Overview of the Immune System
Introduction
Anyone who has had the good fortune to hear an orchestra brilliantly perform a symphony composed by one of the great masters knows that each of the carefully tuned musical instruments contributes to the collective, harmonious sound produced by the musicians. In many ways, the normally tuned immune system continuously plays an orchestrated symphony to maintain homeostasis in the context of host defenses. However, as William Shakespeare noted, “Untune that string, and, hark, what discord follows!” (Troilus and Cressida). Similarly, an untuned immune system can cause discord, which manifests as autoimmunity, cancer, or chronic inflammation. Fortunately for most of us, our immune system is steadfastly vigilant in regard to tuning (regulating) itself to ensure that its cellular components behave and interact symbiotically to generate protective immune responses that ensure good health. In many ways the immune system can be described in anthropomorphic terms: Its memory allows it to remember and recognize pathogens years or decades after initial exposure; it can distinguish between the body’s own cells and those of another organism; and it makes decisions about how to respond to particular pathogens—including whether or not to respond at all, as will be discussed in Chapters 2 and 3.
In his penetrating essays, scientist–author Lewis Thomas, discussing symbiosis and parasitism, described the forces that would drive all living matter into one huge ball of protoplasm were it not for regulatory and recognition mechanisms that allow us to distinguish self from nonself. The origins of these mechanisms go far back in evolutionary history, and many, in fact, originated as markers for allowing cells to recognize and interact with each other to set up symbiotic households. Genetically related sponge colonies that are placed close to each other, for example, will tend to grow toward each other and fuse into one large colony. Unrelated colonies, however, will react in a different way, destroying cells that come in contact with each other and leaving a zone of rejection between the colonies.
In the plant kingdom, similar types of recognition occur. In self-pollinating species, a pollen grain landing on the stigma of a genetically related flower will send a pollen tubule down the style to the ovary for fertilization. A pollen grain from a genetically distinct plant either will not germinate or the pollen tubule, once formed, will disintegrate in the style. The opposite occurs in cross-pollinating species: self-marked pollen grains disintegrate, whereas nonself grains germinate and fertilize.
The nature of these primitive recognition mechanisms has not been completely worked out, but almost certainly it involves cell-surface molecules that are able to specifically bind and adhere to other molecules on opposing cell surfaces. This simple method of molecular recognition has evolved over time into the very complex immune system that retains, as its essential feature, the ability of a protein molecule to recognize and bind specifically to a particular shaped structure on another molecule. Such molecular recognition is the underlying principle involved in the discrimination between self and nonself during an immune response. It is the purpose of this book to describe how the fully mature immune system—which has evolved from this simple beginning—makes use of this principle of recognition in increasingly complex and sophisticated ways.
Perhaps the greatest catalyst for progress in this and many other biomedical areas has been the advent of molecular biologic techniques. It is important to acknowledge, however, that certain technological advances in the field of molecular biology were made possible by earlier progress in the field of immunology. For example, the importance of immunologic methods (Chapter 6) used to purify proteins as well as identify specific cDNA clones cannot be understated. These advances were greatly facilitated by the pioneering studies of Köhler and Milstein (1975), who developed a method for producing monoclonal antibodies. Their achievement was rewarded with the Nobel Prize in Medicine. It revolutionized research efforts in virtually all areas of biomedical science. Some monoclonal antibodies produced against so-called tumor-specific antigens have now been approved by the US Food and Drug Administration for use in patients to treat certain malignancies. Monoclonal antibody technology is, perhaps, an excellent example of how the science of immunology has transformed not only the field of medicine but also fields ranging from agriculture to the food science industry.
Given the rapid advances occurring in immunology and the many other biomedical sciences and, perhaps most important, the sequencing of the human genome, every contemporary biomedical science textbook runs a considerable risk of being outdated before it appears in print. Nevertheless, we take solace from the observation that new formulations generally build on and expand the old rather than replacing or negating them completely. Let’s begin, therefore, with an overview of innate and adaptive immunity (also called acquired immunity) which continue to serve as a conceptual compass that orients our fundamental understanding of host defense mechanisms.
Innate and Adaptive Immunity
The Latin term immunis, meaning “exempt,” gave rise to the English word immunity, which refers to all the mechanisms used by the body as protection against environmental agents that are foreign to the body. These agents may be microorganisms or their products, foods, chemicals, drugs, pollen, or animal hair and dander.
Innate Immunity
Innate immunity is conferred by all those elements with which an individual is born and that are always present and available at very short notice to protect the individual from challenges by foreign invaders. The major properties of the innate immune system are discussed in Chapter 2. Table 1.1 summarizes and compares some of the features of the innate and adaptive immune systems. Elements of the innate system include body surfaces and internal components, such as the skin, the mucous membranes, and the cough reflex, which present effective barriers to environmental agents. Chemical influences, such as pH and secreted fatty acids, constitute effective barriers against invasion by many microorganisms. Another noncellular element of the innate immune system is the complement system. As in the previous editions of this book, we cover the subject of complement in Chapter 14.
Table 1.1. Major Properties of the Innate and Adaptive Immune Systems
| Property | Innate | Adaptive |
|---|---|---|
| Characteristics | Antigen nonspecific | Antigen specific |
| Rapid response (minutes to hours) | Slow response (days) | |
| No memory | Memory | |
| Immune components | Natural barriers (e.g., skin, mucous membranes) | Lymphocytes |
| Phagocytes and natural killer cells | Antigen recognition molecules (B and T cell receptors) | |
| Soluble mediators (e.g., complement) | Secreted molecules (e.g., antibody) | |
| Pattern recognition molecules |
Numerous other components are also features of innate immunity: fever, interferons (Chapter 12), other substances released by leukocytes, and pattern-recognition molecules (innate receptors), which can bind to various microorganisms (e.g., Toll-like receptors or TLRs; Chapter 2), as well as serum proteins such as β-lysin, the enzyme lysozyme, polyamines, and the kinins, among others. All of these elements either affect pathogenic invaders directly or enhance the effectiveness of host reactions to them. Other internal elements of innate immunity include phagocytic cells such as granulocytes, macrophages, and microglial cells of the central nervous system, which participate in the destruction and elimination of foreign material that has penetrated the physical and chemical barriers.
Adaptive Immunity
We introduce the subject of adaptive immunity in Chapter 3. Later chapters provide more details about the cellular and molecular features of this arm of the immune system. Adaptive immunity came into play relatively late, in evolutionary terms, and is present only in vertebrates. Although an individual is born with the capacity to mount immune responses to foreign substances, the number of B and T cells available for mounting such responses must be expanded before one is said to be immune to that substance. This is achieved by activation of lymphocytes bearing antigen-specific receptors following their contact with the antigen. Antigenic stimulation of B cells and T cells together with antigen-presenting cells (APCs) initiates a chain of events that leads to proliferation of activated cells together with a program of differentiation events that generate the B- or T-effector cells responsible for the humoral or cell-mediated responses, respectively. These events take time to unfold (days to weeks). Fortunately, the cellular and noncellular components of the innate system are rapidly mobilized (minutes to hours) to eliminate or neutralize the foreign substance. One way to think about this host defense strategy is to consider this as a one-two punch launched initially by innate cells and noncellular elements of the immune system that are always available to quickly remove or cordon off the invader, followed by a round of defense that calls into play cells of the adaptive immune system (B and T cells) that are programmed to react with the foreign substance by virtue of their antigen-specific receptors. Moreover, the clonal expansion of these cells—a process first explained by the clonal selection theory discussed in the section below—gives rise to an arsenal of antigen-specific cells available for rapid responses to the same antigen in the future, a phenomenon referred to as memory responses. By this process, the individual acquires the immunity to withstand and resist a subsequent attack by, or exposure to, the same offending agent.
The discovery of adaptive immunity predates many of the concepts of modern medicine. It has been recognized for centuries that people who did not die from such life-threatening diseases as bubonic plague and smallpox were subsequently more resistant to the disease than were people who had never been exposed to it. The rediscovery of adaptive immunity is credited to the English physician Edward Jenner, who, in the late eighteenth century, experimentally induced immunity to smallpox. If Jenner performed his experiment today, his medical license would be revoked, and he would be the defendant in a sensational malpractice lawsuit: He inoculated a young boy with pus from a lesion of a dairy maid who had cowpox, a relatively benign disease that is related to smallpox. He then deliberately exposed the boy to smallpox. This exposure failed to cause disease! Because of the protective effect of inoculation with cowpox (vaccinia, from the Latin word vacca, meaning “cow”), the process of inducing adaptive immunity has been termed vaccination.
The concept of vaccination or immunization was expanded by Louis Pasteur and Paul Ehrlich almost 100 years after Jenner’s experiment. By 1900, it had become apparent that immunity could be induced against not only microorganisms but also their products. We now know that immunity can be induced against innumerable natural and synthetic compounds, including metals, chemicals of relatively low molecular weight, carbohydrates, proteins, and nucleotides.
The compound to which the adaptive immune response is induced is termed an antigen, a term initially coined due to the ability of these compounds to cause antibody responses to be generated. Of course, we now know that antigens can generate antibody-mediated and T-cell–mediated responses.
Clonal Selection Theory
A turning point in immunology came in the 1950s with the introduction of a Darwinian view of the cellular basis of specificity in the immune response. This was the now universally accepted clonal selection theory proposed and developed by Jerne and Burnet (both Nobel Prize winners) and by Talmage. The clonal selection theory had a truly revolutionary effect on the field of immunology. It dramatically changed our approach to studying the immune system and stimulated research carried out during the last half of the twentieth century. This work ultimately provided us with knowledge regarding the molecular machinery associated with activation and regulation of cellular elements of the immune system. The essential postulates of this theory are summarized below.
As we have discussed earlier, the specificity of the immune response is based on the ability of B and T lymphocytes to recognize particular foreign molecules (antigens) and respond to them in order to eliminate them. The process of clonal expansion of these cells is highly efficient, but there is always the rare chance that errors or mutations will occur, resulting in the generation of cells bearing receptors that bind poorly or not at all to the antigen, or, in a worse-case scenario, cells that have autoreactivity. Under normal conditions, nonfunctional cells may survive or be aborted with no deleterious consequences to the individual. In contrast, the rare self-reactive cells are clonally deleted or suppressed by other regulatory cells of the immune system charged with this role among others. If such a mechanism were absent, autoimmune responses might occur routinely. It is noteworthy that during the early stages of development, lymphocytes with receptors that bind to self-antigens are also produced, but fortunately they are also eliminated or functionally inactivated. This process gives rise to the initial repertoire of mature lymphocytes that are programmed to generate antigen-specific responses with a relatively minute population functionally benign, albeit potentially autoreactive cells (Figure 1.1). The circumstances and predisposing genetic conditions that may lead to the latter phenomenon are discussed in Chapter 13.
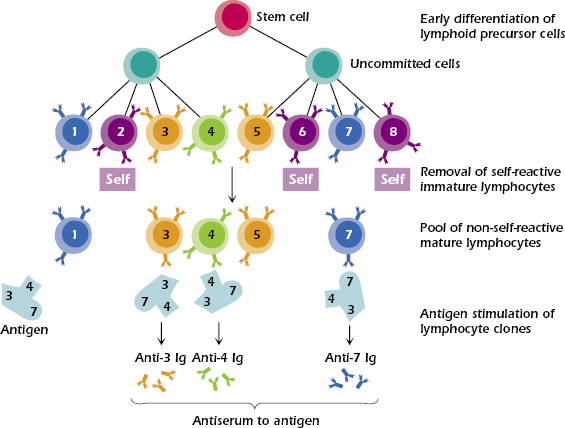
As we have already stated, the immune system is capable of recognizing innumerable foreign substance serving as antigens. How is a response to any one antigen accomplished? In addition to the now-proven postulate that self-reactive clones of lymphocytes are functionally inactivated or aborted, the clonal selection theory proposed the following:
- T and B lymphocytes of a myriad of specificities exist before there is any contact with the foreign antigen.
- Lymphocytes participating in an immune response express antigen-specific receptors on their surface membranes. As a consequence of antigen binding to the lymphocyte, the cell is activated and releases various products. In the case of B lymphocytes, these receptors, so-called B-cell receptors (BCRs), are the very molecules that subsequently get secreted as antibodies following B-cell activation.
- T cells have receptors denoted as T-cell receptors (TCRs). Unlike the B-cell products, the T-cell products are not the same as their surface receptors but are other protein molecules, called cytokines, that participate in elimination of the antigen by regulating the many cells needed to mount an effective immune response.
- Each lymphocyte carries on its surface receptor molecules of only a single specificity as demonstrated in Figure 1.1 for B cells and also holds true for T cells.
- T cells have receptors denoted as T-cell receptors (TCRs). Unlike the B-cell products, the T-cell products are not the same as their surface receptors but are other protein molecules, called cytokines, that participate in elimination of the antigen by regulating the many cells needed to mount an effective immune response.
These postulates describe the existence of a large repertoire of possible specificities formed by cellular multiplication and differentiation before there is any contact with the foreign substance to which the response is to be made. The introduction of the foreign antigen then selects from among all the available specificities those with specificity for the antigen, enabling binding to occur. The scheme shown in Figure 1.1 for B cells also applies to T cells; however, T cells have receptors that are not antibodies and secrete molecules other than antibodies.
The remaining postulates of the clonal selection theory account for this process of selection by the antigen from among all the available cells in the repertoire.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree