culture transport media and processed within 24 hours of collection. The epidemiology of C. pneumoniae is similar to that of M. pneumoniae in that infection with this organism is rare in infancy, and becomes more common through childhood and peaks at adolescence. Most infections are probably asymptomatic.148
At all age points it appears to be somewhat less common than M. pneumoniae.149 Respiratory disease caused by C. pneumoniae is very similar to that caused by M. pneumoniae. A prospective study of 667 college students with acute respiratory disease found 20 patients (3%) with C. pneumoniae and 29 patients (4%) with M. pneumoniae infection; patients with C. pneumoniae were less likely to have a temperature greater than 100° F (37.8° C) and were more likely to present with sore throat as a prominent complaint (80% vs. 52%). Hoarseness was seen in 30% of patients with C. pneumoniae infection but only in 3% of patients with M. pneumoniae.150 Finally, the time from onset of symptoms to presentation for medical care was longer in patients with C. pneumoniae infection.
A variety of serologic tests are available that make a retrospective diagnosis. It may take 3–4 weeks to mount a measurable response; when convalescent titers are ordered too soon the diagnosis may be missed. Polymerase chain reaction (PCR) has been done experimentally, but the results are not always concordant with culture and serology. From 10–90% of patients positive for C. pneumoniae infection by PCR do not mount appropriate serologic responses.151,152
Some people harbor the organism for months after the acute infection. Such patients may be asymptomatic, or they may have problems with periodic wheezing and asthma. It has been suggested that some adults with severe, refractory wheezing may have chronic C. pneumoniae infection. In one study, resolution of infection correlated with improvement in asthma control.153 Another study found that children with persistent C. pneumoniae PCR positivity suffered from more frequent asthma attacks.154 C. pneumoniae-specific immunoglobulin E (IgE) was found in 12 (86%) of 14 culture-positive children with wheezing compared with only 1 (9%) of 11 culture-positive children without wheezing.155 The precise relationship between C. pneumoniae infection and asthma is not understood.
Patients with cystic fibrosis can undergo pulmonary exacerbations in association with C. pneumoniae infection. In the one published study on this topic, M. pneumoniae, by contrast, was not found to be associated with exacerbations.156
Unusual Bacterial Causes
Some bacterial pneumonias are patchy or interstitial and do not respond to macrolides, the usual antibiotic therapy for M. pneumoniae. Tularemia is an example.157
Viruses
RSV is a frequent and important cause of atypical pneumonia, which is usually of the acute bilateral interstitial subgroup but can be patchy or even densely focal. The airway disease is more important than the alveolar involvement (Chapter 7).
A newly discovered virus, termed the human metapneumovirus (hMPV), was first isolated from nasopharyngeal aspirates of 28 children in the Netherlands who presented with respiratory tract symptoms and negative tests for known causes.137 Like RSV, symptomatic infection appears to be most common in the first year of life. Symptoms ranged from mild respiratory problems to severe bronchiolitis or pneumonia, often accompanied by high fever and vomiting. Serologic testing suggests that infection within the first 5 years of life is nearly universal.
Adenoviruses, of which there are more than 40 serotypes, are a frequent cause of atypical pneumonia. Adenoviral pneumonia cannot be distinguished on clinical grounds from mycoplasmal pneumonia.138 Occasionally, epidemics occur with severe disease, often with accompanying gastrointestinal signs.158
Influenza viruses almost always are associated with epidemics or large outbreaks. They can cause an atypical pneumonia usually associated with prominent extrapulmonary manifestations.
Parainfluenza virus type 3 can cause atypical pneumonia, especially in young children. It usually produces bronchiolitis (discussed in Chapter 7),
possibly with minimal patchy pneumonitis, but occasionally produces bilateral interstitial pneumonia or perihilar pneumonia without bronchiolitis.
possibly with minimal patchy pneumonitis, but occasionally produces bilateral interstitial pneumonia or perihilar pneumonia without bronchiolitis.
Rhinoviruses rarely cause atypical pneumonia.159 Likewise, coxsackie A or B viruses are rarely associated. Coxsackievirus B has been recovered from the lungs of patients dying with pneumonia, and in the rare newborn or infantile case, the clinical syndrome is usually that of fulminant disease with concurrent myocarditis.
Measles virus is frequently associated with interstitial infiltrates when there is a classic measles illness.160 It is not ordinarily considered a cause of atypical pneumonia, because the diagnosis is obvious in the classic case. However, pneumonitis is present before the eruption in about 20% of patients with classic measles.160 Measles virus can cause fulminant pneumonia, as described in a later section.
Varicella-zoster virus can produce atypical pneumonia, especially in adults. The diagnosis should present no problem if the typical rash is present. The syndrome usually presents as chickenpox with pneumonia rather than as atypical pneumonia possibly caused by chickenpox virus. The virus can cause fulminating pneumonia in an immunosuppressed patient.
Cytomegalovirus (CMV) can produce an atypical pneumonia but more often produces an acute interstitial pneumonia in a patient with an immunologic problem (especially solid organ or stem cell transplant patients). In this population, CMV pneumonia is characterized by high fever, hypoxemia, and diffuse interstitial infiltrates. This is a serious disease with a high mortality rate. CMV also can cause interstitial pneumonia in very young infants, who appear to become colonized from their mother’s genitourinary tract just before or during delivery.161,162
Herpes simplex can cause interstitial pneumonia, especially in immunosuppressed patients and newborns, that may become severe and extensive.163,164
Late-onset rubella syndrome is a rare cause of bilateral interstitial pneumonia.165 It is typically associated with diarrhea, a skin rash, and hypoglobulinemia. It usually occurs between 3 and 12 months of age, with cough, tachypnea, and cyanosis and is associated with circulating immune complexes.
Infectious mononucleosis can produce hilar adenopathy as a part of the generalized lymphadenopathy, and a few patients have small pulmonary infiltrates.166 Concurrent infection with M. pneumoniae might explain some cases of infectious mononucleosis with pneumonia, as both diseases are common in young adults.167
The recently discovered severe acute respiratory syndrome (SARS) coronavirus is discussed in the section on progressive or fulminating pneumonias.
Bacteria
Bordetella pertussis can be associated with an atypical pneumonia with subacute onset of cough, slight fever, and pulmonary infiltrates, especially linear lower-lobe interstitial infiltrates, perihilar infiltrates (shaggy heart border), or wedge-shaped upper-lobe infiltrates, perhaps with atelectasis. Lymphocytosis is usually present; when it is not, the clinical diagnosis is likely to be atypical pneumonia with perihilar infiltrate. Most children with pertussis have neither fever nor pulmonary infiltrates unless a secondary bacterial pneumonia (e.g., S. aureus) ensues. Pertussis-like illnesses are discussed in Chapter 7.
Other bacteria that can cause atypical pneumonia include the pneumococcus and H. influenzae. They are probably a more frequent cause of atypical pneumonia than are bacteria rarely causing human infection, such as F. tularensis. P. aeruginosa, acquired from water, can rarely cause atypical pneumonia.168 Legionella is an important consideration, particularly in the immunocompromised host.
Mycobacteria
Mycobacterium tuberculosis is an uncommon but important cause of atypical pneumonia with a subacute onset, low-grade fever, and diffuse or linear infiltrates. Most often, the pneumonia is focal and dense, but the rest of the features are atypical, which would fit into the subgroup of a subacute dense focal pneumonia.
Occasionally other mycobacterium species are associated with an atypical pneumonia. Recently, nontuberculous mycobacteria (especially M. avium complex) have been associated with a diffuse pneumonia in patients exposed to hot tubs. Patients present subacutely with dyspnea, cough, hypoxia, and fever.169 Some patients respond to corticosteroids alone, and thus the illness may be due to a hypersensitivity reaction to the organism.170
Rickettsiae
Coxiella burnetii, the cause of Query fever (Q fever), is a rare cause of atypical pneumonia.171 The organism
is excreted in large numbers at the time farm animals, particularly sheep, give birth. Long after the fact, viable organisms remain in the soil, and can be blown about by the wind. Thus, the patient need not have been in attendance at a birth but usually lives in a rural area and has some farm animal exposure history. The condition usually responds to doxycycline or erythromycin, which may be given because of suspected mycoplasmal pneumonia.
is excreted in large numbers at the time farm animals, particularly sheep, give birth. Long after the fact, viable organisms remain in the soil, and can be blown about by the wind. Thus, the patient need not have been in attendance at a birth but usually lives in a rural area and has some farm animal exposure history. The condition usually responds to doxycycline or erythromycin, which may be given because of suspected mycoplasmal pneumonia.
Inhalational Anthrax
In the past, this disease was very rare, and occurred primarily in those exposed to contaminated animal products (wool sorter’s disease). Although still rare, recent bioterrorism-related cases were well publicized.172 The illness presents in two stages. The first stage is that of an influenza-like illness with fever, cough, and chest pain. The second stage presents a few days later with abrupt onset of high fever, severe dyspnea, and shock. Chest x-ray classically demonstrates only a widened mediastinum, but progressive perihilar infiltrates can be present as well.172 At least two drugs active against B. anthracis are recommended for treatment, (such as a fluoroquinolone plus rifampin). Despite appropriate therapy, the mortality rate is more than 50%.
Fungi
Histoplasma capsulatum and Coccidioides immitis are occasional causes of an atypical pneumonia. Patients may present with an acute interstitial pattern when there has been a single overwhelming exposure, but more often they present with a focal infiltrate of subacute onset.
Pneumocystis jiroveci can cause bilateral pulmonary infiltrates in immunocompetent infants 2–12 weeks of age.173 Infants receiving high doses of systemic corticosteroids for prolonged periods of time are at increased risk,173a as are children with HIV infection (Chapter 20), transplant recipients (Chapter 22), and patients with primary cellular immunodeficiency (Chapter 23). Cough, tachypnea, and apneic episodes may occur, but fever is unusual, so that the clinical pattern resembles that of pertussis or chlamydial pneumonia. Serum IgM is usually elevated. Treatment is with trimethoprim-sulfamethoxazole, which is now used as routine prophylaxis for immunocompromised patients.
Miscellaneous Mycoplasma Species
Noninfectious Causes
Congestive heart failure often resembles an atypical pneumonia. Pulmonary infarction or embolism is uncommon in children, as discussed in the section on pneumonia with effusion.
Allergies to Actinomyces species (which may contaminate air conditioners), to pigeons (pigeon breeder’s disease), and to maple bark also may cause noninfectious atypical pneumonia with diffuse interstitial infiltrates. These pneumonias are discussed in the sections on chronic and recurrent pneumonia and PIE syndrome.
Toxic causes include silo filler’s disease, caused by inhalation of nitrogen oxides, and other occupational inhalants. Medications such as phenytoin or nitrofurantoin are a rare cause of acute interstitial pneumonia.175
Collagen diseases occasionally associated with atypical pneumonia include rheumatic fever (Chapter 18) or rheumatoid arthritis (Chapter 10).
Laboratory Tests
Cold Agglutinins
Because the test is neither sensitive nor specific enough to establish a diagnosis, the use of cold agglutinins is in decline. In certain situations, however, the test may be helpful. If a specimen is desired, it may be obtained as soon as the pneumonia is recognized as “atypical,” as agglutinins are often present at this point in the illness. This specimen can also be used as an acute serum for mycoplasmal and viral antibody studies.
Cold agglutinins are found in low titers (about 1:10) in about 10% of normal adults.176 A titer of 1:32 or higher has been regarded as abnormal. M. pneumoniae, C. pneumoniae, and adenoviruses are the most likely causes of cold-agglutinin positive atypical pneumonia. However, RSV, parainfluenza viruses, and coxsackieviruses may also be to blame.177 Cold agglutinin titers greater than 1:10 are unusual in influenza-virus infection but can
occur in patients with pneumococcal pneumonia with bacteremia.176
occur in patients with pneumococcal pneumonia with bacteremia.176
A rapid bedside screening test for cold agglutinins can be performed by placing a few drops of blood into an anticoagulant tube and cooling it on ice. After inserting the specimen in ice, agglutination of red blood cells is visible as clumps when the tube is held up to a light source and rolled slowly. Quantification of a titer of cold agglutinins is not possible at the bedside, although blood with large quantities agglutinates more than does blood with low titers. In one study, 50% of children with acute respiratory disease and 75% of children with pneumonia and serologic evidence of M. pneumoniae infection had a positive screening test for cold agglutinins.178 If a cold agglutinin test is positive in a preschool child, the pneumonia is probably viral. If it is positive in a school-aged child, the pneumonia is probably mycoplasmal. Quantification of cold agglutinin titers is of less utility in children less than 10 years of age.
Rapid Antigen Detection Methods
Some centers offer simultaneous fluorescent-antibody detection of the most common respiratory viruses, including RSV, influenza A and B, the parainfluenza viruses, and adenovirus. Enzyme-linked immunosorbent assay (ELISA) tests are also available for rapid detection of respiratory agents. Such rapid tests are especially useful if there is an established treatment, as for herpes simplex, varicella viruses, or M. pneumoniae, or a treatment that is possibly effective, as for influenza. Commercial ELISA tests for RSV and influenza A virus are readily available.
Serum Antibodies
The serum tested for cold agglutinins can also be used as an acute-phase serum for a battery of antibody studies in comparison with a later serum specimen. Antibodies that may be studied in paired sera include M. pneumoniae, C. pneumoniae, adenovirus, psittacosis, Q fever, influenza virus, and RSV. Occasionally, other agents known by recent laboratory studies to be prevalent in the community will be sought by the reference laboratory.
Often, complement-fixing antibodies to M. pneumoniae are present in low to moderate concentrations in the first serum obtained, which often is a week or so after the onset of the illness. Such low to moderate titers can be regarded as presumptive evidence of current infection with this organism, because these antibodies are usually not detectable in healthy children or adolescents. In one study, an IgM-capture assay drawn in the convalescent phase of illness was the single most reliable test in the diagnosis of M. pneumoniae infection.179 However, in our experience, infections with other organisms can sometimes result in a nonspecific rise in mycoplasma IgM antibodies.
Mycoplasma Cultures
Culture of M. pneumoniae is technically difficult, less sensitive than serology, and not readily available.
Viral Cultures
Culture for viral agents is generally slow. RSV may grow within 3–4 days. Herpes simplex virus, likewise, may be fairly rapidly grown in cell culture. Other respiratory viruses may take a week or two. When virus cultures are available and inexpensive, they may be useful in selected cases, especially to identify viruses prevalent in the community. Some laboratories are able to do a “shell vial” assay for adenovirus, which uses centrifugation and detection of early gene products to speed up the process, in which case results may be available within 48 hours.180
Polymerase Chain Reaction
PCR testing for M. pneumoniae and C. pneumoniae on throat swabs or nasopharyngeal aspirates are available on an experimental basis. The sensitivity of such tests is a matter of debate. Evaluation of these tests is ongoing. PCR testing of cerebrospinal fluid, when positive, can establish the diagnosis of M. pneumoniae encephalitis.181
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree